Abstract
The main object of this current research was to examine transferosomes as a transdermal delivery system for insulin, to overwhelm the difficulties related with its subcutaneous delivery. Transferosomal gel formulations were prepared by rotary evaporation sonication technique. The result revealed that insulin was successfully entrapped (78%) in optimized formulations (2.5 I.U. of the drug and 25% of sodium cholate) with cumulative percent drug release (83.11 ± 3.782). The glucose lowering study revealed that the transferosomal gel with chemical penetration enhancer showed better glucose lowering effect as compared to the control gel. Consequently, this study authenticated that the transferosomal gel can be used as a possible substitute to the conventional formulations of insulin with progressive permeation characteristics for transdermal application.
Introduction
Diabetes mellitus is a long-lasting metabolic disorder categorized by high blood glucose concentration (fasting plasma glucose more than 126 mg/dL) initiated by insulin deficiency, repeatedly collective with insulin resistance. Transdermal delivery of insulin is a better-suited alternative to overcome the difficulties related with its subcutaneous delivery. The transdermal route, as well being convenient and safe, has several advantages over conventional ones, such as local pain, inconvenience of multiple injections and occasional hypoglycemia. Lastly, it was revealed that by providing insulin treatment with the aid of injections, it was difficult to achieve prolonged glycaemic control in significant number of patients due to numerous reasons which include, noncompliance improved patient compliance, and rapid cessation of drug response.
Despite periods of research, the major obstacle in transdermal drug delivery systems is function of the stratum corneum, which leads to some interesting challenges in improvement of new transdermal drug delivery systems. Vesicular systems have been widely reconnoitered as alternate vehicles for drug delivery via topical and transdermal route. Their assistances in enhancement of drug permeation have been well recognized. Even though the strong justification for the use of vesicles in transdermal drug delivery, the major difficulty for developing vesicular systems at industrial and clinical stages is the poor stability of these carrier systems (Brown et al., Citation2006). Various tactics have been projected to impart higher stability to the vesicular system. Transferosomes compromise a versatile delivery perception for improving the stability and the prospective for being used with a wide range of active compounds. They are metastable forms, which create the ultra-flexible vesicular membrane, which are easily deformable and can easily squeeze via pores in the stratum corneum. It is mainly due to strong membrane flexibility that permits the transferosomal vesicles to lodge in a restricting pore, and thus permeate that pore. But, the low viscosity of transferosomal suspensions helps in transdermal delivery due to its rare usage. Biocompatible gels of pluronicF-127 characteristics of reversible thermal gelation property which offer advantages of handling and ease of application (Pillai & Panchagnula, Citation2003). From the viewpoint of development of peptide/protein formulation, the negative thermo rheological behavior lead to preparation of a homogeneous peptide formulation, which can be stored easily under refrigerated conditions. Therefore, the combination of the transferosomal suspension with the gel matrix can lead to formulation of a transferosomal gel, which may prove to be more pertinent for transdermal drug delivery (Sachan et al., Citation2013). The use of chemical enhancer “Iodophor” will be helpful in moderating delivery and enhance the transport of large peptide Insulin by its peculiar action on the vessel walls, as it increases their penetrability, which results in destruction of their epithelial coat, quickly penetrates into the structures, and produces sensible irritation, thereby relieving pain which may be present in the deep-seated tissues. Also, enlarges the walls of the various vessels and promotes absorption (Sintov & Wormser, Citation2007).
This study is designed to incorporate insulin in the transferosomal gel system for transdermal administration to avoid problems related with its parenteral delivery, and to improve the drug permeation through the skin and finally increase the bioavailability. To assess the transdermal delivery of insulin by using the developed formulation ex-vivo permeation study was also conducted. In addition to this, an in-vivo efficacy of the optimized transferosomal formulation was also examined by carrying out glucose-lowering study in wistar rats.
Materials and methods
Materials
Insulin was obtained from Himedia Laboratories Pvt. Ltd., Mumbai, India. Sodium cholate and soya lecithin were obtained from CDH Analytical Reagents, New Delhi, India. All other chemicals used in this study were of analytical grade. Wistar rats (150–250 g) were obtained from the Central Animal House facility of the I.S.F. College of Pharmacy, Moga, Punjab, India. The experimental protocols were reviewed and approved by the Institutional Animal Ethics Committee (IAEC) as per guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Formulation of insulin loaded transfersomes
Transferosomes were fabricated by using conventional rotary evaporation sonication method. In this technique, transferosomes containing phospholipids (soya lecithin), surfactant (sodium cholate), and the drug (Insulin) were formulated. The phospholipids and surfactant were added to round-bottom flask and dissolved chloroform:methanol (3:1). Then by using rotary evaporation method at 40 °C under reduced pressure (Gupta et al., Citation2012). Then film deposited by above process of rotary evaporation was hydrated with pH 5.5 phosphate buffer i.e. solution of the different drug concentrations by rotating at 60 rpm for 1 h. The vesicles formed by above process were kept for 2 h at ambient temperature for swelling and were sonicated with probe sonicator for 2 min to attain smaller vesicles.
Characterization of elastic liposomal formulations
Morphology and structure of transferosomes
The morphology characteristics of the drug-loaded transferosomes were evaluated by Transmission Electron Microscopy (TEM) (Hitachi H7500, Tokyo, Japan). In this technique, formulation to be tested is converted to a diluted suspension, a drop of which can be directly deposited on the holey film grid, tainted by 1% aqueous solution of phosphotungstic acid, and observed after drying (Sivannarayana et al., Citation2012).
Vesicle size and size distribution
Transferosomes vesicle size and Polydispersity Index (PDI) was analyzed by using dynamic light scattering with the Zetasizer DLS 4C (Beckamn Coulter, Fullerton, CA). All samples were sonicated priorto their PDI determination (Gupta et al., Citation2007).
Entrapment efficiency
Entrapment efficiency was evaluated by first parting of the free or unentrapped drug by use of centrifugation technique. After centrifugation, 0.1% Triton X-100 was added for disruption of the vesicles. The percentage drug encapsulation was calculated by the following equation:
where Ct is the concentration of total drug and Cf is the concentration of unentrapped or free drug.
Development of a secondary topical vehicle (Gel)
All of the developed formulations were observed to be in the nano size range and were incorporated into the gel matrix for formulating transferosomal gel. Poloxamer 407 was used as polymer for formation of the gel matrix base. Transferosomal suspension and small amount of iodophor was slowly added to the viscous pluronic F-127 solution under magnetic stirring.The various concentration of pluronic F-127 was ranging from 20% to 30%. The pH values were successively maintained to 6–9 by using triethanolamine.
Preparation of iodophor (5%)
An accurately measured 50 mg of iodine, 1 g of povidone and sufficient amount of water was added to produce not less than 30 ml of iodophor and titrated immediatly with 0.02 M sodium thiosulfate using 1 ml of starch solution as indicator to carry out blank titration (1 ml of 0.02M Sodium thiosulfate ∼0.002538 g of Iodine).
Optimization of iodophor
Preparation of goat skin for permeation studies
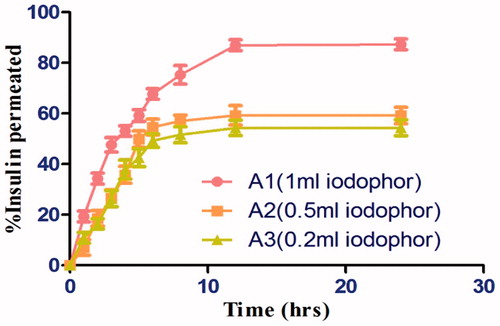
The permeation study was carried out on Hairless animal (goat) skin. The skin was washed with PBS pH 6.8. Different concentration (1 ml, 0.5 ml, 0.2 ml) of 5% iodophor were taken with drug loaded transfersomal gel were studied for percentage release property of drug in accordance to concentraton of iodophor were exposed to permeation studies by utilizing the Franz Diffusion cell in a similar manner as through the cellophane membrane through goat skin. The cumulative amount of drug was permeated through transferosomal gel with different concentrations of iodophor. The cumulative amount of drug permeated across the goat skin was plotted against time and the % release was calculated.
Optimization of transfersomal hydrogel
Optimization of gelation temperature
Gelation temperature of the examined formulations was determined using a ‘Visual Tube Inversion Method’. In brief, 2 ml of the glass vials containing the different types and concentration of thermo sensitive transfersomal hydrogel formulations containing 10 ml of gel in which 5 ml of transfersomes and 0.5 ml of iodophor is added and were placed on a temperature controlled water bath. The temperature was progressively increased with an increment of 1 °C. No fluidity was observed for 5 min, and then we evaluated the phase-transition temperature. The samples were equilibrated for at least for 5 min at each temperature point. The gelation temperature of each formulation was measured at least in triplicate.
Optimization of the gelation time
Gelation time of the each thermo sensitive transferosomal hydrogel formulation was noted down, by injecting the refrigerated hydrogel solution into the empty glass vial which was maintained at 36–37 °C by the Visual Tube Inversion Method. Samples were regarded as hydrogels, if no flow occurred and time was noted down. Each formulation was measured at least in triplicate for its gelation time (Ur-Rehman et al., Citation2011).
Characterization of optmized transfersomal hydrogel
Morphology of transfersomal gel formulations
Transfersomal gel can be characterized for its morphology by the usage of Transmission electron microscopy (TEM) providing two dimensional image 6 order magnified images of a sample under vacuum. The sample was placed on the grid, allowed to dry at ambient temperature and viewed under Transmission electron microscope at suitable magnification. Average diameters of all type of formulations studied were calculated based on the sizes measured from the TEM images (Guo et al., Citation2000).
Differential scanning calorimetry
Differential scanning calorimetry (DSC) is a thermo analytical technique, which involves maintenance of almost same temperature for both the sample and reference throughout the experiment. Usually, the temperature sequencer for a DSC analysis is planned such that the sample holder temperature upsurges linearly as a function of time. The reference sample should have a distinct heat capacity over the numerous range of temperatures which are to be scanned (Brandts & Lin, Citation1990).
Circular dichroism
Circular dichroism (CD) spectroscopy is a spectroscopic technique, which involves measurement of the CD of molecules over numerous ranges of wavelengths. In this technique, a ballpark starting concentration would be 0.5 mg/mL protein formulation, insufficient CD signal (< 5–10 mdeg), increased the concentration of the sample. However, since CD measures the difference between absorbance of orthogonally polarized light, using arbitrarily high concentrations prevented enough light from passing through the sample for meaningful measurements. The HT (dynode) voltage is > 600 V, the total absorbance of sample was too high. Shorter path length (0.5–0.1 mm) cuvettes were used to solve this problem. Wavelength was set to 562 nm and path length was adjusted.
Rheological studies
Rheological properties of thermo sensitive hydrogels were monitored using a rotating rheometer (Brookfield Rheometer, Brookfield Engineering Laboratories Inc., Middleboro, MA) equipped with a temperature controller. A CP 75 spindle was selected for use, which required for samples. The prepared formulation was allowed to form gel and then viscosity was evaluated. Viscosity of samples was measured at different shear rates. The volume of the hydrogel solution was about 300–400 μL, the samples were thermo stated at the required temperature, by circulating bath connected to the viscometer. The shear rate was increased from 0 to 5.4 s − 1 in 5 min. Then from the flow curve which was obtained at different values of shear rate, the viscosity was determined. Shear stress (Pa) was noted down at room temperatures for different samples at an oscillatory frequency of 0.01 Hz. All measurements were made in triplicate (Gupta et al., Citation2012).
In-vitro drug release study through cellophane membrane
The in vitro permeation behavior of insulin from transferosomal gel with iodophor, plain gel with iodophor, transferosomal suspension with iodophor formulations were investigated by using cellophane membrane (Molecular weight cut of 12000–14000, HI Media Ltd, Mumbai, India). The receptor compartment contained 60 mL of phosphate buffer pH 5.5, which was used as the receptor fluid and agitated at 100 rpm, and was maintained at temperature of 37 ± 0.5 °C. The prepared formulation was applied to the membrane in the donor compartment and samples were withdrawn at appropriate time intervals and were replaced immediately with equal volume of fresh diffusion medium. The calculated cumulative amount of drug, which permeated through the cellophane membrane, was plotted against time. To study the release kinetics, the data obtained from in vitro permeation studies were fitted in various kinetic models.
Ex-vivo drug permeation using a diffusion cell
Preparation of goat skin for permeation studies
On the basis of entrapment efficiency, drug content, and permeation through the cellophane membrane the selected formulations were subjected to permeation studies through goat skin by using the Franz Diffusion cell similarly as through the cellophane membrane. The cumulative amount of drug permeated from the developed formulation was compared with the transferosomal suspension, control gel, and drug solution. The cumulative amount of drug which permeated through the goat skin was plotted against time and the flux of the drug was calculated as drug permeated per cm2 per hour.
Confocal laser scanning microscopy (CLSM)
Confocal laser scanning microscopy (CLSM or LSCM) is a technique, which is used for location of high-resolution optical images with depth selectivity. The formulation containing Fluorescein isothiocyanate were applied to abdominal shaved skin of wistar rats. After 24 hrs, rats were dissected and skin was removed and was kept in deep freezer under −78 °C temperature. The sectioning of pre-freezed abdominal skin was done using cryostat MICROM HM 520 (MN). The section was mounted using frozen section medium which contains PEG AND PVA. The following prepared slides were observed under confocal microscope Olympus FV-1000 (Tokyo, Japan) which due to fluorescence property detects skin sample (Meyer et al., Citation2006).
In-vivo characterization of transferosomal hydrogel
Drug loaded transfersomal hydrogel was evaluated using animal models. Diabetes status is characterized by measuring blood glucose level of animals. Diabetes was induced by a single intraperitoneal (i.p) injection of streptozotocin (50 mg/kg) freshly dissolved in citrate buffer pH 4.5. Rats of similar age received dimethyl sulphoxide (DMSO) 0.5% and served as control. Blood glucose level was measured in all these animals before and at various intervals after injecting STZ. Blood glucose was measured by using glucometer method 72 hrs after STZ induction. Only rats with blood glucose concentration more than 240 mg/dL were considered diabetic and used for the study.
Pharmacodynamics studies
Recipient diabetic rats were shaved at abdominal region and topically insulin loaded transfersomal gel was applied and blood glucose level was determined at specific time period according to the formulation requirement. Blood was collected from tail vein at different time intervals after treatments and the blood glucose levels were determined by using glucometer.
Treatment
Details of the work done on the diabetic rats are given in the following . For the administration of the transfersomal hydrogel via transdermal route, the rats of the respective groups were anesthetized with Ketamine (40 mg/kg) and Diazepam (5 mg/kg), following which the transfersomal hydrogel was applied topically. The blood glucose concentration (both fasted and fed) were determined for each group by taking blood from the tail vein (0.2–0.5 ml) as per the following schedule; 0th, 2nd, 4th, 6th, 8th, 10th, 12th, 18th, 24th hour), after the a fore mentioned treatment was given. The blood glucose concentration was determined using the glucometer and the comparisons between the various groups were made.
Table 1. Formulation evaluation study protocol.
Result and discussions
Sodium cholate is biocompatible and pharmaceutically acceptable, therefore it was selected for being used as the edge activator surfactant for the transferosomal formulation. Phospholipid was used as the bilayer-forming agent and ratio of choloroform and methanol was used as the hydrating agent FTIR data of the pure drug insulin and drug in combination with excipients (sodium cholate and soya lecithin) suggested that the drug and the excipients are compatible with each other and there is no interaction between them.
Effect of sodium cholate concentration on size distribution
Results indicates that sodium cholate at higher concentration produces larger vesicles, could be associated to the micellar formation. As the micellar formation takes place the availability of free sodium cholate decrease. results in the formation of leaky vasculature of transfersomes. From the optimization studies, it has been concluded that sodium cholate and phosphitidylcholine in a ration of 1:3, produced transfersome of desired memberane intensity with narrow size distribution. Thus formulation (trans4) was utilized for evaluating maximum drug loading in the formulation.
Effect of concentration of drug on entrapment efficiency
In the selected transferosomal formulation (trans1), the amount of drug was varied in the range of 0.5–1.8% w/w of lecithin and all the developed drug-loaded transferosomes were examined for maximum entrapment efficiency. Initially, it was found that by increasing drug concentration there is gradual increase in entrapment efficiency. However, insulin concentration beyond 10 mg there is a significant decrease in entrapment efficiency, which could be associated to the vesicle size, where insulin due to its solvation property,contribution of each insulin molecule into the entrapment efficiency, which results with increasing vesicle size with prior entrapment efficiency.
Characterization of transfersomes
Vesicle shape
Optical examination revealed that there were no aggregation irregularities and the vesicles appeared as multilamellar vesicles (). Spherical structures were observed in results of TEM, which authorize the vesicular characteristics.
Vesicle size and size distribution
The size and size distribution of optimized drug loaded formulation were determined by photon correlation spectroscopy method using Zetasizer as shown in . As the surfactant concentration was kept to 25%, the vesicle size found to be lowest was (187.9 nm) with increase in surfactant concentration above 25%, the vesicle size rises significantly due to the formation of micellar structure by surfactant instead of forming vesicles. Polydispersity index values of the formulation were observed to be low (0.224–0.380), which indicate uniform particle size distribution within the formulation.
Table 2. Characterization of elastic liposomal formulations.
Preparation and optimization of iodophor
5% Iodophor was succesfully prepared, and was dark brown in appearance and was optimized with drug loaded Transfersomal gel as shown in (). Optimization of iodophor using different concentrations of iodophor showed that: as the concentration of iodophor was increased, maximum release was observed. Hence, the permeation was concentration dependent.
Characterization of transfersomal hydrogel
Morphology of transfersomal gel
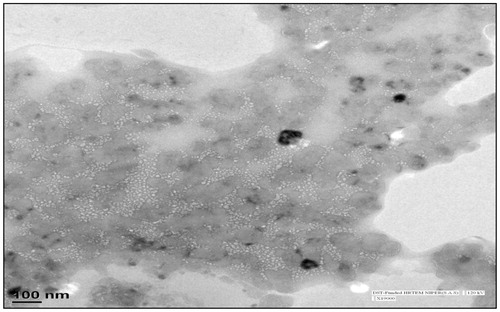
Transfersomal gel can be characterized for its morphology by using Transmission electron microscopy (TEM). Gel consistency and uniformity was analyzed by TEM as shown in (). Photomicrograph TEM results inferred that the transfersomes moniter their original property and uniformily disperse in the developed gel.
Dynamic scattering calorimetry (DSC)
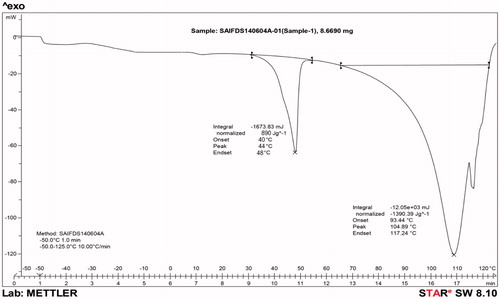
DSC used to determined compatibility between components used in formulation. DSC graph is shown in . Phase transition temperature observed around 111 °C indicated thermodynamic behavior of gel. This could be due to the formation of tri-iodide complex with net decrease to solvation effect;which has been ascribed not only to increase hydrophobicity of developed gel but also adsorption of iodine/iodide into the surface of pluronic facilitates the tri-iodide formation. Increased hydrophobicity and presence of tri-iodide complex decrease the concentration, leading to spontaneous aggregation of polymeric unit with increase in micellization density. Rigid micellar structure results in improved thermodynamic stability of developed formulation.
Circular dichroism (CD)
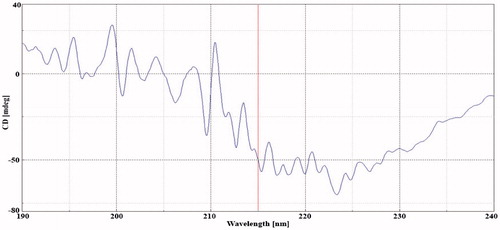
Circular dichroism of insulin is shown in , the characteristic peaks within the scattering wavelength of 190–204 nm. Results further confirms the formulation excipients selected found compatible with insulin. Further, the aqueous core of transfersome, helps to maintain the glassy stage through the hydrogen bonding with the surrounding water. Further under storage, viscoelastic property of gel restrict the mobility of transfersome resulting in a higher formulation stability.
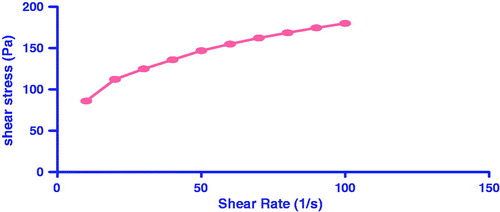
Viscosity measurements and rheological behavior of gel
The viscosity of the transferosomal suspension was found to be low 28.25PaS, which was not appropriate for transdermal application. The transferosomal suspension was combined with a gel matrix, resulting in formation of transferosomal gel having a high viscosities. The viscosity of the transferosomal suspension formulation (trans1) (2.825PaS) was significantly increased in case of the transferosomal gel (8.6paS) due to the incorporation of the pluronic F-127 (2.5%) gel matrix, which lead to increase in suitability for the transdermal administration. The consistency of formulation and behavior of gel formulation was governed by rheological behavior of the gel formulation. The consistency index of transfersomal gel having high viscosity of 8.640 Pa.s and had flow index of (n) 0.389 as shown in . The flow index of a system is measure of deviation of that system from newtonian behavior (n = 1). If the value of n < 1, then flow will be pseudoplastic or shear thickening. Flow index provides an idea about the flow properties of the formulation from the container. The flow index of the gel was found to be 0.389, which indicates pseudoplastic flow. The pseudoplasticity is due to formation of a colloidal network structure, which gets alligned in the directon similar to direction of shear, which lead to decrease in viscosity with increase in shear rate. Because of the pseudoplastic flow, the developed system will require application of some force to expel. The viscosity of transfersomal gel increased with increase in surfactant concentration. Sodium cholate was used as surfactant, which was having high solubility in the external aqueous phase. Increase self-association of these amphiphillic molecules and formed various sizes and shapes of micellar aggregates. With increase in concentration in external phase, there is formation of network between the surfactant molecules, micelles and vesicles. The formation of network leads to increase in density, decrease in distance between the dispersed phase and ultimately increase in viscosity.
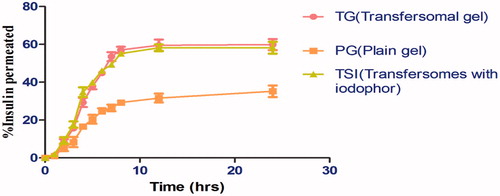
In-vitro drug release study through cellophane memberane
Results revealed that the transferosomal suspension is having the highest cumulative amount of drug release (82.52 ± 1.29%) up to 24 h in comparison to other transferosomal gel and plain gel formulations. The release studies clearly indicated that transferosomal gel release the drug in sustained manner. The highest release was observed in suspension, because of association of surfactant molecule with the phospholipid bilayer leading to better partitioning of the drug, and ultimately increase the drug release from the vesicles as shown in .
A comparitive drug release profile of tested formulation clearly depicts that transfersomal suspension containing iodophor exhibit maximum drug release as compared to transfersomal gel containing iodophor and plain gel containing iodophor. The slower drug release from plain iodophor gel could be attributed to rigid micellar network of polymerization micelles induced by tri-iodide complex leading to an increasing viscosity of the gel. Further there is a significant difference in release rate was observed in presence of transfersomes. Clearly establish the permeability enhancement behavior of transfersomes. Better permeability of transfersomes associated to physical property related to surfactant i.e. sodium cholate,sodium cholate with an HLB value of 18 improves the wetability and solubility of the day in the gel. Moreover, a maximum release was observed in transfersomal suspension and is largely dependent on viscosity. Whereas pluronic force formulation exhibit significant lower viscosity. Release kinetics analysis demonstrated a maximum R2 of 0.9481 corresponding to Higuchi model as shown in , which indicate a delayed drug release behavior of gel formulation. Further, the mechanism of drug release following diffusion and erosion behavior confirmed by korsmeyer peppas exponent value of 0.886.
Table 3. Release kinetics of various formulation.
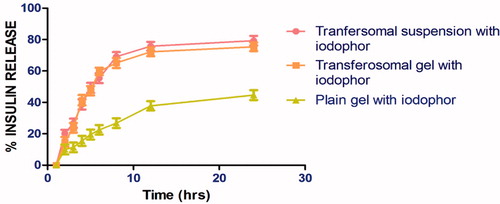
Ex-vivo: skin permeation studies
In-vitro skin permeation studies were performed using hairless goat abdominal skin by Franz diffusion cell. Results of permeation studies are shown in and . In the present study, the transferosomal gel with iodophor was compared with the transferosomal suspension containg iodophor, plain gel. The maximum transdermal permeation was observed for the suspension than that of the transferosomes gel. Furthermore, a significant difference was observed in lag time for the transferosomal suspension and gel, which may be due to the higher viscosity of the gel formulation in comparison to suspension. Further, the iodophor contain gel exhibit better permeation than iodophor free gel. This could be due to increase in micellar density and higher solubilizatio capacity of iodophor containing gel. Iodophor reported to Iodophor are counterirritant, it has a peculiar action on the vessel walls, as it increases their penetrability, further increased micellar density contribute higher skin permeability of iodophor containing gel.
Table 4. Release kinetics of diferent formulations.
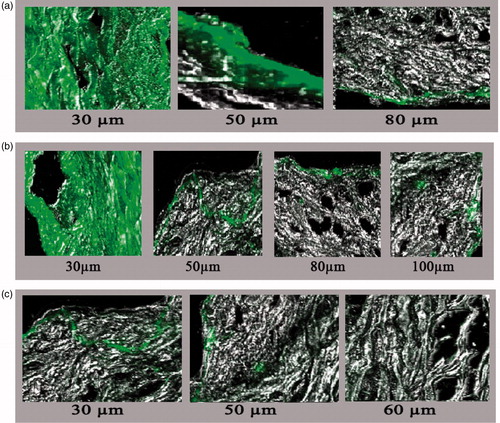
Confocal laser scanning microscopy
In this study, laser confocal scanning microscopy was used to investigate penetration enhancement effects of tested formulations. Results of confocal microscopy suggests that the transfersomal gel containing iodophor have a significantly greater efficacy in the transdermal delivery of insulin. This could be released due to the ability of iodophor to disrupt the hydrogen and disulfide linkage of keratin followed by increased membrane permeability.Further, the membrane fluidization and structural modification of the epidermis is possible mechanism for the high transport of encapsulated drugs in elastic vesicles. Further, the surface property and elasticity of vesicle membrane plays an important role in percutaneous absorption of therapeutic agent.Then, observation are consistent with the proposed hypothesis. Indeed the presence of tri-iodide complex in formulation may enhance both paracellular and transcellular uptake of insulin.Moreover, the membrane elastic behavior of transfersome, may be able to squeeze themselves through intracellular region of stratum corneum deliverying encapsulated drugs in dermis. Further, hydrogel behavior of pluronic F-127 benefits of high skin hydration,which generally favor percutaneous absorption. More of the occlusive matter of the developed gel and membrane. property of pluronic resulting in self absorption in iodophor gel than the plain gel. Further, a relatively high flouroscence in iodophor preparation than the plain gel attributed to better skin permeability in iodophor preparation. Moreover, plain gel exhibit least flouroscence released to poor emulsification of developed system. As shown in .
In-vivo evaluation of transfersomal iodophor containg gel
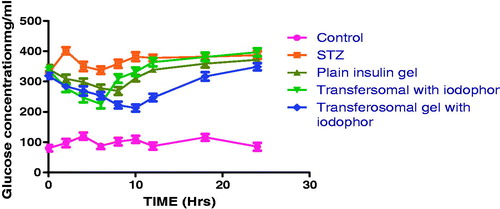
Pharmacodynamic study demonstrated that none of the tested formulation attains the normal blood glucose level. However, there is a significant difference in a blood glucose level was observed with transfersomal gel containing iodophor as compared to disease control. This could be attributed to higher permeability ability of transfersomal gel containing iodophor. As discussed in earlier section, the higher micellar density and ability of iodophor to disrupt epidermal layer and breaking of disulfide linkage to active higher drug permeability. Moreover, a transfersomal suspension containing gel iodophor exhibit relatively higher initial reduction in blood glucose level, followed by a steep increase in blood glucose.This could be related to viscosity which is unable to control the release of drug. Reduction in blood glucose level in plain gel estabilish the penetration enhancer behavior of pluronic gel. A sustained reduction in blood glucose by a gel conatining iodophor further estabilish the controlled release behavior of the formulation ().
Conclusion
The present study revealed that the developed transferosomal gel can be applicable for enhancement of delivery of insulin via skin. Various in-vitro and in-vivo studies clearly indicated that developed transdermal transferosomal formulation may serve as a promising carrier for insulin and other proteins, especially because of their properties such as simple production and simplistic scale-up.
References
- Brandts JF, Lin LN. (1990). Study of strong to ultratight protein interactions using differential scanning calorimetry. Biochemistry 29:6927–40
- Brown MB, Martin GP, Jones SA, Akomeah FK. (2006). Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13:175–87
- Guo J, Ping Q, Sun G, Jiao C. (2000). Lecithin vesicular carriers for transdermal delivery of cyclosporin A. Int J Pharmaceut 194:201–7
- Gupta A, Aggarwal G, Singla S, Arora R. (2012). Transfersomes: a novel vesicular carrier for enhanced transdermal delivery of sertraline: development, characterization, and performance evaluation. Scientia Pharmaceut 80:1061–80
- Gupta A, Prajapati SK, Balamurugan M, et al. (2007). Design and development of a proniosomal transdermal drug delivery system for captopril. Trop J Pharmaceut Res 6:687–93
- Meyer LE, Otberg N, Sterry W, Lademann J. (2006). In vivo confocal scanning laser microscopy: comparison of the reflectance and fluorescence mode by imaging human skin. J Biomed Opt 11:044012
- Pillai O, Panchagnula R. (2003). Transdermal delivery of insulin from poloxamer gel: ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J Contr Release 89:127–40
- Sachan R, Parashar T, Singh V, et al. (2013). Drug carrier transfersomes: a novel tool for transdermal drug delivery system. Int J Res Dev Pharmacy Life Sci 2:309–16
- Sintov AC, Wormser U. (2007). Topical iodine facilitates transdermal delivery of insulin. J Control Release 118:185–8
- Sivannarayana P, Rani AP, Saikishore V, et al. (2012). Transfersomes: ultra deformable vesicular carrier systems in transdermal drug delivery system. Res J Pharmaceut Dosage Forms Technol 4:243–55
- Ur-Rehman T, Tavelin S, Gröbner G. (2011). Chitosan in situ gelation for improved drug loading and retention in poloxamer 407 gels. Int J Pharmaceut 409:19–29