Abstract
Recently, microneedle arrays (MAs) have been developed for painless inoculation of vaccines and possess many prominent advantages, including convenience for inoculation, and exact delivery of vaccine to the exact epidermal and dermal or mucosal compartments which teem with antigen-presenting cells (APCs). Among different types of MAs, while the micro-environmental stimulus-responsive MAs represent one of the developmental trends in the field, the MAs combined with the conventional vaccines that are based on nonvirulent viruses, such as live attenuated or whole inactivated viruses, and antigen-encoding DNA viral vectors, have developed rapidly into the advanced stages, with certain products already on clinical trials. The pre- and clinical research outcomes showed that the painless MA delivery of the conventional vaccines through mammalian skin or mucosa can not only elicit robust systemic and even mucosal immunity to pathogens but also, in certain circumstances, redirect the immune response toward a specific Th1 pathway, resulting in cytotoxic T lymphocytes (CTL) to erase the cell-hidden pathogens, thanks to the robust adjuvant function of MAs exerted through damaging the contacted cells to release dangerous signals. This paper focuses on reviewing the latest research and advancements in MA delivery of the conventional vaccines that are based on nonvirulent viruses, underlining MA enhancement of the overall vaccine performance and the most advanced MA vaccine products that are relatively close to markets.
Introduction
Vaccination is widely recognized as the most cost-effective and the best prophylactic strategy for the treatment of many diseases, such as pathogenic infections, inflammations, and even malignant tumors (Ozawa et al., Citation2012). Unfortunately, most of the available vaccines are administered by intramuscular (i.m.) or intradermal (i.d.) injection and are, thus, inevitably associated with numerous unwanted disadvantages, such as causing needle phobia and low patient compliance, the risk of potential contamination by needles, a need for highly trained personnel for inoculation and, especially, triggering poor mucosal immunity (Levine, Citation2011). Obviously, it is just the mucosal immunity that is one of the first barriers to the entry of the vast majority of human pathogens, which are transmitted mainly through crossing mucus membranes, and, thus, mucosal immunity is highly desired by people, especially, in the areas with poor sanitation (Chen & Zehrung, Citation2012; Wang et al., Citation2014b,c). To overcome the drawbacks associated with injections, researchers have tried novel strategies to allow inoculation of vaccines through alternative routes, such as oral uptake, inhalation, intranasal and intra-oral cavity administration, and even skin patching (Childress et al., Citation2014; Domingues et al., Citation2014; Low et al., Citation2015). Encouragingly, some of the noninjection vaccines have been marketed for prophylaxis of corresponding infections (Childress et al., Citation2014), offering great benefits to recipients and, especially, to the ones with needle phobia.
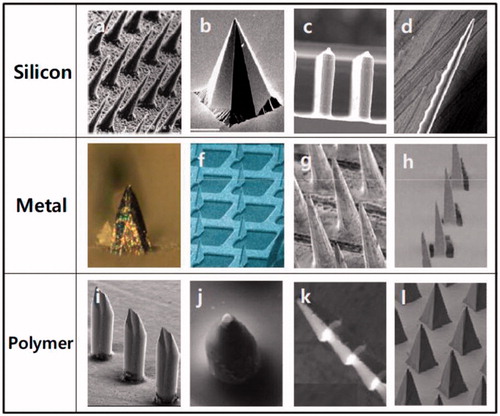
Notably, in the past two decades, microneedles have also been developed for delivery of various vaccines via cutaneous or mucosal routes and have attracted much attention of researchers and pharmaceutical developers (Henry et al., Citation1998; Kim et al., Citation2012; Cheung & Das, Citation2016). Due to limited cargo-loading capacity, usually many an identical microneedle is assembled on a fixing pedestal forming a microneedle array (MA), which is often made of metal, silicon, and polymers, to enlarge loading content and enhance delivery efficiency (Kim et al., Citation2012). Microneedles on a MA are needle-like structures that have a variety of shapes () and are usually shorter than 1 mm for piercing the stratum corneum or stratified squamous epithelia to facilitate the delivery of agents into the skin or mucosa (Wang et al., Citation2015d; Zhen et al., Citation2015). Moreover, for effective vaccine delivery, microneedles should be long enough to pierce the cutaneous or mucosal surfaces, but preferably short enough to avoid bleeding and pain-causing, rendering them advantages over other devices in that, they pierce the skin or mucosa painlessly while deliver vaccines efficiently to the cutaneous or mucosal compartments enriched in APCs (antigen-presenting cells), such as Langerhans cells, dendritic cells, and macrophages, providing the basis for robust immunostimulatory effects and a significant antigen-sparing potential (Prausnitz et al., Citation2009; Pearton et al., Citation2010; Pattani et al., Citation2012; Norman et al., Citation2014). In particular, MA vaccines possess the potential to simplify immunization programs by eliminating the use of hypodermic metal needles, allowing self-administration of vaccine during pandemics and thus facilitating large-scale immunization in developing nations with a critical shortage of health-care workers (Al-Zahrani et al., Citation2012; Zipursky et al., Citation2014).
Figure 1. Different type of MAs made of silicon, metal, and polymer with microneedles of different shapes. Reprinted with permission from Butler (Citation2015).
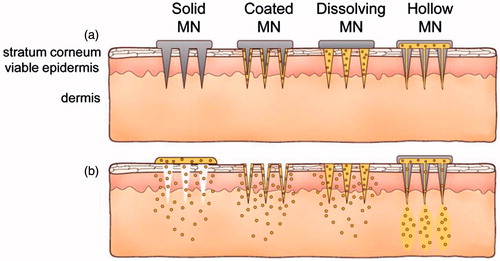
To increase immunization efficacy, different types of MAs have been designed for the delivery of vaccines and can be classified into two categories: dissolvable and nondissolvable ones (Kim et al., Citation2012). Usually, the nondissolvable MAs include either the hollow metal microneedles for injection of the enclosed contents or the solid microneedles that are made of stiff inorganic materials, such as metal, silicon, glass, and ceramics, bearing, or not, active ingredients on their surfaces and, therefore, function mainly as poking or penetration (). The dissolvable MAs are usually fabricated with the biocompatible materials that can be degraded or dissolve in body fluids, and are either formulated as a homogeneous matrix entrapping antigen/adjuvant or engineered into specific multifunctional particles carrying pathogenic antigens (Wang et al., Citation2015d; Zhen et al., Citation2015). Both dissolvable and nondissolvable MAs have been developed for delivering a variety of vaccines, such as the conventional live attenuated or whole inactivated pathogens, plasmid DNA, and the subunit vaccines (Qiu et al., Citation2016). In particular, MAs bearing the conventional vaccines, such as live attenuated and whole inactivated viruses, have been developed into more advanced stages than those bearing other types of vaccines, with some products already on clinical trials (Wang & Wang, Citation2015). Pre- and clinical studies demonstrated that MA vaccination through, in most cases, mammal skin which is enriched in professional APCs, can elicit robust systemic and even mucosal immunity against the related pathogens and can even direct the immunoresponses toward a specific Th1 pathway, resulting in the cytotoxic T lymphocytes (CTLs) with specific killing activity to erase the infected cell-hidden pathogens (Suh et al., Citation2014). This paper focuses on reviewing the current advances and latest research in MA delivery of conventional vaccines that are mainly comprised of the live attenuated or whole inactivated pathogens to provide readers certain knowledge on MA vaccines.
Figure 2. Methods of drug delivery to the skin using different MAs. Microneedles are first applied to the skin (a) and then used for drug delivery (b). Solid microneedle are used as a pretreatment, after which drug can diffuse through residual holes in skin from a topical formulation (solid MA). After insertion of drug-coated microneedles into the skin, the drug coating dissolves off the microneedles in the aqueous environment of the skin (coated MA). Drug-loaded microneedles are made of water-soluble or biodegradable materials encapsulating drug that is released in the skin upon microneedle dissolution (dissolving MN). Hollow microneedles are used to inject liquid formulations into the skin (hollow MA). Reprinted with permission from Butler (Citation2015).
Preparation of vaccine-loaded MAs
The nondissolvable MAs made of inorganic materials, such as metal, silicon, glass, and ceramics, are usually prepared by the microelectromechanical systems (MEMS) technology including photolithography and reactive ion etching (RIE; Donnelly et al., Citation2010; Qin et al., Citation2010; Kim et al., Citation2012). The dissolvable MAs are usually fabricated using the specific microneedle array inverse molds (MAIMs), which defining the number, geometry, and size of the microneedles. To make the MAs, the materials are firstly prepared with or without vaccine ingredients and then casted onto the MAIMs, and after removal of water and any other solvents by drying, the solidified MAs are formed (Kim et al., Citation2012). Though this casting and drying process for making MAs is simple, production of either the vaccines or the MAIMs is never an easy thing, requiring, especially in making MAIMs, a comprehensive design and – many rather complicated techniques in the field of precision apparatus and MEMS (Wendorf et al., Citation2011; van der Maaden et al., Citation2012).
While one of the most commonly used materials for preparing MAIMs is polydimethylsiloxane (PDMS) (Kim et al., Citation2012; van der Maaden et al., Citation2012), other materials such as ceramics and even purple sands have also been tried on making the molds with the advantage of simplifying the process by saving several steps as well as some types of special precision instruments (Bystrova & Luttge, Citation2011; Yang et al., Citation2012). Recently, Jin’s group has successfully fabricated the MAIMs using the purple sands which are the most often used materials for making the teakettles and stewpots in Chinese kitchens (Yang et al., Citation2012). In their process, the purple sands are first wetted into the plastic mud to generate a soft adobe, which is then poked with tips of the orderly arrayed metal needles to form soft MAIMs, and then the final hard MAIMs following the drying and calcinating steps. The purple sand MAIMs have advantages in that they are energy-saving and environment-friendly products involving no organic solvents during making, while the MAIM entity is “breathable” but water impermeable, which is favorable for filling the microholes with liquid vaccine formulations using a vacuum applied to the opposite side of the MAIMs. By comparison, preparation of the MAIMs with PDMS is rather complex and involves toxic solvents and many energy-consuming steps (Park et al., Citation2005; Wilke et al., Citation2005; Donnelly et al., Citation2011). Generally, a MAIM of PDMS is fabricated from the master mold, of which the MA to be made is actually an exact replica. The master molds may be made of different materials, such as silicon, titanium, stainless-steel, and glass, and are often fabricated by photolithography of the MEMS technology, of which the detailed process can be found in previous papers (Qin et al., Citation2010; Wang & Wang, Citation2015).
Both the dissolvable and nondissolvable MAs have been employed for the delivery of conventional virus-based vaccines, which are defined here as the live attenuated or whole activated viruses or the nonvirulent adapted viruses as a vector for antigen-encoding DNAs. For delivery of vaccines, the nondissolvable solid MAs are usually coated with vaccine ingredients on their microneedle surface or used nakedly for poking and penetration to allow the independent vaccines to pass through stratum corneum (Depelsenaire et al., Citation2014). In most cases, the metal MAs with hollow microneedles are designed into a special syringe device that may, or not, be prefilled with vaccines for injection into superficial layer of skin (Laurent et al., Citation2010). For delivery by dissolvable MAs, vaccines are either coated on preformed microneedle surface or are incorporated within individual microneedles during MA fabrication (Chu et al., Citation2016). Thus, a vaccine-loaded dissolvable MA generally comprises two parts: a pedestal and a number of identical microneedles fixed on the pedestal. While the basement is, in most cases, composed of inert materials as matrix, the microneedles contain vaccines, adjuvants, and the excipients that are well-chosen for shaping, protecting, and increasing the adjuvanticity and efficacy of the vaccines. The currently developed MA vaccines include the subunit ones with isolated antigenic proteins expressed on the pathogens, the live attenuated or inactivated pathogens such as viruses and bacteria, and recombinant virus vectors carrying DNA encoding pathogenic antigens (Carey et al., Citation2014; Edens et al., Citation2015; Wang et al., Citation2015d). In addition, MA vaccines are often entrapped or integrated into the biocompatible materials which are functionally engineered to increase the delivery efficiency and immunization efficacy of the vaccines (Wang et al., Citation2015b,c).
MA delivery of nonvirulent virus-based vaccines
The nonvirulent virus-based vaccines defined here include both the conventional vaccines composed of whole inactivated viruses, live attenuated viruses, and the vaccines made of the genetically engineered viruses that carry DNAs encoding the pathogen antigens. Although numerous novel types of vaccines have been developed in recent years, the conventional vaccines composed of inactivated or attenuated viruses occupy most of the immunization products that are currently used for prophylaxis of the infectious diseases, and therefore their development and production represent the most sophisticated and matured methods and techniques in vaccinology field (Arya & Prausnitz, Citation2015). However, MAs as a novel VADS have been in development for only a little more than a decade, with the early appearance of reports on using microneedles for vaccine delivery at the beginning of this new century (Mikszta et al., Citation2002; Martanto et al., Citation2004). Obviously, MAs combined with the conventional vaccines, for which researchers have already developed relatively matured technologies and procedures, are expected to have the best chance of winning the clinical race at the highest pace, as evidenced by the fact that several licensed vaccines based on nonvirulent virus strains are approaching or have already reached clinical trials to evaluate the pros and cons of MA delivery (Arya & Prausnitz, Citation2015; Hotez et al., Citation2016).
Influenza vaccines with MA delivery
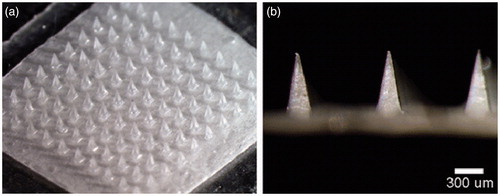
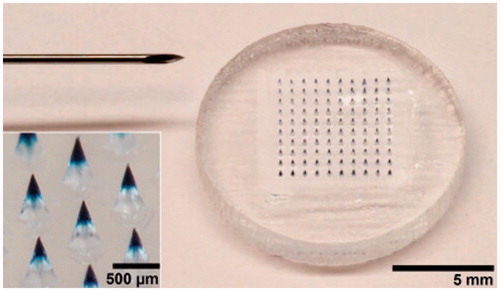
Influenza vaccines are most frequently used products that are administered in humans to defend the viral infections that occur most frequently in all ranges of population, including children, adults as well as the olds. Recently, Prausnitz’s group successfully formulated a thermostable MA influenza vaccine that has an increased stability and has the potential to be used in the districts lack of the integrated cold chain (Chu et al., Citation2016). The researchers casted 1.8 μL of the formalin-inactivated influenza virus vaccine (A/Puerto Rico/8/34) solution into a PDMS MAIM containing 10 × 10 arrays of pyramidal cavities with each cavity (300 × 300 × 600 μm: L × W × H) having a center-to-center spacing of 640 μm. Then the molds filled with the vaccine casting solution were partially dried and reloaded with a PVA/trehalose matrix casting solution to fill the remaining space in the mold cavities and form a backing that connected all the microneedles into a single patch. Finally, the influenza vaccine-entrapped MAs () were obtained through lyophilization and evaluated on the stability by in vitro and in vivo assays of antigenicity and immunogenicity after storage for up to 3 months at 4, 25, 37, and 45 °C. The experimental results showed that while liquid vaccine completely lost the potency of hemagglutination (HA) activity within 1–2 weeks outside of refrigeration, MA vaccine lost 40–50% HA activity during or shortly after fabrication and had no additional loss of activity over 3 months of storage, independent of temperature and oxygen presence but requiring reduced humidity. Also, further immunogenicity stability assays showed that vaccination with MA vaccine stored for 1 month at all three of the temperatures tested generated HAI titers that were indistinguishable from the freshly made microneedle patches and the intramuscular injection of liquid vaccine stored for 1 d at 4 °C. Similar to the HAI responses, IgG titers were similar among animals vaccinated with microneedle patches stored for 1 month at various temperatures compared to microneedle patches or liquid vaccine stored for 1 d at 4 °C . And 4 weeks after immunization, all mice vaccinated with MA vaccines stored at any temperature were completely protected against the virus challenge with lethal dose of live, mouse-adapted A/Puerto Rico/8/34 virus, which contrasted the mice immunized with intramuscular injection of liquid vaccines. Thus, the MA influenza vaccine has an remarkably increased stability fully satisfying being dispatched and vaccinated in the controlled temperature chain (CTC) instead of the integrated cold chain, which is sometimes unavailable in less-developed countries (Zipursky et al., Citation2014).
Figure 3. Microneedle patch encapsulating inactivated influenza vaccine. (a) A patch containing a 10 × 10 array of pyramidal microneedle viewed from above. (b) A side view of individual microneedles. Reprinted with permission from Hotez et al. (Citation2016).
Interestingly, these researchers further found that drying vaccines on PDMS MAIM led to increased stability compared with drying on stainless-steel and that a number of excipients, such as polysaccharides and some amino acids, could further stabilize the vaccine during the drying process (Mistilis et al., Citation2015). Thus, formulation of inactivated influenza vaccines into MAs can increase not only the vaccine delivery efficiency but also the stability of the anhydrous vaccine products. These primary and extensive explorations are key steps in advancing MA-based vaccines into the practical application products, which may greatly facilitate global vaccination against many lethal infectious diseases.
To reveal the mechanisms underlying the microneedle-piercing-skin-mediated “adjuvant” effect, researchers fabricated high-density silicon MAs with >20 000 cm−2 100-μm-long microneedles, which were then coated with inactivated trivalent influenza vaccine (15 μg of HA per dose of inactivated split-virion influenza virus) for immunization of mice (Depelsenaire et al., Citation2014). These researchers demonstrated that dynamic application of silicon MA vaccines to mouse ear skin generated localized transient stresses, invoking cell death around each projection, and yielded anti-HA IgG endpoint titers, all at significantly higher levels than did i.d. injection of vaccines with a hypodermic needle. More importantly, the researchers proved that colocalization of cell death and the delivered antigen nearby live skin cells was necessary for immunogenicity enhancement, indicating a correlation between the cell-death caused by the microneedle piercing and the increased immunogenicity. Thus, the investigation revealed that it is the localized cell death that served as a “physical immune enhancer” for the adjacent viable skin cells, which is believed to be, at least partial, the mechanisms underlying blank MA adjuvant effects on vaccination.
Interestingly, to explore the “damaged cell adjuvant” effect to enhance i.d. vaccination efficacy, Wu’s group combined MA vaccine delivery with the nonablative fractional laser (NAFL; Wang et al., Citation2015a). NAFL can engender by illumination in the inoculation site an array of microthermal zones (MTZs) beneath the stratum corneum, wherein a controllable skin injury serves as a safe “adjuvant” thanks to the within dying cell-released “danger” signals which can provoke sterile inflammation constrained within individual MTZs (Wang et al., Citation2014a). The researchers fabricated the dissolving MAs incorporating the inactivated PR8 influenza viruses or BCG (bacillus Calmette–Guérin) vaccine in the 6 × 9 microneedles (600 μm in height and 200 μm in base diameter) with PVP as a matrix and pedestal, which were formed by irradiation with 300 nm-UV light of a mixture of vinylpyrrolidone, free radical initiator azobisisobutyronitrile, and vaccine ingredients in PDMS MAIMs for polymerization in situ. It was found that, when the inoculation site was treated by the FDA-approved NAFL before insertion of a PR8 model influenza vaccine-packaged MAs, mice displayed vigorous antigen-uptake and established strong Th1-biased immunity, which completely protected mice from homologous viral challenges, but fully and partially protected mice from heterologous H1N1 and H3N2 viral challenges, respectively, whereas mice receiving MAs alone suffered from severe illnesses or died of similar viral challenges. Additionally, with the approach of combining MA delivery with NAFL, animal models exhibited strong protective immunity without incurring any appreciable skin irritation, in sharp contrast to the overt skin irritation caused by i.d. injections. Further exploring disclosed that NAFL-mediated adjuvanticity was ascribed primarily to dsDNA and other “danger” signals released from laser-damaged skin cells, as also supported by the observation that mice deficient in dsDNA-sensing pathway, but not TLR or inflammasome pathways, showed poor responses to prior NAFL treatment. These results indirectly rationalize Matzinger’s “danger hypothesis” for vaccine adjuvants which are supplemented by others with apoptotic and necrotic cells acting as immunostimulants by releasing damage/danger associated molecular patterns (DAMPs), enhancing cellular and humoral immune responses to antigen (Matzinger, Citation1994; Marichal et al., Citation2011). Thus, combination of MA vaccine delivery with NAFL can not only significantly augment vaccine efficiency but also broaden the cross-protection against homologous and heterologous influenza viral infections, which is pivotal for the development of influenza vaccines since mismatches occur frequently between vaccine viral strains and circulating viruses (Dormitzer, Citation2015), representing a novel strategy for lesion-free cutaneous vaccination which deserves further development toward clinical use.
Although in preclinical studies, MAs hold great promise for vaccine delivery; their potential economic and epidemiologic impacts should have been evaluated in practice and clinical application. Recently, Lee et al. evaluated the potential economic and epidemiologic impacts of MA immunization using an economic model by assessing the value of MA delivery of the seasonal influenza vaccine (Lee et al., Citation2015). Through analysis with a susceptible-exposed-infectious-recovered transmission model linked to an economic influenza outcomes model, the researchers concluded that when health-care providers administered the vaccines, the MA introduction would be cost-effective or dominant (i.e. less costly and more effective) in the majority of scenarios assessed; if self-administration were available, MA introduction would also be cost-effective on the condition that MA increased compliance enough to overcome any decrease in self-administration success or that the MA presentation afforded an increase in efficacy over current delivery methods for influenza vaccines. Thus, when MA vaccines are developed, the vaccine delivery efficiency as well as the compliance should be sufficiently concerned at the beginning design stages of the products.
Measles vaccines with MA delivery
Despite the widespread availability of inexpensive and effective vaccines for prophylaxis, measles virus infection is still one of the leading causes for the vaccine-preventable morbidity and mortality among children worldwide; especially, in recent years measles cases abruptly rise even in some developed countries due to reduced vaccination rate (Butler, Citation2015). Currently, the measles vaccine is delivered by subcutaneous injection using a needle and syringe and thus requires specifically trained health-care personnel to administer each vaccine dose, typically at centralized locations. Obviously, if MA vaccines against measles were available, the vaccination could be performed by minimally trained medical personnel and thereby vaccination rate would be markedly enhanced to facilitate the worldwide measles reduction and elimination programs.
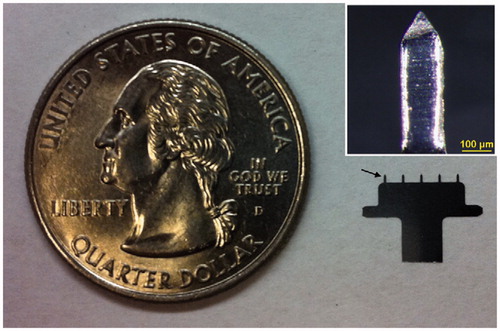
Recently, Edens et al. engineered a product of the live attenuated measles (Edmonston–Zagreb strain) vaccine which was then coated together with trehalose as a stabilizer onto the microneedle surface of a steel MA containing a single row of five pyramidal microneedles with a 750-μm length and a −200 × 50-μm2 – base (; Edens et al., Citation2013). As a result, the viability of vaccine virus dried on MAs was well maintained, and the loss of viral titer was less than 1 log10(TCID50) (TCID50, 50% tissue culture infective dose) after storage for at least 30 d at room temperature, meeting the current WHO standard for the stability of lyophilized measles vaccine which is set at less than 1 log10(TCID50) unit of infectivity loss after 30 d at 25 °C or after 1 week at 37 °C (Galazka et al., Citation1998). Cotton rats i.d.ly received the MA measles vaccine at doses equaling to the standard human dose or one-fifth the human dose generated neutralizing antibody levels equivalent to those of a subcutaneous immunization at the same dose (Edens et al., Citation2013). The results indicate convincingly that, with trehalose as a stabilizer, live-attenuated measles vaccine can be stabilized on the steel MA microneedles and then can be efficiently reconstituted in vivo after vaccination having the ability to effectively elicit mice to generate a neutralizing antibody response equivalent to that elicited by subcutaneous injection.
Figure 4. A five-needle MA next to a U.S. quarter coin with a diameter of 24 mm. The arrow points at one of the microneedles mounted on the holder. Inset: a single microneedle coated with measles vaccine in a trehalose-based coating formulation. Reprinted with permission from Merkle (Citation2015).
Going further, the researchers incorporated the measles vaccine into microneedles of the dissolving MAs which were fabricated with sucrose, threonine, and carboxymethyl cellulose as a pedestal and matrix and evaluated the immunogenicity of the biodegradable MA vaccine in nonhuman primates (; Edens et al., Citation2015). Also, the MA measles vaccine maintained full potency for almost 4 months at 25 °C, and had less than a 10-fold decrease in potency after almost 4 months at 40 °C, contrasting the commercial lyophilized measles vaccine of which the potency at 40 °C was reduced by more than100-fold after 28 d and more than 1000-fold within 3 months. When the dissolving MA measles vaccine was i.d.ly administered to rhesus macaques, the microneedles dissolved in skin within 10 min with side effects of only mild and transient skin erythema. In addition, the macaques generated neutralizing antibody responses to measles that were consistent with protection and the neutralizing antibody titers, which could fully protect the nonhuman primates against the virus challenges. Obviously, the results obtained from nonhuman primates provide valuable and closely direct information to the development of human MA measles vaccines that have the potential to increase vaccination coverage due to the painless and convenient administration mode.
Figure 5. Dissolvable MA for measles vaccination. A microneedle patch is shown next to a 25-gauge hypodermic needle. The patch contains 100 solid microneedles made of water-soluble excipients that encapsulate measles vaccine for delivery to the skin. The inset photo shows a magnified view of the microneedles. To facilitate imaging, the microneedles encapsulated dye (Trypan blue) instead of vaccine. Reprinted with permission from Jacoby et al. (Citation2015).
Polio vaccines with MA delivery
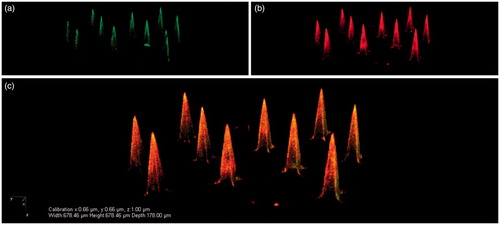
The recent advent and advancement of combinatory and click chemistry make it possible for scientists to synthesize functional materials that can be employed to construct the drug/vaccine-loaded multifunctional carriers that can serve as vehicles for targeting delivery, sustained or controlled release, and even microenvironment stimulus-responsive cargo dump (Merkle, Citation2015). Recently, Bouwstra’s group made pH-sensitive silicon MAs by coating 200-μm-long microneedles with inactivated polio vaccine (IPV) particles and N-trimethyl chitosan chloride (TMC) via electrostatic interactions () (van der Maaden et al., Citation2015), providing an alternative strategy to dispersing particles within microneedles for preparing MA vaccines. After modification with pH-sensitive (pyridine) groups, the microneedle surfaces were alternately coated with negatively charged IPV and a positively charged polymer TMC. Finally, the pH-sensitive microneedles were homogeneously coated with 10 layers of both IPV and TMC, resulting in 45 D-antigen units IPV (1 D-antigen unit contains ∼13 ng viral protein, D-antigen represents a distinct type of antigen associated with infectious polio virus) and 700 ng of TMC per silicon MA. It was demonstrated that both IPV and TMC were released into ex vivo human skin upon application, while in vivo application of IPV/TMC-coated pH-sensitive MA vaccine in rats resulted in the production of high levels of IPV-specific antibodies. The results illustrate that the pH-sensitive microneedles coated with polyelectrolyte multinanolayers of antigens and oppositely charged polymers are practically applicable and may be a useful approach for preparing the MA-based vaccines. Also, this study exemplifies the sophisticated consideration in the MA design to endow the vaccine entity with multifunctional features to improve both the delivery efficiency and the vaccination effectiveness, representing one of the developmental trends in the field of novel VADS (Suh et al., Citation2014).
Figure 6. Representative CLSM images of pH-sensitive MA with 24 × 24 microneedles that were coated via a layer-by-layer approach with 10 layers of IPV alternated with TMC: IPV-fluo-488 (a), TMC-rhodamin B (b), and an overlay of these images (c). Reprinted with permission from Neutra & Kozlowski (Citation2006).
Virus-vectored vaccines with MA delivery
For decades, recombinant replication-defective adenovirus (AdV)-vectored vaccines have been developed as candidates for induction of T-cell responses against intercellular pathogens such as HIV-1, liver-stage malaria parasites (Draper & Heeney, Citation2010). However, it is now recognized that antivector immunity prevents repeated use of the same serotype vectors within a short time-frame, as confirmed by the experimental evidence that even heterologous AdV vectors have shown disappointing clinical results when used together in a prime-boost regimen against intracellular virus (Barnes et al., Citation2012). Recently, heterologous prime-boost immunization regimens, involving AdV as the prime induction and the poxvirus modified vaccinia virus Ankara (MVA) as boost immunization (vice versa), have shown particular promise in antibody as well as T-cell induction in preclinical animal models with blood-stage malaria vaccines (de Cassan & Draper, Citation2013); however, the cost and logistic problem of requiring heterologous vaccines present yet another challenge to be addressed.
To erase the obstacle of induction of antivector response in developing AdV-based vaccines, Carey et al. fabricated silicon MAs with microneedles ranging from 100 to 300 μm in height and 16–100 in number for delivery of independent malaria vaccines (Carey et al., Citation2014). The researchers demonstrated that i.d. delivery of a human AdV5-vectored malaria vaccine, so-called HAdV5-PyMSP142, to mice using silicon MAs by post-penetration (administration of vaccines followed by MA patching) induced equivalent or enhanced antibody responses to the encoded antigen with, however, the decreased anti-vector responses, compared to i.d. needle injection, allowing repeated use of the same AdV vaccine vector. In addition, boosting with a heterologous vaccine, MVA-PyMSP142 also resulted in significantly greater antibody responses in mice primed with HAdV5-PyMSP142 using MA delivery than using i.d. needle injection. Notably, the highest protection against blood-stage malaria challenge was, however, achieved when a heterologous route of immunization with MA and i.d. injection was used. Thus, the silicon MA-mediated delivery may potentially get around antivector adverse effects allowing the repeated use of the same adenovirus-vectored vaccines. Also this MA post-penetration strategy may possibly reduce manufacturing costs of multiple vaccines, overcome some of the logistic obstacles surrounding needle-and-syringe-based immunization (Arya & Prausnitz, Citation2015), and bring humans big benefits by expanding the clinical use of AdV-based vaccines.
Recently, Irvine’s group developed a novel type of biodegradable MAs made of PLA with base pedestal of 1 cm in diameter bearing 78 conical microneedles, each 650 μm in height and 250 μm in diameter at the base (DeMuth et al., Citation2013). The PLA MAs were coated with a sucrose sugar-glass matrix entrapping Ad5 vectors encoding model HIV antigens, SIV-gag or -env, which were confirmed stable at room temperature for several months after coating and to be effectively delivered into the skin of mice without loss of bioactivity, as evidenced by the fact that the Ad5-HIV MAs elicited systemic and even mucosal immunoresponses in mice largely equivalent to traditional syringe injections. Compared to i.m. vaccines, Ad5-HIV MA vaccines induced mice to generate the modestly increased frequencies of peripheral antigen-specific central memory T-cells but remarkably elevated levels of vaginal wash IgG titers. Going further, the researchers assessed vector delivery and immunogenicity of Ad-MA patches in rhesus macaques and observed reliable microneedle insertion in the epidermis of the nonhuman primates. When rhesus macaques were immunized with Ad5-HIV MAs encoding the model HIV antigens, SIV-gag or –env, by applying four patches for each vector to the shaved deltoid skin, followed by a boost with the same patch administration regimen at 12 weeks, robust systemic as well as mucosal immunoresponses against the antigens were stimulated. The researchers proved that MA i.d. delivery of adenovirus-vectored vaccines could induce systemic cellular responses equivalent to traditional intramuscular injection of an adenoviral vaccine, but eliciting strong humoral and cellular as well as mucosal immunity in macaques. This contrasts the general conception that i.d. immunization can rarely elicit mucosal immunity in mammals, as confirmed by a recent report, but using a mouse model, showing that only through mucosal administration can a MA vaccine induce a wide mucosal immune response (Wang et al., Citation2015d).
Other researchers have also confirmed that vaccination with MAs containing recombinant AdV-vectored vaccines can induce strong immunoresponses and prime high-frequency cytolytic CD8 + T cells in mammals (Bachy et al., Citation2013; Becker et al., Citation2015). Beker et al. explored the capacity of CMC-Na/lactose MAs coated with AdV encoding HIV-1 gag to program the antigen-experienced T cells to provide long-term memory and to respond rapidly to antigen re-encounter, which is the hallmark of an effective vaccine (Becker et al., Citation2015). Currently, it is argued that the potential of vaccines to program memory is dependent on the initial encounter of naïve CD8 + T cells with antigens (Sarkar et al., Citation2008), while the differences in signals derived from antigen, inflammatory cytokines, and co-stimulation contribute to the fate commitment of naïve CD8 + T cells to short-lived terminal effectors or long-lived memory cells, which are defined by homing molecular characteristics as central memory (CMT) and effector memory (EMT; Obar & Lefrancois, Citation2010). It is proved that biophysical differences in vaccine delivered i.d.ly in liquid suspension via a single puncture point or by MA as a dry matrix through multiple microneedle punctures confer differences in the rate of antigen dissemination and DC acquisition and also provide a distinct local tissue stress response (Depelsenaire et al., Citation2014), all of which influence the kinetics and quality of signals encountered by naïve CD8 + T cells, as confirmed by the observation that certain MA designs induced a significantly higher proportion of TCM phenotype CD8 + T cells than the i.d. injection did (Carey et al., Citation2013). However, Beker et al. found that although i.d. injection of AdV vaccine programed an earlier expansion of CD8 + T cells in draining lymph nodes (LN) relative to MA immunization, the absolute number of CD8 + T cells expressing an effector memory (CD62L − CD127+) and central memory (CD62L + CD127+) phenotype during peak expansion were comparable after MA or i.d. injection vaccination of mice with the HIV-1 gag-encoding AdV vaccine. The authors also observed that the immune induction effects with either MA or i.d. injection method were comparable in certain pivotal aspects, including the antigen-specific CD8 + T cells expressing CD62L and CD127 in the early phase of the response, and the activated memory CD8 + T cells that retained functionality and the capacity to respond on antigen reencounter 2 years post-vaccination (Becker et al., Citation2015). These results suggest that CD8 + T-cell effector/memory generation and long-term memory are largely unaffected by physical differences in vaccine delivery to the skin via dried MA or i.d. injection liquid suspension, further supporting the practical feasibility of MA delivery of recombinant virus-vectored vaccines.
Clinical trials on MA vaccines
The advantages of using MAs for vaccine delivery have increasingly attracted the collaborative research interests of scientists in several fields, such as immunology, bioengineering, and MEMS, and their incessant efforts and work have brought several MA vaccine products into clinical trials (Arya & Prausnitz, Citation2015). Searching “ClinicalTrials.gov” website for studies (visit on 18 December 2015, https://clinicaltrials.gov/ct2/search/index) with the term of “microneedle” results in 46 hits, showing the number of clinical studies related to microneedles that are being undertaken or have been completed. However, looking through the contents under each listed item tells that the trials are performed mostly on drug delivery or cosmetic application but rarely on vaccination, hinting the difficulty and the cautious attitudes of researchers and developers in the advancement of MA vaccines into clinical trials (Jacoby et al., Citation2015). Herein, we introduce the available information related to MA vaccine clinical studies that we acquired by searching both the general websites and the searching engines of biomedical specialty.
Clinical trials on nondissolvable MA vaccines
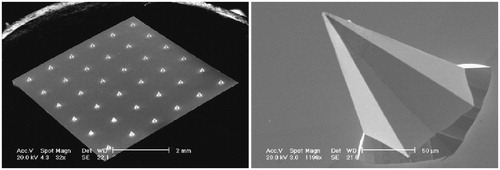
One of the first clinical trials on nondissolvable MAs was carried out in UK and reported in 2009 by Birchall’ group from Welsh School of Pharmacy in Cardiff University. This clinical trial aimed to determine whether the wet-etch silicon MAs with 0.3-μm platinum thin film-coated microneedles could elicit pain on application to human volunteers, and whether the solid silicon MAs could be used in such a way as to reliably penetrate the human stratum corneum, and also to provide a preliminary indication of how skin responds to microneedle injury with time (Haq et al., Citation2009). The fabricated silicon MAs comprised 36 pyramidal-shaped microneedles of either 280 or 180 μm length with a base diameter of approximately 180 μm and needle tips less than 1-μm wide (). Pain intensity was scored using a visual analog scale and sensory perception determined using an adapted McGill Pain Questionnaire Short Form (Melzack, Citation1987), and skin penetration was determined by the external staining and the measurement of trans-epidermal water loss. The pilot trial showed that the silicon MAs caused significantly less pain and discomforting sensation in participants than the hypodermic needle. The microchannels formed actually in the skin following microneedle application and repaired and resealed apparently at 8–24 h post-application. Thus, the silicon MAs with pyramidal microneedles can penetrate human skin with minimal pain and sensory discomfort, creating transient pathways for potential drug, vaccine, and DNA delivery. Though the process for preparing this kind of MAs is rather complex involving photolithography patterning, RIE and coating of platinum thin films using sophisticated instruments and apparatus, which may limit production scale, this first primary trial provides important lessons for further development of vaccines based on MA delivery (Jacoby et al., Citation2015).
Figure 7. Representative SEM images of platinum thin film-coated silicon MA with 36 pyramidal 180 or 280 μm length MAs. The left image shows a complete MA with 280 μm length microneedles, and the right image is a single-magnified 180-μm microneedle. Reprinted with permission from Resik et al. (Citation2013).
Compared to nondissolvable solid MAs, the hollow microneedle injection device received more clinical trials due to, perhaps, the large delivery capacity. The first two commercially marketed MN-based products are Intanzia® and Micronjet® which are based on metal and silicon MN, respectively (Alkilani et al., Citation2015). One of such trials was carried out to evaluate the safety, efficacy, and dose-sparing potential of the microneedle device, MicronJet® which has an array of four hollow microneedles, each 0.45 mm in length, for intradermal influenza vaccination in healthy adults (van Damme et al., Citation2009). The results revealed that the low-dose influenza vaccines i.d.ly delivered using microneedles elicited immunogenic responses similar to those elicited by the full-dose intramuscular vaccination, indicating that the metal MA injection device was effective, safe, and reliable in vaccine delivery.
A previous Phase ‖ clinical trial on trivalent virosomal-adjuvanted influenza vaccine was conducted in healthy adults using MicronJet™ for i.d. vaccination (Levin et al., Citation2014). The clinical results showed that i.d. injection of 3 μg (hemagglutinin [HA]/strain in 0.1 ml) using the microneedle device was well-tolerated and showed a statistically higher geometric mean fold rise than the same dose using a conventional needle for the H1N1 and B strains or a 15 μg (HA/strain in 0.5 ml) intramuscular injection for the H3N2 strain, proving that i.d. microneedle injection is, at least in the case of virosomal influenza vaccine, a dose-sparing vaccination method. This conclusion was also supported by a recent preclinical i.d.-versus-intranasal comparative study on the inactivated swine-origin influenza A/H1N1 virus vaccine. The study showed that mice i.d.ly received only 1 μg HA influenza vaccine coated on stainless steel MA microneedles produced high levels of HA-specific antibodies and HA-inhibition titer, and all survived the lethal virus challenge; in contrast, the mice that received intranasal influenza vaccine could obtain similar protective immunity only at the HA dose of 20 and 40 μg (Shin et al., Citation2015). Thus, the researchers argued that MA vaccination induced more potential immune response and protection than intranasal vaccination at the same vaccine dose of the inactivated swine-origin influenza A/H1N1 virus vaccine.
Based on these results, a project was launched to reduce the costs of maintaining a poliovirus immunization in low-income areas involving evaluation of the extent of priming immune responses after administration of inactivated poliovirus vaccine (IPV) with MicronJet® (Resik et al., Citation2013). One of several clinical trials in this project on polio vaccines by using microneedle injection for vaccination was carried out in infants at age 6–7 weeks in Bangladesh (Anand et al., Citation2015). Though vaccination of liquid vaccines by means of microneedle injection is less relevant to the general MAs that are usually referred to, the results provide a useful reference to conducting clinical studies on real MA vaccines. In Bangladesh clinical trial, physicians administered all the study polio vaccines and other routine vaccines for infants as recommended by local government. Healthy 6-week-old infants were randomized to one of five study arms: receipt of trivalent OPV (tOPV) or bivalent OPV (bOPV) at ages 6, 10, and 14 weeks, intramuscular IPV or i.d. one-fifth fractional dose IPV (f-IPV) at ages 6 and 14 weeks, or f-IPV at ages 6 and 14 weeks with bOPV at age 10 weeks (f-IPV/bOPV). All participants received tOPV at age 18 weeks. Intramuscular IPV (0.5 ml) was administered using a standard needle and syringe, while i.d. f-IPV (0.1 ml) was administered using the MicronJet™, a microneedle device with three microneedles (600 μm in length) that attaches to an i.d. syringe. IPV and f-IPV were administered in the anterolateral thigh, opposite the side used for routine immunization of injectable vaccines. In the study, 95% (922) of 975 randomized infants completed follow-up, and seroconversions to types 1 and 3 after 2 IPV doses at ages 6 and 14 weeks were no different than after three doses of tOPV or bOPV at ages 6, 10, and 14 weeks, suggesting vaccination with intramuscular IPV can save at least one dose compared to that with OPV. As for the priming response, seroconversion, 1 week after IPV at 14 weeks among those who did not seroconvert after IPV at 6 weeks, was observed against poliovirus types 1, 2, and 3 in 91%, 84%, and 97%, respectively. Notably, compared with IPV, f-IPV failed noninferiority tests for seroconversion with one or two doses and priming after one dose, suggesting at least that the immunogenicity of one-fifth f-IPV is inferior to that of full IPV and that the dose-saving objective for polio vaccination with microneedle injection device was not fully achieved yet in this trial, which is in agreement with results from another clinical trial on reducing the costs of maintaining polio immunization which was carried out in Cuba involving i.d. injection of f-IPV with a needle-free device (Biojector 2000, Bioject Medical Technologies) (Resik et al., Citation2013).
Though dose-saving effect by microneedle injection was not observed in these clinical trials, the cause of this failure should be found out and might well be deduced from the fact that the polio vaccine dose for microneedle vaccination was set to too low to elicit the immune responses noninferior to the normally used IPV dose. Notably, in contrast to these results, the dose-sparing potential of the microneedle device was clearly confirmed in a pilot study using BD microneedle device (with a single microneedle of 1–3 mm in length) for i.d. immunization of a rabies vaccine, which showed that that i.d. delivery using BD microneedle delivery of, notably, 1/4 the i.m. antigen dose is safe, efficient, and reliable, resulting in a protective seroconversion rate (Laurent et al., Citation2010).
Currently, there are few vaccine products that can be routinely self-administered, despite the fact that convenient self-vaccination may enormously expand vaccination coverage and reduce administration costs. Recently, Norman et al evaluated the usability and acceptability of steel MAs for self-vaccination against influenza through conducting a randomized, repeated measures study with the venue-recruited adult volunteers, who inserted patches with thumb pressure alone or used snap-based devices that closed shut at a certain force (Norman et al., Citation2014). It was observed that the best usability was seen with the snap device, with users inserting a median value of 93–96% of microneedles over three repetitions, and when a self-administered MA patch was offered, intent to vaccinate increased from 44% to 65%, with the majority preferring to self-vaccination. Also, there were no serious adverse events associated with the use of microneedle patches by the participants themselves, indicating that MA patches for self-vaccination against influenza are usable and may lead to improved vaccination coverage.
Clinical trials on dissolvable MA vaccine
To date, numerous types of dissolving MAs have been developed by researchers for vaccine delivery; however, just like the overwhelming majority of novel tumor-targeting DDS with amazing cure efficacy in small animals, few of these MA products advanced into clinical trial phase. In particular, it was reported on several websites that clinical trials on MA vaccines, including influenza, polio, and measles, are going to be or have been sponsored (Korschun, Citation2015; Meek, Citation2015; Toon, Citation2015). However, unfortunately, up to now there are almost no published results related to these news-website-reported trials.
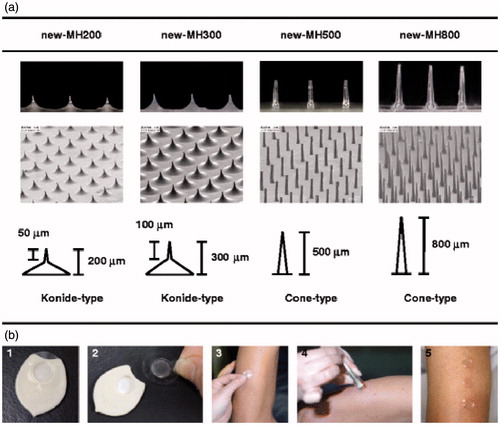
Notably, two clinical trials on dissolvable MA vaccines have recently been reported by Nakagawa’s group from Osaka University in Japan (Hirobe et al., Citation2013,Citation2015). Using sodium hyaluronate, dextran, and povidone as matrix, Nakagawa’s group developed a new type of dissolving MAs (called MHs) with microneedles ranging from 200 to 800 μm in length and incorporating 20 μg tetanus toxoid (TT) and 10 μg diphtheria toxoid (DT) within () (Hirobe et al., Citation2013). Through testing the MHs on healthy volunteers in a pilot trial, they confirmed that the microneedles of MH could easily pierce stratum corneum without severe skin irritation and induced increased antigen-specific antibody titers in participants, suggesting that MHs are safe enough for i.d. vaccination in human. Going further, this group investigated the clinical safety and efficacy of a novel transcutaneous influenza vaccine using MHs which contains trivalent influenza hemagglutinins (15 μg) in healthy adults (Hirobe et al., Citation2015). The trial results showed that no severe local or systemic adverse events were detected, while immune responses against A/H1N1 and A/H3N2 strains were induced equally in the participant received either the MHs influenza vaccine or the influenza vaccine in aqueous suspension by i.d. injection; moreover, the efficacy of the vaccine against the B strain in the MH group was stronger than that in the i.d. injection group. From these clinical studies on MH influenza vaccine, it is acceptable to make conclusions that vaccination with biocompatible material-constituted MAs is promising for practical use as an easy and effective method to replace conventional injection systems. Though more trials are needed to further evaluate MH vaccines in many aspects, such as long-term safety, wide-use efficacy, the cost of MH vaccination, and the real acceptability of the novel products, the primary pilot clinical studies results in valuable information in the advancement of various MA vaccines and are beginning to open the door to clinical use of MA vaccines (Lutton et al., Citation2015; Marshall et al., Citation2016).
Figure 8. The new-MHs and procedure of new-MH application to the human skin. (a) The new-MHs contain 200 microneedles in an area of 0.8 cm2. New-MH200 and new-MH300 have 200 μm and 300 μm cone-type microneedles of 50 μm and 100 μm lengths, respectively. New-MH500 and new-MH800 contain cone-type microneedles of 500 μm and 800 μm length, respectively.(b) New-MH was fixed to the plastic case as a new-MH formulation (1) was adhered to the center of a 2.3 cm2 adhesive film (2) was put on skin of the lateral upper arm (3) was applied by impact of a handheld spring-type applicator (4) and was removed 6 h after application (5).Reprinted with permission from Hirobe et al. (Citation2013).
Perspectives
MA vaccines have big advantages over others in that they pierce the skin or mucosa painlessly, can deliver vaccines to the cutaneous or mucosal compartments enriched in APCs, and have the potential to spare antigens (Prausnitz et al., Citation2009; Pearton et al., Citation2010; Pattani et al., Citation2012; Norman et al., Citation2014) and to simplify immunization programs even allowing self-administration of vaccines (Al-Zahrani et al., Citation2012; Zipursky et al., Citation2014). Therefore, more and more academic and company researchers are committed to the development of MA vaccines, which, unfortunately, are still not ready up to now for use for prophylaxis of the existing infectious pathogens.
Dissolvable MA versus nondissolvable MA for vaccination
Among different types of nondissolvable MAs, the microneedle injection devices that have already been approved as a medical instrument for clinical use can yet be immediately tried for vaccination of the marketed vaccines, such as influenza, polio, and measles, engendering the possibility of the rapid approval by authorities of the combination of two kinds of commercialized products as a new entity (Anand et al., Citation2015). A prominent advantage of nondissolvable MAs over dissolvable MAs exists in that they are tough enough in texture to withstand the friction subjected during packaging and to pierce various skin stratum corneum encountered when vaccination. However, using nondissolvable MAs including microneedle injection device for wide vaccination confronts still several drawbacks, such as needle pollution, pathogen dissemination potential, and high costs, all of which should be taken into full consideration before their eventual clinical use.
In contrast to nondissolvable MAs, dissolvable MAs can deliver vaccines without sharp wastes left behind after vaccination and can incorporate vaccine ingredients within microneedles enhancing vaccine stability by water removal and lyoprotectant addition (Zhen et al., Citation2015). However, dissolvable MAs are made of polymers and saccharides and, therefore, are relatively brittle and frail, needing sufficient protection during packaging and transporting and also requiring careful operation for vaccination. For vaccine delivery or to be an effective VADS, MAs must be fabricated with appropriate ingredients selected through formulation optimization to ensure their sufficient hardness for skin penetration and high resistance to friction (Lutton et al., Citation2015). In this aspect, a variety of materials and chemicals have been successfully used in combination to construct MAs that can efficiently pierce mammalian skin; however, up to now, there is still no reports on MA resistance to possible damages exerted by packaging and transportation before vaccination. As summarized from literature, usually the MAs will be tough enough to pierce mammalian stratum corneum when constructed with an optimized combination of biocompatible polymers, such as PVP, PLA, PLGA and chitosan, and/or sugars, such as sucrose and trehalose, and the highly active adhesives, such as CMC-Na, starch, and gelatin (Suh et al., Citation2014).
Mucosal MA vaccination versus cutaneous MA vaccination
Although most of the currently developed MAs are vaccinated via cutaneous administration, oral cavity mucosa provides a promising immunization site for MA vaccines. It has been proved that vaccination of dissolvable MA vaccines via oral cavity mucosa has numerous advantages: good compliance, convenience, vaccination by self-administration or by minimally trained personnel, production of zero pollution thanks to uptake of the MA rests into gastrointestinal tract, and, especially, induction of systemic as well as mucosal immunity, which is crucial to defend the body against many infectious pathogens (Wang et al., Citation2015d). In addition, oral mucosa is much easier to pierce with MA than the tough mammalian skin (Prausnitz et al., Citation2009; van der Maaden et al., Citation2012; Carey et al., Citation2013), and this markedly reduces the hardness requirement for microneedles and, thus, greatly expands the scope of materials and procedures for producing MAs (Zhen et al., Citation2015). Obviously, i.d. MA vaccination will leave behind, after vaccination on the vaccinated skin, the MA pedestal and residues which may be rather adhesive and is very likely to stain clothes; in contrast, mucosal MA vaccines not only do not cause these troubles but also have a high potential to elicit robust mucosal immunity, which is usually believed not able to be efficiently elicited by vaccination via routes other than mucosa (Neutra & Kozlowski, Citation2006).
MA vaccination: pros and cons
Reasonably, using MA for vaccine delivery is highly beneficial for wider vaccination (Wang & Wang, Citation2015). First, the increased stability of MA vaccines makes the products able to be dispatched in a CTC, even outside the cold chain, in remote districts where the integrated cold chain is often unavailable (Zipursky et al., Citation2014). Second, MA vaccines can be conveniently and painlessly administered increasing inoculation compliance which markedly influences global vaccination rates. Third, MA vaccination needing no professionally trained personnel, who are often very scarce in less-developed countries, will enormously expand the inoculation coverage (Zipursky et al., Citation2014; Wang et al., Citation2015d). Fourth, the dissolving MA vaccines do not leave behind metal needle pollution, which is usually associated with conventional injection vaccination and has a potential for infectious disease dissemination and therefore requires the post-disposal, adding a remarkable extra cost to global vaccination. Fifth, when administered at oral cavity mucosa, MA vaccines with sugar in the pedestal and matrix taste sweet and are, thus, much suitable and acceptable for children, which will positively influence parents’ attitude toward vaccination expanding vaccination coverage. Finally, cutaneous or mucosal MA vaccination can efficiently elicit robust systemic and even mucosal immunity thanks to delivering antigens to the right site, where affluent professional APCs are patrolling on surveillance of the exogenous pathogenic invaders, and therefore may potentially reduce the dose and dosing times for a vaccine (Wang et al., Citation2015b).
However, preparation of MAs, especially the dissolvable MAs, is in fact rather complex and usually involves numerous fine steps using a variety of precision instruments (Wilke et al., Citation2005; Wang & Wang, Citation2015). Generally, the bottleneck of manufacturing the dissolvable MA products is argued to lie in the large-scale production of MAIMs as preliminary reserves using MEMS technology, such as deposition, lithography pattern, and RIE (Wang & Wang, Citation2015), of which recent remarkable progress and advances make it possible to produce the MAIMs on a large scale but at the cost of production costs. Another barrier to the widespread use of dissolving MA vaccines is regarded as the requirement of exquisite packaging, which must fully protect the products from damage, ensuring the frail microneedles remain intact before their penetration into skin or mucosa of the recipients and allowing the inoculation to be completed by self-administration or by minimally trained workers(Kolli, Citation2015). Obviously, the aseptic MA vaccines, which can rarely be sterilized by either γ-ray irradiation or high-temperature procedure, must be guaranteed and thus will also enormously enhance product costs, because numerous steps are involved in the MA manufacturing process but have to carry out in a sterile condition.
MA vaccination: barriers and hopes
Nowadays more and more academic and company researchers are committed to the development of MA vaccines; however, big barriers to wide vaccination with MAs still exist in several aspects (Lutton et al., Citation2015). First, large-scale production of the MA vaccines and, especially, MAIMs for dissolvable MAs is still confronting resource shortage and technical challenges, which may not vanish until a big input of resources and efforts converged from governments, academy, and even microelectronic and biomedical industries are devoted. Second, although MA delivery can reduce the cost of vaccination, and increased thermostability may lower dispatch spending by omitting the cold chain, the production costs of MA vaccines can hardly be expected at levels offsetting the overall expenditures associated with final clinical use of MAs. Third, it is key attributes of MA vaccines, such as usability, wear time, and proper disposal that control the product acceptability in the marketplace, which, in turn, influences the resource inputs from social and industrial communities (Jacoby et al., Citation2015).
Recently, Jacoby et al interviewed key opinion leaders in the United States for insights into the opportunities and challenges associated with MA delivery of influenza vaccines, and particularly its potential for self-administration (Jacoby et al., Citation2015). All interviewees expressed high support for administration of influenza vaccine in MAs by health-care providers and for self-administration in groups supervised by a provider. Also, the interviewees highlighted priorities that should be considered in the ongoing development of an MA influenza vaccine, such as confirming efficacy and ensuring safety for self-administration, ease of use, short wear times, and an easily accessible application site. As expected, it was agreed that using MAs can help increase coverage, facilitate easy and safe delivery, and reduce the cost of vaccination. Moreover, it is believed that the prospect of reduced provider training requirements, increased thermostability, and high patient and provider acceptability will make it an attractive option for MA use in remote and low-resource settings worldwide. But all these are desirable prospects for MA vaccines, which are, at least in the present status, still a little like a concept car and need still to pass numerous intensive and extensive clinical examinations.
Encouragingly, up to now, several vaccines based on inactivated or attenuated viruses, such as influenza and measles, have been tried to combine with MA for delivery by Prausnitz group under collaboration with the Centers for Disease Control and Prevention (CDC) (Korschun, Citation2015; Meek, Citation2015; Toon, Citation2015). This practical action indicates that the feasibility of using MA for vaccine delivery is widely recognized and its attention by certain governments begin arising. In particular, pilot clinical trials on both dissolvable and nondissolvable MA vaccines have already been carried out by pioneering researchers and physicians, and the safety and effectiveness of the microneedle-based vaccination have been primarily confirmed by the updated results (Levin et al., Citation2014; Hirobe et al., Citation2015), although much more advanced trials are still needed to confirm the long-term final effects of different MA vaccines. Nevertheless, it is reasonable to believe that MA vaccines that have numerous merits and advantages may eventually be used for wide and even global vaccination against numerous infectious diseases, bringing big benefits to human health, on the condition that collaborative efforts on the development of this novel product are continuously committed by researchers, manufacturers as well as the governments.
Declaration of interest
The authors report no declarations of interest. The authors acknowledge that this work was supported by Chinese Domestic Advanced Visiting Scientist Program jointly funded by Ministry of Education and AMU awarded to T.W. who conducts the research work as a visiting scientist in the University of Science and Technology of China (AMU-VS201507/201607). This work was also supported by the Fundamental Research Funds for the Central Universities awarded to N.W. (HUT2014). Additionally, this work was also supported by the University Natural Science Foundation by the Department of Education of Anhui Province (Grant number KJ2016SD28).
References
- Al-Zahrani S, Zaric M, McCrudden C, et al. (2012). Microneedle-mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy. Expert Opin Drug Deliv 9:541–50
- Alkilani AZ, McCrudden MT, Donnelly RF. (2015). Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 7:438–70
- Anand A, Zaman K, Estivariz CF, et al. (2015). Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: a randomized controlled trial. Vaccine 33:6816–22
- Arya J, Prausnitz MR. (2015). Microneedle patches for vaccination in developing countries. J Control Release. [Epub ahead of print]. doi:10.1016/j.jconrel.2015.11.019
- Bachy V, Hervouet C, Becker PD, et al. (2013). Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci USA 110:3041–6
- Barnes E, Folgori A, Capone S, et al. (2012). Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 4:115ra111
- Becker PD, Hervouet C, Mason GM, et al. (2015). Skin vaccination with live virus vectored microneedle arrays induce long lived CD8(+) T cell memory. Vaccine 33:4691–8
- Butler D. (2015). Measles by the numbers: a race to eradication. Nature 518:148–9
- Bystrova S, Luttge R. (2011). Micromolding for ceramic microneedle arrays. Microelectron Eng 88:1681–4
- Carey JB, Pearson FE, Vrdoljak A, et al. (2013). Microneedle array design determines the induction of protective memory CD8+ T cell responses induced by a recombinant live malaria vaccine in mice. PLoS One 6:e22442
- Carey JB, Vrdoljak A, O’Mahony C, et al. (2014). Microneedle-mediated immunization of an adenovirus-based malaria vaccine enhances antigen-specific antibody immunity and reduces anti-vector responses compared to the intradermal route. Sci Rep 4:6154
- Chen D, Zehrung D. (2012). Desirable attributes of vaccines for deployment in low-resource settings. J Pharm Sci 102:29–33
- Cheung K, Das DB. (2016). Microneedles for drug delivery: trends and progress. Drug Deliv. [Epub ahead of print]. doi:10.3109/10717544.2014.986309
- Childress BC, Montney JD, Albro EA. (2014). Making evidence-based selections of influenza vaccines. Hum Vaccin Immunother 10:2729–32
- Chu LY, Ye L, Dong K, et al. (2016). Enhanced stability of inactivated influenza vaccine encapsulated in dissolving microneedle patches. Pharm Res 33:868–78
- de Cassan SC, Draper SJ. (2013). Recent advances in antibody-inducing poxviral and adenoviral vectored vaccine delivery platforms for difficult disease targets. Expert Rev Vaccines 12:365–78
- DeMuth PC, Li AV, Abbink P, et al. (2013). Vaccine delivery with microneedle skin patches in nonhuman primates. Nat Biotechnol 31:1082–5
- Depelsenaire AC, Meliga SC, McNeilly CL, et al. (2014). Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J Invest Dermatol 134:2361–70
- Domingues CM, de Fatima Pereira S, Cunha Marreiros AC, et al. (2014). Introduction of sequential inactivated polio vaccine-oral polio vaccine schedule for routine infant immunization in Brazil’s national immunization program. J Infect Dis 210:S143–1
- Donnelly RF, Majithiya R, Singh TRR, et al. (2011). Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharmaceut Res 28:41–57
- Donnelly RF, Raj Singh TR, Woolfson AD. (2010). Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv 17:187–207
- Dormitzer PR. (2015). Rapid production of synthetic influenza vaccines. Curr Top Microbiol Immunol 386:237–73
- Draper SJ, Heeney JL. (2010). Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol 8:62–73
- Edens C, Collins ML, Ayers J, et al. (2013). Measles vaccination using a microneedle patch. Vaccine 31:3403–9
- Edens C, Collins ML, Goodson JL, et al. (2015). A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine 33:4712–18
- Galazka A, Milstien J, Zaffran M. (1998). Thermostability of vaccines. In: The global programme for vaccines and immunization. Geneva Switzerland: World Health Organization, 2–4
- Haq MI, Smith E, John DN, et al. (2009). Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices 11:35–47
- Henry S, McAllister DV, Allen MG, Prausnitz MR. (1998). Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharmaceut Sci 87:922–5
- Hirobe S, Azukizawa H, Hanafusa T, et al. (2015). Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials 57:50–8
- Hirobe S, Azukizawa H, Matsuo K, et al. (2013). Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharm Res 30:2664–74
- Hotez PJ, Bottazzi ME, Strych U. (2016). New vaccines for the world’s poorest people. Annu Rev Med 67:405–17
- Jacoby E, Jarrahian C, Hull HF, Zehrung D. (2015). Opportunities and challenges in delivering influenza vaccine by microneedle patch. Vaccine 33:4699–704
- Kim YC, Park JH, Prausnitz MR. (2012). Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 64:1547–68
- Kolli CS. (2015). Microneedles: bench to bedside. Ther Deliv 6:1081–8
- Korschun H. (2015). Clinical study at Emory tests microneedle skin patches as alternative to flu shot, Researchers are enrolling volunteers in a clinical study at the Hope Clinic of the Emory Vaccine Center. Woodruff Health Sciences Center, Emory University
- Laurent PE, Bourhy H, Fantino M, et al. (2010). Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine 28:5850–6
- Lee BY, Bartsch SM, Mvundura M, et al. (2015). An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. Vaccine 33:4727–36
- Levin Y, Kochba E, Kenney R. (2014). Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: are all delivery methods the same? Vaccine 32:4249–52
- Levine MM. (2011). “IDEAL” vaccines for resource poor settings. Vaccine 29:D116–25
- Low N, Bavdekar A, Jeyaseelan L, et al. (2015). A randomized, controlled trial of an aerosolized vaccine against measles. N Engl J Med 372:1519–29
- Lutton RE, Moore J, Larraneta E, et al. (2015). Microneedle characterisation: the need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug Deliv Transl Res 5:313–31
- Marichal T, Ohata K, Bedoret D, et al. (2011). DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med 17:996–1002
- Marshall S, Sahm LJ, Moore AC. (2016). Microneedle technology for immunisation: perception, acceptability and suitability for paediatric use. Vaccine 34:723–34
- Martanto W, Davis SP, Holiday NR, et al. (2004). Transdermal delivery of insulin using microneedles in vivo. Pharm Res 21:947–52
- Matzinger P. (1994). Tolerance, danger, and the extended family. Annu Rev Immunol 12:991–1045
- Meek G. (2015). Microneedle patch for measles vaccination could be a game changer, promises to increase reach of immunization coverage globally. CDC Newsroom Releases
- Melzack R. (1987). The short-form McGill pain questionnaire. Pain 30:191–7
- Merkle HP. (2015). Drug delivery’s quest for polymers: where are the frontiers? Eur J Pharm Biopharm 97:293–303
- Mikszta JA, Alarcon JB, Brittingham JM, et al. (2002). Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med 8:415–19
- Mistilis MJ, Bommarius AS, Prausnitz MR. (2015). Development of a thermostable microneedle patch for influenza vaccination. J Pharmaceut Sci 104:740–9
- Neutra MR, Kozlowski PA. (2006). Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 6:148–58
- Norman JJ, Arya JM, McClain MA, et al. (2014). Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine 32:1856–62
- Obar JJ, Lefrancois L. (2010). Early events governing memory CD8+ T-cell differentiation. Int Immunol 22:619–25
- Ozawa S, Mirelman A, Stack ML, et al. (2012). Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: a systematic review. Vaccine 31:96–108
- Park JH, Allen MG, Prausnitz MR. (2005). Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release 104:51–66
- Pattani A, McKay PF, Garland MJ, et al. (2012). Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release 162:529–37
- Pearton M, Kang SM, Song JM, et al. (2010). Influenza virus-like particles coated onto microneedles can elicit stimulatory effects on Langerhans cells in human skin. Vaccine 28:6104–13
- Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. (2009). Microneedle-based vaccines. Curr Top Microbiol Immunol 333:369–93
- Qin D, Xia Y, Whitesides GM. (2010). Soft lithography for micro- and nanoscale patterning. Nat Protoc 5:491–502
- Qiu Y, Guo L, Zhang S, et al. (2016). DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug Deliv. [Epub ahead of print]. doi:10.3109/10717544.2014.992497
- Resik S, Tejeda A, Sutter RW, et al. (2013). Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med 368:416–24
- Sarkar S, Kalia V, Haining WN, et al. (2008). Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med 205:625–40
- Shin JH, Park JK, Lee DH, et al. (2015). Microneedle vaccination elicits superior protection and antibody response over intranasal vaccination against swine-origin influenza A (H1N1) in mice. PLoS One 10:e0130684
- Suh H, Shin J, Kim YC. (2014). Microneedle patches for vaccine delivery. Clin Exp Vaccine Res 3:42–9
- Toon J. (2015). Polio vaccination with microneedle patches receives funding for patch development, clinical trial, clinical trial will evaluate polio vaccination using microneedle patch. Atlanta (GA): Georgia Institute of Technology News Center, Georgia Institute of Technology
- van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, et al. (2009). Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 27:454–9
- van der Maaden K, Jiskoot W, Bouwstra J. (2012). Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release 161:645–55
- van der Maaden K, Sekerdag E, Schipper P, et al. (2015). Layer-by-layer assembly of inactivated poliovirus and n-trimethyl chitosan on ph-sensitive microneedles for dermal vaccination. Langmuir 31:8654–60
- Wang J, Li B, Wu MX. (2015a). Effective and lesion-free cutaneous influenza vaccination. Proc Natl Acad Sci USA 112:5005–10
- Wang J, Shah D, Chen XY, et al. (2014a). A micro-sterile inflammation array as an adjuvant for influenza vaccines. Nat Commun 5:4447.
- Wang N, Wang T, Zhang M, et al. (2014b). Using procedure of emulsification-lyophilization to form lipid A-incorporating cochleates as an effective oral mucosal vaccine adjuvant-delivery system (VADS). Int J Pharm 468:39–49
- Wang N, Wang T, Zhang ML, et al. (2014c). Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur J Pharm Biopharm 88:194–206
- Wang T, Wang N. (2015). Biocompatible mater constructed microneedle arrays as a novel vaccine adjuvant-delivery system for cutaneous and mucosal vaccination. Curr Pharm Design 21:5245–55
- Wang T, Zhen Y, Ma X, et al. (2015b). Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids Surf B Biointerfaces 126:520–30
- Wang T, Zhen Y, Ma X, et al. (2015c). Phospholipid bilayer-coated aluminum nanoparticles as an effective vaccine adjuvant-delivery system. ACS Appl Mater Interfaces 7:6391–6
- Wang T, Zhen YY, Ma XY, et al. (2015d). Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloid Surface B Biointerfaces 126:520–30
- Wendorf JR, Ghartey-Tagoe EB, Williams SC, et al. (2011). Transdermal delivery of macromolecules using solid-state biodegradable microstructures. Pharm Res 28:22–30
- Wilke N, Mulcahy A, Ye SR, Morrissey A. (2005). Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron J 36:650–6
- Yang S, Feng Y, Zhang L, et al. (2012). A scalable fabrication process of polymer microneedles. Int J Nanomed 7:1415–22
- Zhen YY, Wang N, Gao ZB, et al. (2015). Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine 33:4330–40
- Zipursky S, Djingarey MH, Lodjo JC, et al. (2014). Benefits of using vaccines out of the cold chain: delivering meningitis A vaccine in a controlled temperature chain during the mass immunization campaign in Benin. Vaccine 32:1431–5

