Abstract
This study was designed to investigate whether polymerized human placenta hemoglobin (PolyPHb) pretreatment provided protection to the heart after ischemia/reperfusion (I/R) injury. After 10-min basal perfusion, isolated Sprague-Dawley rat hearts were pretreated with 0.1 gHb/dL PolyPHb and subjected to I/R injury. PolyPHb pretreatment greatly reduced the decreases in left ventricular developed pressure (LVDP), maximum LVDP increase and decrease rate (±dp/dt), and the increase in left ventricular end-diastolic pressure (LVEDP) as compared to the control group. Moreover, the myocardial infarction and troponin-I release were significantly reduced in the pre-HBOCs group. Therefore, PolyPHb pretreatment was protective to the isolated I/R heart.
INTRODUCTION
Ischemia/reperfusion (I/R) injury may lead to myocardial contractile dysfunction, cardiac arrhythmias, myocardial infarction, as well as cardiomyocyte apoptosis [Citation1,Citation2]. Extensive studies have been performed to find therapeutic strategies that protect the heart from I/R injury, but most of them fail to be applied to the patient in clinical settings for lack of significant effect. Additionally, with the population aging around the world, more and more old patients come to hospitals for medical therapy, which requires us to further improve the techniques of cardioprotection [Citation3,Citation4].
Polymerized human placenta hemoglobin (PolyPHb), one type of hemoglobin-based oxygen carrier (HBOC), was initially developed by the research group of Professor Chengmin Yang to treat patients with trauma and hemorrhagic shock [Citation5,Citation6]. In our previous studies, we found that PolyPHb had a potential protective effect to rat hearts after cold storage and reperfusion, and the mechanism was closely related to down-regulation of iNOS expression and NO/O2·− production, preservation of mitochondrial function, and thereby attenuation of NO-mediated myocardial apoptosis and restoration of nitroso-redox balance in the I/R heart [Citation7,Citation8]. As a promising HBOC, PolyPHb allows the transportation of more oxygen to the hypoxia tissues owing to its higher oxygen affinity, lower viscosity, and smaller mean diameter than human red blood cells; thus it can be helpful to microcirculation perfusion and alleviate myocardial I/R injury [Citation5,Citation9–11]. Therefore, we designed this study to investigate the effect of PolyPHb pretreatment to isolated rat hearts after 35-min lethal ischemia and 2-hr reperfusion.
MATERIALS AND METHODS
The present study was performed in adherence with the Guidelines on the Use of Laboratory Animals published by the National Institutes of Health and approved by the Animal Care and Use Committees in Sichuan University.
PolyPHb Preparation
PolyPHb was prepared as we previously described [Citation5,Citation6]. Briefly, hemoglobin from fresh human placenta blood (donated by Tianjin Union Stem Cell and Genetic Engineering Ltd., Tianjin, China) was purified and viral inactived by heat treatment, then intra- and inter-molecularly cross-linking were performed by using pyridoxal phosphate (PLP) and glutaraldehyde (GDA), respectively. After that, ultrafiltration and molecular sieve chromatography were performed to harvest PolyPHb with molecular weight range from 64 KD to 600 KD. Before use, the prepared PolyPHb solution was added into the Krebs-Henseleit buffer (KHB: 120.0 mM NaCl, 4.5 mM KCl, 20.0 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgCl2, 2.5 mM CaCl2 and 10.0 mM glucose, pH 7.4, 37°C) to a final concentration of 0.1 gHb/dL, and bubbled with 100% oxygen gas for 10 mins. As a result, the oxygen tension of the above PolyPHb solution was 581+20 mmHg, which was significantly higher than that of the KHB alone (532±25 mmHg, P<0.05).
Experimental Proposal
Thirty male Sprague-Dawley rats, weighing 250-300g, were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) and heparin (500 IU). The hearts were quickly excised and perfused on a Langendorff apparatus with KHB at a constant pressure of 100 cmH2O. A thin-wall latex balloon was inserted into the left ventricle (LV) through the left atrium to continuously monitor the LV function, including heart rate (HR), left ventricular developed pressure (LVDP), maximum LVDP increase (+dp/dt) and decrease rate (–dp/dt), and LV end-diastolic pressure (LVEDP) (ADInstruments Pty Ltd., Bella Vista, NSW, Australia). After 10 mins of basal perfusion, hearts were randomly assigned to sham, control (without pretreatment), and pre-HBOCs groups (pretreatment with PolyPHb). In the pre-HBOCs group, the hearts were perfused with 0.1 gHb/dL PolyPHb for 10 mins and KHB for another 10 mins, then subjected to 35 mins of lethal ischemia at 37°C and 2 hr reperfusion (). The control group hearts were basal perfused for 30 mins by KHB without HBOCs treatment and followed by ischemia and reperfusion. Coronary flow rate (CF) was calculated from the coronary effluent, sampling time, and cardiac wet weight.
Figure 1. The experimental protocol of this study. After 10 mins of basal perfusion, the pre-HBOCs group hearts were perfused with 0.1 gHb/dL PolyPHb for 10 mins and KHB for another 10 mins and the control group hearts were perfused with KHB alone for 20 mins, then subjected to 35 mins of lethal ischemia at 37°C and 2 hr reperfusion. Hearts perfused with KHB for 150 mins without pretreatment and ischemia were used as sham control. KHB: Krebs-Henseleit buffer.
Myocardial Infarction Size Determination
Myocardial infarction size in the three group hearts was estimated by 2,3,5-triphenyltetrazolium chloride (TTC) staining. Briefly, after reperfusion, the hearts were weighed, frozen, and cut into 2-mm-thick slices parallel to the atrioventricular groove. The slices were thawed and stained by incubation in 1% TTC solution in phosphate buffer (0.1M) at 37°C for 10 to 20 mins, then fixed in 4% paraformaldehyde solution for 24 hr. After fixation, the myocardial infarction size was determined by Image-Pro Plus 4.5 software.
Cardiac Troponin-I Release Measurement
The cardiac troponin-I release was measured by an ELISA kit (Life Diagnostics, Inc., West Chester, PA) from the coronary effluents collected at the end of 30-min baseline and 2-hr reperfusion.
Statistical Analysis
All values in the text and figures were presented as mean ± SEM. The values of HR, LVDP, ± dp/dt, LVEDP, and CF were analyzed by 2-factor ANOVA with repeated measures. The myocardial infarct size and the release of troponin-I were analyzed by one-way ANOVA followed by LSD correction for post hoc t test (SPSS 13.0 software). P values < 0.05 were considered statistically significant.
RESULTS
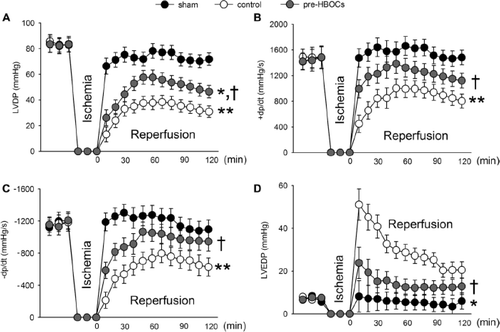
PolyPHb Pretreatment Improved Cardiac LV Function
At basal perfusion, there were no significant differences in HR, LVDP, ± dp/dt, LVEDP, and CF among the 3 groups. However, during reperfusion, the LVDP was greatly improved in the pre-HBOCs group when compared to the control group (P<0.05, ). The ± dp/dt were also greatly increased in the pre-HBOCs group as compared with the control group (P<0.05 and P<0.05, respectively, and ). Consistently, the LVEDP elevation after I/R injury was also significantly attenuated in the pre-HBOCs group (P<0.05 vs. the control group, ). There were no significant differences in the HR and CF among the three groups during reperfusion (data not shown). Even though the PolyPHb pretreatment improved the cardiac function during reperfusion, it still cannot totally reverse the damage caused by I/R injury, as the LVDP was greatly decreased when compared to the sham group (P<0.05).
Figure 2. The LVDP (A), ±dp/dt (B and C), and LVEDP (D) of the three group hearts. Values were presented as mean ± SEM (n=8 to10). *P<0.05 and **P<0.01 vs. the sham group. †P<0.05 vs. the control group. LVDP: left ventricular development pressure, ± dp/dt: maximum LVDP increase and decrease rate, LVEDP: left ventricular end-diastolic pressure.
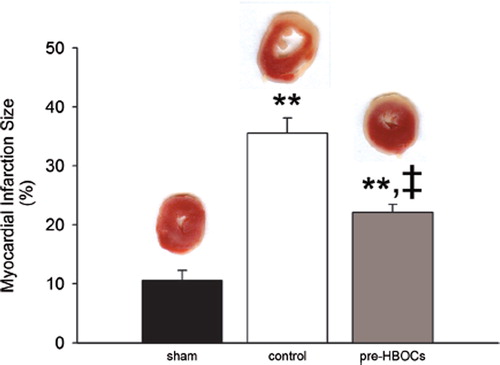
PolyPHb Pretreatment Limited Infarction Size
As shown in , the sham group hearts exhibited minimal myocardial infarction (10.58 ± 1.70%). After I/R injury, the myocardial infarction size was greatly increased (35.54± 2.56% in the control group, P<0.01 vs. the sham group). PolyPHb pretreatment significantly reduced such an increase in the infarction size (22.08±1.38% in the pre-HBOCs group, P<0.01 vs. the control group).
Figure 3. Myocardial infarct size determined by TTC staining. Red staining areas indicate viable tissue and non-stained pale areas indicate infarct tissue. Representative TTC-stained myocardial sections were shown at the top. Values were presented as mean ± SEM (n=5, 5 to 6 slices per heart). **P<0.01 vs. the sham group, ‡P<0.01 vs. the control group.
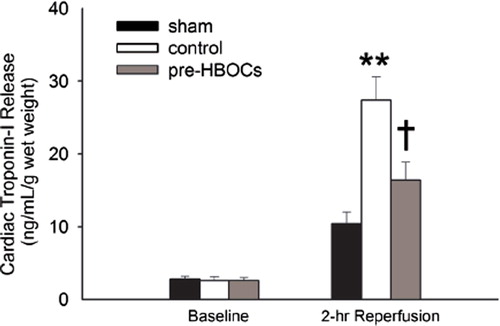
PolyPHb Pretreatment Reduced Cardiac Troponin-I Release
At baseline, the release of troponin-I was low and showed no significant difference among the three groups. After 2-hr reperfusion, the level of troponin-I release was greatly elevated in the control group (27.40±3.23 vs. 10.41 ± 1.63 ng/mL/g wet weight in the sham group, P<0.01, ). PolyPHb pretreatment markedly reduced the release of troponin-I (16.39 ±2.50 ng/mL/g wet weight, P<0.05 vs. the control group).
DISCUSSION
HBOCs were initially developed as blood substitutes [Citation12, Citation13]. However, there were several complications associated with HBOCs systemic administration, such as renal toxicity, coronary and cerebral vasospasm, gastrointestinal side-effects, chest and abdominal pain [Citation14,Citation15]. Some recently published clinical trials indicated that HBOCs may induce hypertension and myocardial infarction, and probably increase the mortality of the patient, which have placed HBOCs in a disadvantageous situation [Citation16,Citation17]. Therefore, in addition to improving the quality of HBOCs itself, we have started to explore the alternative clinical uses of HBOCs from several years ago [Citation7–9].
In the present study, we pretreated the isolated rat hearts with 0.1gHb/dL PolyPHb to investigate the potential effect of PolyPHb on the I/R heart. The results demonstrated that PolyPHb pretreatment greatly improved the cardiac functional recovery, as evidenced by the elevated contractile performance during reperfusion when compared to the control group. In addition, the myocardial infarction and necrosis were also significantly reduced in the pre-HBOCs group, for the TTC non-stained areas in the cardiac sections were largely decreased and the cardiac troponin-I release was greatly inhibited, which further proved the protective effect of PolyPHb pretreatment. The reasons for the cardioprotection we observed are not very clear but may be associated with the myocardium oxygen preload before lethal ischemia. As we know, ischemic preconditioning is performed by brief and repetitive exposure to I/R before sustained ischemia, which can markedly enhance the ability of the heart to withstand a subsequent I/R injury. Conversely, in the present study, the heart was exposed to a brief hyperoxia/reperfusion by oxygen-rich PolyPHb solution before sustained ischemia, which could be termed “hyperoxic preconditioning.” The results clearly indicated such a “hyperoxic preconditioning” from PolyPHb pretreatment also provided protection to the heart against I/R injury. Recently, some animal studies of HBOCs have indicated their potential cardioprotective effects in I/R hearts [Citation13,Citation18,Citation19]. Our previous study found that a PolyPHb protected rat heart after cold storage and reperfusion and the mechanism was closely related to attenuation of NO-mediated myocardial apoptosis and restoration of myocardial nitroso-redox balance. The results of this study provided additional evidence that PolyPHb, a type of HBOCs, was a promising agent for cardioprotection.
In conclusion, the results of the present study demonstrated that PolyPHb pretreatment is beneficial to the recovery of cardiac function and can greatly reduce the myocardial infarct size and cardiac troponin-I release, thus providing protective effect to the isolated rat heart against myocardial infarction caused by I/R injury.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Moens, A. L., Claeys, M. J., Timmermans, J. P., Vrints, C. J. (2005). International Journal of Cardiology. 100:179–190.
- Ferdinandy, P., Schulz, R., Baxter, G. F. (2007). Pharmacological Reviews. 59:418–458.
- Jahangir, A., Sagar, S., Terzic, A. (2007). J Appl Physiol. 103:2120–2128.
- Boengler, K., Schulz, R., Heusch, G. (2009). Cardiovasc Res. 83:247–261.
- Li, T., Yu, R., Zhang, H. H., Wang, H., Liang, W. G., Yang, X. M., Yang, C. M. (2006). Artif Cells Blood Substit Immobil Biotechnol. 34:175–188.
- Li, T., Zhang, H., Wang, H., Yu, R., Yang, C. (2006). Journal of Biomedical Engineering. 23:640–644.
- Li, T., Li, J., Liu, J., Zhang, P., Wu, W., Zhou, R., Li, G., Zhang, W., Yi, M., Huang, H. (2009). Free Radical Biology and Medicine. 46:397–405.
- Li, T., Liu, J., Yang, Q., Wu, W., Zhang, P., Yang, J., Li, J., Zhang, W., Yang, C. (2009). Artif Cells Blood Substit Immobil Biotechnol. 37:48–52.
- Wu, W., Yang, Q., Li, T., Zhang, P., Zhou, R., Yang, C. (2009). Artif Cells Blood Substit Immobil Biotechnol. 37:163–165.
- Standl, T., Freitag, M., Burmeister, M. A., Horn, E. P., Wilhelm, S., Am Esch, J. S. (2003). J Vasc Surg. 37: 859–865.
- McNeil, C. J., Smith, L. D., Jenkins, L. D., York, M. G., Josephs, M. J. (2001). J Trauma. 50:1063–1075.
- D'Agnillo, F., Chang, T. M. (1998). Nat Biotechnol. 16:667–671.
- Burmeister, M. A., Rempf, C., Standl, T. G., Rehberg, S., Bartsch-Zwemke, S., Krause, T., Tuszynski, S., Gottschalk, A., Schulte am Esch, J. (2005). Br J Anaesth. 95:737–745.
- Jahr, J. S., Nesargi, S. B., Lewis, K., Johnson, C. (2002). American Journal of Therapeutics. 9:437–443.
- Winslow, R. M. (2003). J Intern Med. 253:508–517.
- Chen, J. Y., Scerbo, M., Kramer, G. (2009). Clinics. 64: 803–813.
- Natanson, C., Kern, S. J., Lurie, P., Banks, S. M., Wolfe, S. M. (2008). JAMA. 299:2304–2312.
- Caswell, J. E., Strange, M. B., Rimmer, D. M., 3rd, Gibson, M. F., Cole, P., Lefer, D. J. (2005). Am J Physiol Heart Circ Physiol. 288:1796–1801.
- George, I., Yi, G. H., Schulman, A. R., Morrow, B. T., Cheng, Y., Gu, A., Zhang, G., Oz, M. C., Burkhoff, D., Wang, J. (2006). Am J Physiol Heart Circ Physiol. 291:1126–1137.