Abstract
Abstract: Gastroretentive floating microspheres have a potential for enhancing the bioavailability and controlled delivery of drugs. The present study involves development of rifampicin floating microspheres in order to increase the gastric retention time. The microspheres were prepared by solvent evaporation technique and characterized for particle size, shape, zeta-potential, entrapment, and release kinetics. The developed systems were almost spherical in shape. The entrapment efficiency was found to be 86.34%. The percentage buoyancy after 8 hours was found to be 61.06. The prepared microspheres exhibited prolonged drug release in gastric medium and hence could be utilized for sustained delivery of anti-tubercular drugs.
INTRODUCTION
The oral route is the most common and preferable route for the delivery of drugs. This may be due to ease of administration, patient compliance, and flexibility in formulation [Citation1]. However, it has limitations because of the broad diversity in biochemical and physiological atmospheres of the gastrointestinal tract. Thus, it can't be possible to get the same absorption pattern of orally administered drugs at different parts of the gastrointestinal tract (GIT). Moreover, first-pass metabolism of drugs in the intestinal wall and liver has also been a limiting factor for exploring the potential of oral dosage forms. These problems can be overcome by using oral controlled release (CR) formulations that provide controlled release of the drugs in GIT, maintain a constant drug concentration in the serum for longer periods of time, provide better bioavailibility, therapeutic efficacy, and possible reduction of the dose size. Moreover, oral control release systems can also be utilized to improve the gastric retention time (GRT). Prolonged gastric retention helps to retain the CR system in the stomach for a longer time in a predictable manner [Citation2]. The gastro-intestinal residence time determines the time period available for drug release from oral controlled release delivery systems within the GIT [Citation3]. Several approaches have been utilized to increase the gastric retention time, i.e. floating systems, mucoadhesive dosage forms, high-density systems, superporous hydrogel, swelling and expanding systems, and magnetic systems. These systems have more flexibility in dosage design than conventional dosage forms [Citation4–6].
Gastroretentive formulations are a device that remains buoyant in gastric juice for a longer time and releases drugs in a predetermined and predictable manner. Floating Drug Delivery Systems (FDDS) can be designed in both single and multiple unit systems. Generally, single unit floating devices are not suitable for control release formulation because of variability release patterns and bioavailability in GIT [Citation7,Citation8]. However, multiple unit particulate dosage forms (e.g. microspheres) have shown better applicability due to increased surface area of dosage forms that will provide uniform distribution of drugs in GIT and provide a desired release pattern, which reduces variability in absorption and other associated problems [Citation8–11]. These microspheres are characteristically free flowing powders having a size less than 200 μm and remain buoyant in gastric content for a prolonged period. As the system floats in gastric fluids, the drug releases slowly at the desired rate, resulting in enhanced gastric absorption with reduced fluctuations in plasma drug concentration [Citation8,Citation12]. Kawashima et al. [Citation13] prepared the hollow microspheres of ibuprofen by the emulsion solvent diffusion method. Soppimath et al. [Citation14] prepared the floating microspheres using natural polymers such as chitosan, xanthan gum, gelatin, and pectin. Muthuswamy et al. [Citation15] also prepared the floating microspheres of lanzoprazole and cimetidine using semi-synthetic polymers such as methyl cellulose and hydroxyl methyl cellulose. Dennis et al. [Citation16] proposed a loose powder-filled floating capsule that will float on gastric juices and thus improve the drug availability. Muller et al. [Citation17] patented a floating drug delivery system that has lower density than gastric juice, due to incorporation of at least one porous structural element, such as foam or a hollow body.
Table 1. Micromeritics properties of developed rifampicin microspheres.
The objective of the present investigation was to prepare a floating microsphere of rifampicin in order to achieve an extended release in the upper GIT, which may result in an enhanced absorption and thereby improved bioavailability. Rifampicin is a first-line anti-tubercular agent that is used with other anti-tubercular agents to treat tuberculosis caused by mycobacterium tuberculosis. It is mainly absorbed through the stomach and is better absorbed in the acidic environment of the stomach. But in combination with isoniazid, rifampicin forms 3-formyl rifampicin, thereby reducing the bioavailability of rifampicin from oral route [Citation18]. Gastro-retentive systems can remain in the gastric region for several hours and hence significantly improve the gastric residence time of drugs. Prolonged gastric retention further improves the bioavailability of rifampicin and reduces drug waste by minimizing the interaction of rifampicin with isoniazid.
In the present study, rifampicin loaded floating microspheres were prepared by the solvent evaporation method and characterized for their size, shape, percent yields, flow properties, and in-vitro buoyancy and in-vitro release.
MATERIALS AND METHODS
Rifampicin was received as a gift sample from Lupin Pharmaceutical (India) Ltd., Mumbai. Hydroxy propyl methyl cellulose (HPMC) and ethyl cellulose (EC) were purchased from Himedia Pvt. Ltd., India. All the other ingredients used were of analytical grade.
PREPARATION OF FLOATING MICROSPHERES
Rifampicin loaded floating microspheres were prepared by using the solvent evaporation method as employed by Srivastava et al. [Citation8]. EC, HPMC, and Rif were dissolved in a mixture of ethanol and dichloromethane having the ratio of 1:1. This solution was poured slowly into the water containing 0.01% Tween 80 and stirred for 40 minutes to allow the volatile solvent to evaporate. The microspheres were filtered, washed with water, and dried in vacuum.
CHARACTERIZATION OF MICROSPHERES
Size and Shape of Microspheres
The size of microspheres was determined using a microscope fitted with an ocular micrometer and stage micrometer (Motic Microscope, India). Mean particle size and zeta potential of prepared systems were determined by a photon correlation spectroscopy using a Delsa Nano (Beckman Coulter, UK).
Determination of Percent Yield. Thoroughly dried microspheres were collected and weighed accurately. The percentage yield was then calculated.
In-vitro Buoyancy Percentage. Floating microspheres (50 mg) were placed in 0.1 N HCI (100 ml) and stirred at 100 rpm. The floating microspheres were separated by filtration. The microspheres were dried in a desiccator overnight [Citation19]. The % buoyancy was calculated using the formula below.
ENTRAPMENT EFFICIENCY
To determine the entrapment efficiency, 50 mg microspheres were taken, thoroughly triturated, and suspended in a minimal amount of alcohol. The suspension was suitably diluted with water and filtered. Drug content was analyzed spectrophotometrically at 475 nm.
IN-VITRO RELEASE
The drug release of rifampicin loaded microspheres was determined using a USP XXIV basket-type dissolution apparatus [Citation20]. A weighed amount of microspheres equivalent to 50 mg drug was placed in the basket. Simulated gastric fluid was used as the dissolution medium and maintained at 37°C at 100 rpm. 5 ml sample was withdrawn after a 30 min interval for 1 hour and was passed through a membrane filter. Other samples were withdrawn hourly for 8 hours. The samples were then analyzed spectrophotometrically at 475 nm to determine the concentration of drug present in the dissolution medium. The initial volume of the dissolution fluid was maintained by adding 5 ml of fresh dissolution fluid after each withdrawal.
RESULTS AND DISCUSSION
Floating microspheres were prepared by solvent diffusion to create the hollow inner core. Polymers were dissolved in an organic solvent and then emulsified with an aqueous drug solution containing surfactant to form oil in water emulsion. After the formation of a stable emulsion, the organic solvent was evaporated by continuous stirring. The solvent removal lead to polymer precipitation at the o/w interface of droplets, forming a cavity, and thus imparted the floating properties in the developed microspheres.
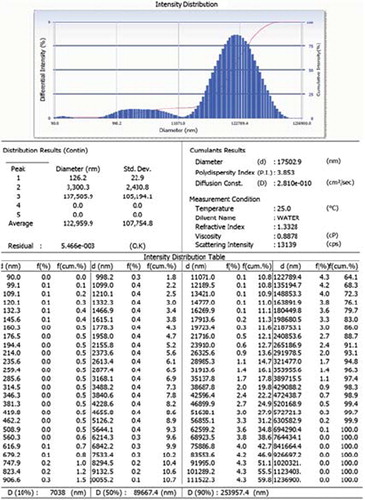
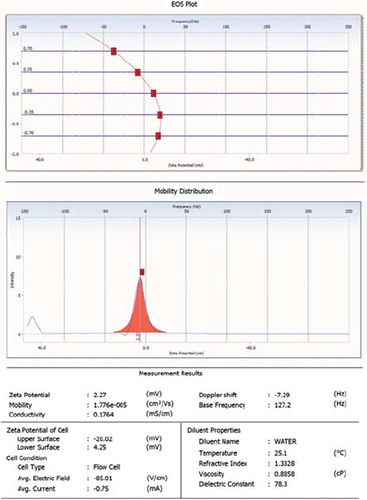
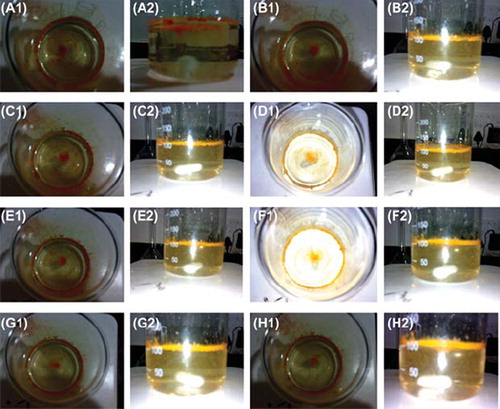
In the present study, floating microspheres were prepared by the solvent evaporation technique using HPMC and EC in the 1:6 ratio. The photomicroscopic images confirm that floating microspheres were almost spherical in shape with no visible major surface irregularity (). The particle sizes of prepared formulation were found to be 17.5 ± 4.45 μm; however, zeta potential of developed formulation was found to be 2.5 ± 0.1 mV ( and ). The percentage yield of the developed formulation was calculated and found to be 78.57%. The high entrapment efficiency of rifampicin is believed to be due to its poor aqueous solubility. The drug entrapment was found to be 86.34% ± 2.34.
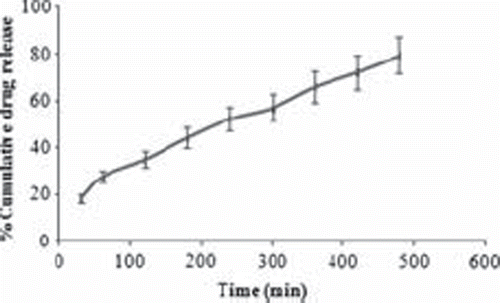
In-vitro buoyancy was carried out to investigate the floatability of the prepared floating microspheres. The floating behavior of the rifampicin microspheres was carried out in simulated gastric fluid. The lag time of floating microspheres was found to be zero. The HPMC and EC in the ratio of 1:6 showed the excellent buoyancy time with no lag time. The formulation was buoyant for more than 8 hours. A photomicrograph of the developed formulation has also confirmed the floatability properties of the microspheres (). 61.6 % of the formulation remained buoyant after 8 hours, indicating that the bulk density of the formulations remained unchanged. This could be due to poor wettability of ethyl cellulose. Floating microspheres showed sustained release of the drug in an acidic environment and the drug release was found to be approximately linear. The percentage of rifampicin released from the formulated products is shown in . One of the objectives of the present study was to control the release of the rifampicin in the acidic medium to minimize the concentration-dependent degradation of rifampicin when administered along with isoniazid. Approximately 18% of the drug was released initially within half an hour. The total drug release from the microspheres after 8 hour in the simulated gastric fluid was 79.45% (). The study demonstrated that floating microspheres are useful for the delivery of sparingly soluble and insoluble drugs. The positioned gastric release is useful for efficient absorption of drugs through the stomach, thus enhancing their bioavailability.
CONCLUSION
For better management of tuberculosis and in order to sustain the release of rifampicin in the stomach, an attempt was made to prepare floating microspheres of rifampicin using HPMC and EC. Studies demonstrated better entrapment and sustained release patterns. Moreover, the developed system has shown good buoyant ability. It is known that, as the solubility of a drug decreases, the time available for drug dissolution becomes less adequate and thus the transit time becomes a significant factor affecting drug absorption. This could be advantageous to improve the bioavailability of rifampicin for the efficient management of tuberculosis. The microspheres could be compressed into tablets, filled into capsules, or formulated into oral suspensions for reconstitution.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Desai, S., Bolton, S.A. (1993). Floating controlled-release drug delivery system: In vitro-in vivo evaluation. Pharm Res 10: 1321–1325.
- Soppimath, K.S., Kulkarni, A.R., Aminabhavi, T.M. (2001). Development of hollow microspheres as floating controlled-release systems for cardiovascular drugs: Preparation and release characteristics. Drug Dev Ind Pharm 27: 507.
- Singh, B.M., Kim, K.H. (2000). Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J Control Release 63: 235–259.
- Seth, P.R., Tossounian, J. (1984). The hydrodynamically balanced system HBSTM: A novel drug delivery system for oral use. Drug Dev Ind Pharm 10: 313–339.
- Moes, A.J. (1993). Gastroretentive dosage forms. Crit Rev Ther Drug Carrier Syst 10: 143–195.
- Deshpande, A.A., Rhodes, C.T., Shah, N.H., Malick, A.W. (1996). Controlled-release drug delivery systems for prolonged gastric residence: An overview. Drug Dev Ind Pharm 22: 531–539.
- Whitehead, L., Fell, J.T., Collett, J.H., Sharma, H.L., Smith, A.M. (1998). Floating dosage forms: An in vivo study demonstrating prolonged gastric retention. J Control Release 55: 3–12.
- Srivastava, A.K., Ridhurkar, D.R., Wadhwa, S. (2005). Floating microspheres of cimetidine: Formulation, characterization and in vitro evaluation. Acta Pharm 55: 277–285.
- Kawashima, Y., Niwa, T., Takeuchi, H., Hino, T., Itoh, Y. (1992). Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J Pharm Sci 81: 135–140.
- Stithit, S., Chen, W., Price, J.C. (1998). Development and characterization of buoyant theophylline microspheres with near zero order release kinetics. J Microencapsulation 15: 725–737.
- Struebel, A., Siepmann, J., Bodmeier, R. (2003). Multiple units gastroretentive drug delivery systems: A new preparation method for low density microspheres. J Microencaps 20: 329–347.
- Khar, R.K., Vyas, S.P. (2002). Targeted and Controlled Drug Delivery Novel Carrier System, CBS Publishers and Distributors, New Delhi, 417–441.
- Kawashima, Y., Niwa, T., Takechi, H., Hino, T., Itoh, Y. (1992). Hollow microspheres for use as floating controlled drug delivery systems in the stomach. J Pharm Sci 81: 135–140.
- Soppimath, K.S., Kulkarni, A.R., Rudzinski, W.E., Aminanhavi, T.M. (2002). Microspheres as floating drug delivery systems to increase gastric retention of drugs. Drug Metab Rev 33: 149–160.
- Muthuswamy, K., Govindrazan, G., Ravi, T.K. (2005). Preparation and evaluation of lansoprazole floating micropellets. Ind J Pharm Sci 67: 75–79.
- Dennis, A., Timmins, P., Lee, K. (1992). Buoyant controlled release powder formulation. U.S. Patent 5, 169, 638.
- Muller, W., Anders, E. (1989). Floating system for oral therapy. W.O. Patent 89, 06956.
- Shishoo, C.J., Shah, S.A., Rathod, I.S., Savale, S.S., Kotecha, J.S., Shah, P.B. (1999). Stability of rifampicin in dissolution medium in presence of isoniazid. Int J Pharm 190: 109–123.
- Junyaprasert, V.B., Pornsuwannapha, S. (2008). Drug Delivery 15: 331–341.
- The United States Pharmacopoeia XXIV, United States Pharmacopoeial Convention, Rockville, MD. 1941–1943.