Abstract
This study was designed to investigate whether polymerized human placenta hemoglobin (PolyPHb) attenuated hemorrhagic shock-induced lung injury. A mean arterial pressure (MAP) of 30mmHg was maintained for 60 min. Then, all the rats were randomly resuscitated with hetastarch, whole blood, or PolyPHb. The result indicated that PolyPHb greatly improved the MAP and pulmonary function, and significantly reduced the release of inflammatory cytokines, histopathological changes, and pulmonary edema. Therefore, our findings suggest that PolyPHb could reduce pulmonary injury after hemorrhagic shock, and this effect was probably associated with the depressed inflammatory response.
Introduction
Approximately one-third of acute lung injury was caused by non-infectious systemic inflammatory response syndrome from various pathological situations, including hemorrhagic shock (Matthay et al. Citation2003, Spragg et al. Citation2010). The proinflammatory mediators and reactive oxygen species induced by hemorrhagic shock play a significant role in the development of acute lung injury (Fink Citation2002, Maier et al. Citation2007). Despite the fact that fluid resuscitation markedly improved short-term survival of patients with hemorrhagic shock, acute lung injury was frequent and threatened the lives of these patients (Cohn et al. Citation2007, Yu et al. Citation2011).Thus, finding an agent that can both resuscitate a patient from hemorrhagic shock and preserve pulmonary function is urgently required.
Polymerized human placenta hemoglobin (PolyPHb) is one of the hemoglobin-based oxygen carriers developed in China (Li et al. Citation2006a, Li et al. Citation2006b). The primary application of PolyPHb is to treat patients suspected of suffering from ischemia, such as in trauma and hemorrhagic shock. Owing to its excellent oxygen-transporting capacity, PolyPHb has been demonstrated to be effective in the reduction of cardiac ischemia/reperfusion (I/R) injury and reversal of anaerobic metabolism (Li et al. Citation2011, McNeil et al. Citation2001, Standl et al. Citation2003). The underlying mechanisms include attenuation of NO-mediated myocardial apoptosis, restoration of nitroso-redox balance, and preservation of mitochondria function (Li et al. Citation2009a, Li et al. Citation2009b, Li et al. Citation2010). However, the effect of PolyPHb on acute lung injury has never been reported. Therefore, by use of a rat hemorrhagic shock model, we sought to investigate whether PolyPHb could also attenuate acute lung injury after hemorrhagic shock.
Materials and Methods
This study was approved by the Institutional Animal Care and Use Committee of Sichuan University, and all animals received human care in compliance with The Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Hemoglobin-based oxygen carrier
The PolyPHb employed in this study was prepared as we reported previously (Li et al. Citation2006a, Li et al. Citation2006b). Briefly, purified and viral inactivated fresh human placenta hemoglobin (Tianjin Union Stem Cell Genetic Engineering Ltd, Tianjin, China) was modified with pyridoxal phosphate to achieve optimal O2 affinity. After crosslinkage with glutaraldehyde, PolyPHb was subject to ultrafiltration and molecular sieve chromatography. The final product had a molecular weight of 64–600 kDa and concentration of 10 gHb/dL (pH 7.4).
Animal preparation
Sixty male Sprague-Dawley rats, weighing 200-250g, were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) and heparin (500 IU). After endotracheal intubation, all the rats were mechanically ventilated with 95% oxygen at a respiratory rate (RR) of 30 bpm and tidal volume (Vt) of 6 ml/kg (DH-150, Medical Instrument Company of Zhejiang University, Hangzhou, Zhejiang, China). Anesthesia was maintained by injection of 15 mg/kg sodium pentobarbital into the tail vein. Body temperature was maintained at 36–37°C during the experiment with a heating blanket. A polyethylene catheter was placed in the left femoral artery to withdraw blood and measure mean arterial pressure (MAP) via a PowerLab data-acquisition system (ADInstruments Pty, Bella Vista, NSW, AUS). Another polyethylene catheter was placed in the left femoral vein for infusion of blood or solutions.
Protocol
A rat hemorrhagic shock model was established as we described previously (Li et al. Citation2012). Briefly, MAP of 30 mmHg was achieved by blood withdrawal and kept for 60 min. After that, the rats were randomly resuscitated with a 10 min infusion of equivalent volume of hetastarch (HES group, n = 20), shed blood (whole blood group, n = 20) or PolyPHb solution (PolyPHb group, n = 20), and then observed for 2 h. After the experiment, all the rats were sacrificed with a bolus of I.V. sodium pentobarbital (120 mg/kg).
Pulmonary function
The arterial oxygen tensions (PaO2) of blood samples collected at baseline, 0, 30, 60, and 120 min after resuscitation were measured by a portable blood gas analyzer (i-STAT Corporation, Windsor, NJ). The pulmonary function was assessed by the ratio of arterial oxygen tension /fraction of inspire oxygen (PaO2/FiO2).
Determination of inflammatory cytokine release
The white blood cell (WBC) and neutrophils counts were measured by an auto hematology analyzer (BC-3000 Plus, Mindray, Shenzhen, China) from blood samples collected at baseline and 2 h after resuscitation. Lung tissue (100 mg, wet weight) was homogenized and the protein concentration of its supernatant was determined by the BCA method (Pierce, Rockford, IL, USA). Then the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-8 in the supernatant were determined by commercial ELISA kits (R&D, Minneapolis, MN).
Histological examination and pulmonary edema
The left lower lung tissues of rats were placed in 10% neutral formalin and embedded in paraffin, then sectioned into 5-μm intervals and stained with Hematoxylin and Eosin (H&E). The results were assessed in a blinded fashion by a pathologist for alveolar wall thickness and neutrophil infiltration. The remaining left lower lobe was excised and immediately weighed, and then dried at 60oC for 72 h in an oven. The edema of pulmonary was determined by the ratio of wet weight/dry weight (W/D ratio).
Statistical analysis
All values in the text and figures were presented as mean ± SD and analyzed by one-way ANOVA followed by LSD correction for post hoc t test (SPSS 13.0 software). P values < 0.05 were considered statistically significant.
Results
Five rats in the HES group, two rats in the whole blood group, and three rats in the PolyPHb group were removed from this protocol for sudden cardiac arrest or uncontrolled blood loss during operation.
PolyPHb improved the recovery of MAP and pulmonary function after hemorrhagic shock
As shown in , the recovery of MAP in the PolyPHb group was significantly improved when compared with the HES group (P< 0.001), even though it was not as high as that in the whole blood group (P< 0.05). Similarly, the PaO2/FiO2 ratio was greatly increased in the PolyPHb group as compared to the HES group, but it was still lower than that of the whole blood group ().
Figure 1. The PaO2/FiO2 at baseline and during the period of resuscitation. Values were presented as mean ± SD (n = 15–18). ***P< 0.001 and **P< 0.01 vs. the HES group; †P< 0.05 vs. the whole blood group. PaO2/FiO2: the ratio of arterial oxygen tension /fraction of inspire oxygen.
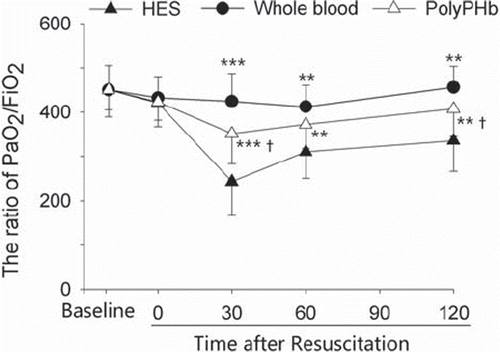
Table I. Mean arterial pressure throughout the study of the three groups.
PolyPHb attenuated pulmonary inflammatory after hemorrhagic shock
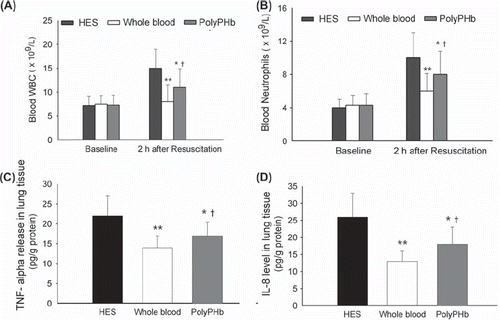
At baseline, the counts of WBC and neutrophils in blood were similar among the three groups. However, after 2 h of resuscitation, they were greatly increased in the HES group. PolyPHb markedly depressed the elevation of these cytokines (P 0.05, respectively, /B). Meanwhile, the TNF-α release and IL-8 level in the lung tissue were also significantly decreased by PolyHb (P 00.05, respectively). But it is noteworthy that these inflammatory cytokines in the PolyPHb group were still higher than these in the whole blood group (P 0.05, respectively, /D).
Figure 2. The counts of WBC (A) and neutrophils (B) in blood. The levels of TNF-α (C) and IL-8 (D) in the lung tissue. Values were presented as mean ± SD (n = 15–18). **P< 0.01 and *P< 0.05 vs. the HES group; †P< 0.05 vs. the whole blood group. IL: interleukin; TNF: tumor necrosis factor; WBC: white blood cell.
PolyPHb reduced pulmonary histopathological changes and edema after hemorrhagic shock
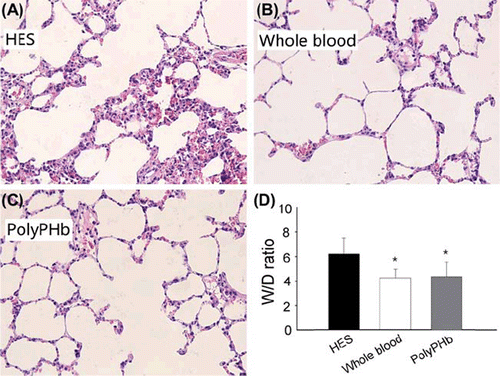
Typical pulmonary inflammation induced by hemorrhagic shock was further confirmed by increases of neutrophil infiltration and thickness of alveolar membrane in the HES group. These histopathological changes were remarkably limited in the PolyPHb and whole blood groups. In addition, the elevation of W/D ratio in the HES group was significantly decreased by treatment either with PolyPHb or with whole blood ().
Figure 3. A-D, Representative photomicrographs of H&E-stained left lung tissue sections (40x) harvested after 2 h of resuscitation. E, the W/D ratio of the three groups. Values were presented as mean ± SD (n = 15–18). *P< 0.05 vs. the HES group. H&E: Hematoxylin and Eosin; W/D ratio: the ratio of wet weight/dry weight.
Discussion
We sought to find an agent that can reduce acute lung injury related to hemorrhagic shock. PolyPHb seems to be a reasonable candidate because, in many other studies, it has been proven to be organ protective, especially for the in vivo and in vitro heart (Li et al. Citation2011, McNeil et al. Citation2001, Standl et al. Citation2003, Wu et al. Citation2009). PolyPHb was developed by the research group of Professor Yang and initially used for treatment of patients in hemorrhagic shock and trauma (Li et al. Citation2006a, Li et al. Citation2006b). Several years ago, great effort was put on investigations of its clinical alternative uses (Burmeister et al. Citation2005, D’Agnillo and Chang Citation1998). The property of high oxygen affinity, low viscosity, and small mean diameter of PolyPHb makes it possible to freely diffuse in microcirculation and transport oxygen to hypoxia tissues. Thus, in theory, PolyPHb is a prospective agent for treatment of organ I/R injury.
Consistent to our hypothesis, the present study demonstrated that resuscitation by PolyPHb infusion can greatly ameliorate the MAP recovery and pulmonary function and depress the elevation of inflammatory cytokines release, including WBC, neutrophils, TNF-α and IL-8, after hemorrhagic shock, even though this effect was not as good as the whole blood. Also, the data of this study clearly showed that PolyPHb can reduce histopathological changes and pulmonary edema induced by hemorrhagic shock, indicating a promising protective effect on lung injury.
In contrast, our previous study indicated that there was no positive cardiac effect provided by PolyPHb in hemorrhagic shock. The exact mechanism for this different effect observed in this study was not clear, but we believe the following reasons may be involved. Firstly, during hemorrhagic shock, lung I/R injury is unavoidable because the systemic circulation is dramatically altered. As an oxygen carrier, PolyPHb is able to facilitate oxygen delivery to hypoxia tissues, thus facilitating the restoration of oxygen supply-demand balance and minimizing the ischemia injury. Secondly, the proinflammatory mediators and reactive oxygen species induced by hemorrhagic shock play a significant role in the development of acute lung injury. In the early phase of hemorrhagic shock, the release of cytokines like IL-1, IL-6, IL-8, IL-10, and TNF-α secreted by alveolar macrophages may be greatly increased. These increased cytokines will act locally to stimulate chemotaxis and activate neutrophils, and finally bring great damage to the lung (Abraham et al. Citation2000, Strieter et al. Citation1999, Takala et al. Citation2002). The data of this study showed that PolyPHb depressed the hemorrhagic shock induced systemic and pulmonary inflammation, as evidenced by reduced inflammatory cytokines release in blood and lung tissue. Therefore, the depressed inflammatory response may probably contribute to the protective effect of PolyPHb on the lung.
In conclusion, our findings suggest that PolyPHb provided a protective effect on lung injury in a rat hemorrhagic shock model, and this effect was probably related to the depressed inflammatory response.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by grants from the National Nature Science Foundation of China (81100180 and 81070117), the China Postdoctoral Specialized Science Foundation (201003700), the Specialized Research Fund for the Doctoral Program of Higher Education (20100181120090), the Major Program of the Clinical High and New Technology of PLA (2010gxjs039), and the Scientific Research Staring Foundation for Young Teachers of Sichuan University (2010SCU11022).
References
- Abraham E, Carmody A, Shenkar R, Arcaroli J. 2000. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 279:L1137-L1145.
- Burmeister MA, Rempf C, Standl TG, Rehberg S, Bartsch-Zwemke S, Krause T, Tuszynski S, Gottschalk A, Schulte am Esch J. 2005. Effects of prophylactic or therapeutic application of bovine haemoglobin HBOC-200 on ischaemia-reperfusion injury following acute coronary ligature in rats. Br J Anaesth 95:737–745.
- Cohn SM, Nathens AB, Moore FA, Rhee P, Puyana JC, Moore EE, Beilman GJ, Investigators tSiTPT. 2007. Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. J Trauma 62:44–55.
- D’Agnillo F, Chang TM. 1998. Polyhemoglobin-superoxide dismutase-catalase as a blood substitute with antioxidant properties. Nat Biotechnol 16:667–671.
- Fink MP. 2002. Reactive oxygen species as mediators of organ dysfunction caused by sepsis, acute respiratory distress syndrome, or hemorrhagic shock: Potential benefits of resuscitation with Ringer’s ethyl pyruvate solution. Curr Opin Clin Nutr Metab Care 5:167–174.
- Li T, Jiang Y, Zhang Z, Zhang S, Wu W, Liao D, Chen Y, Yang C, Xu X, Liu J. 2012. Effect of polymerized human placenta hemoglobin on hemodynamic parameter and cardiac function in a rat hemorrhagic shock model. Artif Cells Blood Substit Immobil Biotechnol. DOI: 10.3109/10731199.2012.663384.
- Li T, Li J, Liu J, Zhang P, Wu W, Zhou R, Li G, Zhang W, Yi M, Huang H. 2009a. Polymerized placenta hemoglobin attenuates ischemia/reperfusion injury and restores the nitroso-redox balance in isolated rat heart. Free Radic Biol Med 46:397–405.
- Li T, Liu J, Yang Q, Wu W, Zhang P, Yang J, Li J, Zhang W, Yang C. 2009b. Polymerized Placenta hemoglobin improves cardiac functional recovery and reduces infarction size of isolated rat heart. Artif Cells Blood Substit Immobil Biotechnol 37:48–52.
- Li T, Yu R, Zhang HH, Wang H, Liang WG, Yang XM, Yang CM. 2006a. A method for purification and viral inactivation of human placenta hemoglobin. Artif Cells Blood Substit Immobil Biotechnol 34:175–188.
- Li T, Zhang H, Wang H, Yu R, Yang C. 2006b. Purification and viral inactivation of hemoglobin from human placenta blood. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 23:640–644.
- Li T, Zhang P, Liu J, Zhou R, Li Q, You Z, Dian K. 2010. Protective effects of hemoglobin-based oxygen carrier given to isolated heart during ischemia via attenuation of mitochondrial oxidative damage. Free Radic Biol Med 48:1079–1089.
- Li T, Zhou R, Xiang X, Zhu D, Li Y, Liu J, Wu W, Yang C. 2011. Polymerized human placenta hemoglobin given before ischemia protects rat heart from ischemia reperfusion injury. Artif Cells Blood Substit Immobil Biotechnol 39:392–397.
- Maier B, Lefering R, Lehnert M, Laurer HL, Steudel WI, Neugebauer EA, Marzi I. 2007. Early versus late onset of multiple organ failure is associated with differing patterns of plasma cytokine biomarker expression and outcome after severe trauma. Shock 28:668–674.
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. 2003. Future research directions in acute lung injury: Summary of a national heart, lung, and blood institute working group. Am J Respir Crit Care Med. 167:1027–1035.
- McNeil CJ, Smith LD, Jenkins LD, York MG, Josephs MJ. 2001. Hypotensive resuscitation using a polymerized bovine hemoglobin-based oxygen-carrying solution (HBOC-201) leads to reversal of anaerobic metabolism. J Trauma 50:1063–1075.
- Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, Hall J, Israel E, Jain M, Needham DM, Randolph AG, Rubenfeld GD, Schoenfeld D, Thompson BT, Ware LB, Young D, Harabin AL. 2010. Beyond mortality: Future clinical research in acute lung injury. Am J Respir Crit Care Med. 181:1121–1127.
- Standl T, Freitag M, Burmeister MA, Horn EP, Wilhelm S, Am Esch JS. 2003. Hemoglobin-based oxygen carrier HBOC-201 provides higher and faster increase in oxygen tension in skeletal muscle of anemic dogs than do stored red blood cells. J Vasc Surg 37:859–865.
- Strieter RM, Kunkel SL, Keane MP, Standiford TJ. 1999. Chemokines in lung injury. Chest 116:103S–110S.
- Takala A, Jousela I, Takkunen O, Kautiainen H, Jansson S-E, Orpana A, Karonen S-L, Repo H. 2002. A prospective study of inflammation markers in patients at risk of indirect acute lung injury. Shock 17:252–257.
- Wu W, Yang Q, Li T, Zhang P, Zhou R, Yang C. 2009. Hemoglobin-based oxygen carriers combined with anticancer drugs may enhance sensitivity of radiotherapy and chemotherapy to solid tumors. Artif Cells Blood Substit Immobil Biotechnol 37:163–165.
- Yu HP, Hsieh PW, Chang YJ, Chung PJ, Kuo LM, Hwang TL. 2011. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic Biol Med 50:1737–1748.
