Abstract
Objective: Surgical resection of skull base tumors can be associated with significant morbidity. In cases where the risks outweigh the benefits, radiation therapy can offer an alternative means to effectively control tumor growth. However, the optimal dose regime for radiation therapy remains controversial. The objective of this study was to assess the neurological outcome, local control rate and morbidity associated with a 5-fraction regime of hypofractionated stereotactic radiotherapy (HSRT) for benign skull base tumors.
Methods: Twenty-six patients presenting with two of the most prevalent benign skull base tumors were included in the study. The tumors comprised 16 meningiomas and 10 acoustic neuromas. All patients exhibited preserved cranial nerve function prior to treatment, and a detailed audiological assessment was performed pre- and post-treatment for those patients with acoustic neuroma. Stereotactic radiosurgery was administered with the frameless CyberKnife Robotic Radiosurgery System. In each case, a 5-fraction HSRT regime was used: a dose of 5 Gy × 5 = 25 Gy to 6 Gy × 5 = 30 Gy was prescribed for skull base meningiomas, and 5 Gy × 5 = 25 Gy was prescribed for acoustic neuromas.
Results: The clinical and radiographic median follow-up was 22 months (range: 6-54 months). Radiological assessment showed local control in all 26 tumors (100%), and in 5/26 patients (20%) the tumor showed a decrease in size. Cranial nerve function was preserved in all cases thus far studied; however, 28% of patients had transient Grade II side effects, including fatigue, headaches, unsteadiness and transient subjective worsening of hearing. In two of these patients, the period of transient worsening of hearing was associated with a temporary increase in the size of the tumor on control T2 MR images, consistent with radiation-induced edema. One patient had transient decrease in visual acuity from treatment-related edema. At the last follow-up, 3/16 patients with meningiomas (19%) and 2/10 with acoustic neuromas (20%) showed a decrease in tumor volume and improvement in hearing.
Conclusion: A 5-fraction stereotactic radiotherapy regime, as used in this study, seems to be effective for local control of benign skull base tumors in this early follow-up evaluation. Neurological function preservation is excellent with this short regime in the early post-treatment period, but long-term follow-up is crucial for validation.
Introduction
Meningiomas and acoustic neuromas constitute the majority of benign skull base tumors Citation[1]. Other frequently encountered neoplasms include pituitary adenomas, craniopharyngiomas, glomus tumors and chordomas.
Meningiomas are the most common non-glial intracranial extra-axial tumors and constitute approximately 20% of all intracranial tumors Citation[2]. While most meningiomas occur supratentorially, roughly one in four occurs at the skull base Citation[3]. The most common location for skull base meningiomas is along the sphenoid ridge, followed in frequency by the olfactory grove, the sella/cavernous sinus, the cerebellopontine angle (CPA), the foramen magnum, and around the optic nerve sheath. Approximately 60–75% of skull base meningiomas are considered resectable Citation[4], Citation[5]; however, surgical resection is associated with significant morbidity and, in particular, post-operative cranial neuropathy in 14 to 58% of nerves Citation[4], Citation[6], Citation[7].
Sporadic acoustic neuromas are also rather common benign neoplasms of the skull base in the region of the CPA and represent up to 10% of intracranial tumors in large series Citation[8]. These tumors account for approximately 10–15 cases per million people per year in the US, which translates into approximately 2200 treated patients per year. Around 45% of them grow at an average rate of 1.2 mm/yr Citation[9], although acute growth spurts have been noted. While many tumors are detected incidentally during work-up for unrelated issues, some present with clinical symptoms including hearing loss, tinnitus, vertigo and unsteadiness. Microsurgical resection leads to good tumor control, but facial and hearing function is compromised in a significant number of patients, particularly those with larger tumors Citation[10].
Conventional radiation, conformal radiation (Intensity Modulated Radiation Therapy [IMRT], proton beam irradiation), fractionated stereotactic radiotherapy (FSRT) and stereotactic radiosurgery (SRS) are the common radiation therapy techniques employed for benign skull base tumors. Most radiation therapy techniques result in a high degree of local control, reportedly in the order of 90% or more Citation[11].
Stereotactic radiosurgery has a long track record of good long-term local control rates with relatively good preservation of cranial nerve function Citation[12], Citation[13]; however, hearing function can be compromised with single-fraction radiosurgery. Modern single-fraction SRS has been associated with a 10–40% risk of cranial nerve damage in some series Citation[14], Citation[15], and the incidence has been reported to be as high as 60% when qualitative hearing assessments are used Citation[16], Citation[17]. On the other hand, a conventional fractionated radiation therapy regime with 30–33 treatments over 5–6 weeks is very rarely associated with neurological toxicity Citation[18–21].
It would be desirable to design a radiation technique that offers a high degree of local control and convenience comparable to SRS but also a low level of neurological morbidity comparable to that with fractionated radiation over a prolonged treatment course Citation[22]. To this end, we validated a hypofractionated 5-fraction regime (Hypofractionated Stereotactic Radiotherapy – HSRT) for skull base meningiomas and acoustic neuromas.
Materials and methods
Patients and eligibility
This is a retrospective analysis of 26 consecutively treated patients with radiological diagnosis of benign skull base meningioma or acoustic neuroma. All patients were reviewed at a multidisciplinary brain tumor conference and were radiographically evaluated via gadolinium-enhanced MRI, including T1, T2 and FLAIR sequences with multiplanar reconstruction. Based on the radiographic characteristics, we identified 16 meningiomas and 10 acoustic neuromas for this study. Patients with acoustic neuromas had to have preserved facial nerve function (House-Brackman Grade 1) and preserved objective hearing (Gardner-Robertson Grade 1–3). As an indication to treat, sequential patient evaluation had to demonstrate clinical or radiological progression of the lesion. A radiation oncologist, a neuro-oncologist and a neurosurgeon evaluated all the patients, and all those with acoustic neuromas had pre-treatment and post-treatment follow-up audiograms. None of the patients had undergone previous surgery of the lesion, so histological diagnosis was unavailable. Patients with raised intracranial tension, significant edema or multiple lesions were excluded from the study.
Patients were treated with HSRT at Beth Israel Deaconess Medical Center from September 2005 to October 2010 using the CyberKnife® Robotic Radiosurgery System (Accuray, Inc., Sunnyvale, CA). Patients were prospectively entered into an institutional review board-approved database.
Treatment planning
Thin-cut gadolinium-enhanced axial MPRAGE MRI sequences were acquired for all patients prior to treatment. Patients were simulated and treated in the supine position with their arms down, lying in memory foam placed over a head cup augmented with a thermoplastic mask to ensure a comfortable and reproducible position. A contrast-enhanced CT scan with 1-mm sections was obtained in the treatment position after administration of 100 cc of standard IV contrast. The CT images were transferred to a dedicated MultiPlan™ workstation (Accuray, Inc.) and a CT/MRI fusion was performed. The target volume and critical structures, including the lens, optic globe, brain stem, optic nerves and optic chiasm were contoured. The clinical target volume (CTV) and the planning target volume (PTV) were defined as the enhancing lesion in the MRI and CT images. No expansion margin was used.
HSRT (Hypofractionated Stereotactic Radiotherapy) dose prescription
Skull base meningiomas were prescribed a cumulative dose of 25–30 Gy in 5 fractions of 5–6 Gy each, i.e., 5 Gy × 5 = 25 Gy to 6 Gy × 5 = 30 Gy. Twelve patients received 30 Gy, two received 25 Gy, and two received 27.5 Gy, all in 5 fractions. Normal tissue constraints were employed during treatment planning, such that less than 1 cc of the optic pathway and brain stem would receive >20 Gy in 5 fractions with the maximum dose not exceeding the prescription dose, i.e., there were no hot spots outside the target volume. All meningiomas were planned for 30 Gy in 5 fractions; however, in cases in which the optic pathway or brain stem tolerance was reached, a lower dose per fraction (5.0–5.5 Gy) was prescribed to conform to the dose tolerance limits.
Acoustic neuromas were prescribed 5 Gy × 5 = 25 Gy. They were treated with the smaller prescribed dose because of their abutment of the brainstem. In addition, 6 of the 10 had an intracanalicular component. The dose was prescribed to the isodose line covering at least 95% of the PTV. The radiation oncologist and neurosurgeon delineated all target volumes and reviewed the treatment plans.
Treatment delivery
The CyberKnife® Robotic Radiosurgery System (Accuray, Inc., Sunnyvale, CA) with frameless skull base live image guided tracking was used to treat all patients. The system and its use in frameless image guided robotic radiosurgery are well established and have been described previously Citation[23–25]. All treatments were prescribed for 5 consecutive working days. According to institutional protocol, prophylactic dexamethasone (4 mg p.o. bid 2 days prior to treatment and then maintained and tapered off during the 3 weeks following treatment) and H2 receptor antagonists such as ranitidine (Zantac®) (through the course of dexamethasone) were given. All patients were offered 0.5–1.0 mg of lorazepam 30 minutes prior to treatment.
Follow-up and analysis
Patients were followed by the treating radiation oncologist, neuro-oncologist and neurosurgeon, with follow-up visits at one month after HSRT, at three months after that, then every 6 months for 3 years, and annually thereafter. At each follow-up visit, a neurological examination was performed and a gadolinium-enhanced MRI was obtained. Audiological examinations were repeated at the time of each follow-up visit. Local control was defined as size-stability and non-progression in the MRI scan. Acute toxicity was defined as adverse events occurring within 3 months of HSRT, and long-term toxicity was defined as such events occurring after 3 months.
Descriptive (frequency and percentage) statistics were used for this patient population.
Results
Patients and treatment characteristics
summarizes the patient and treatment characteristics. The locations of skull base meningiomas in this series were as follows: the cavernous sinus (n = 3), the olfactory groove (n =3), the CPA (n =2), the foramen magnum (n =2), the clivus (n =2), Meckel's cave (n =2), the optic nerve sheath (n =1) and the anterior clinoid (n =1).
Table I. Treatment characteristics.
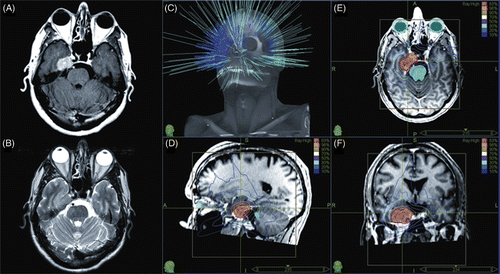
For skull base meningiomas (prescription dose 5 Gy × 5 = 25 Gy to 6 Gy × 5 = 30 Gy), the mean maximum dose was 35.54 Gy (range: 31.2 to 38.9 Gy) and the mean prescription isodose employed was 80%. The mean volume within the prescription isodose was 10.5 cc (range: 3.4 to 43.7 cc), with an average conformality index of 1.4 (range: 1.18 to 1.86) and a homogeneity index of 1.2 (range: 1.18 to 1.28). A representative meningioma treatment plan is shown in .
Figure 1. Representative MRI and treatment plan for skull base meningioma: (A) axial gadolinium-enhanced T1 MRI image; (B) axial T2 MRI image; (C) non-isocentric beam paths; (D) sagittal view of treatment plan; (E) axial view of treatment plan; and (F) coronal view of treatment plan.
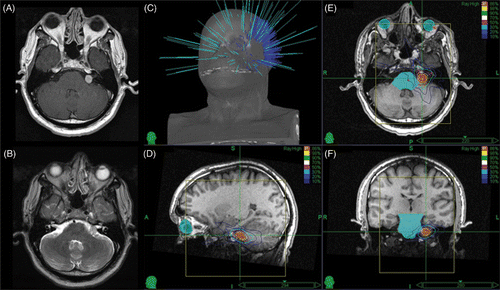
Patients with acoustic neuromas were treated with a prescription dose of 5 Gy × 5 = 25 Gy. The mean maximum dose was 30.1 Gy (range: 28.4 to 32.9 Gy) and the mean prescription isodose employed was 83%. The mean volume within the prescription isodose was 1.9 cc (range: 0.8 to 3.22 cc), with an average conformality index of 1.21 (range: 1.16 to 1.4) and a homogeneity index of 1.24 (range: 1.14 to 1.36). A representative acoustic neuroma treatment plan is shown in .
Figure 2. Representative MRI and treatment plan for acoustic neuroma: (A) axial gadolinium-enhanced T1 MRI image; (B) axial T2 MRI image; (C) non-isocentric beam paths; (D) sagittal view of treatment plan; (E) axial view of treatment plan; and (F) coronal view of treatment plan.
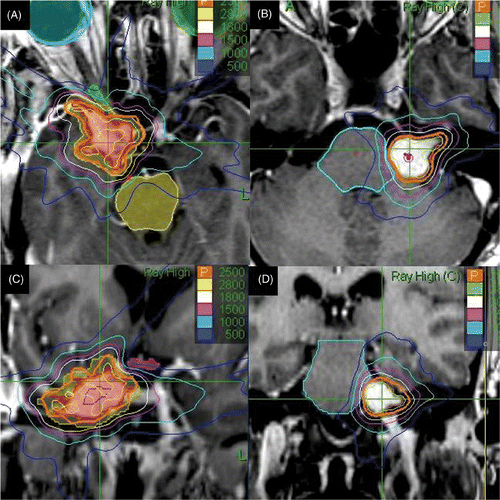
represents dose sculpting of isodose lines around critical structures including the optic nerve, chiasm and brainstem.
Clinical outcomes
At a median follow-up of 22 months (range: 6 to 54 months) for all patients, the radiological local control rate was 100%, as defined by non-progression in follow-up scans. All patients had preserved or improved cranial nerve and neurological function. Three of the 16 patients (19%) with skull base meningiomas (median follow-up: 23 months) had shrinkage of the treated volume. On assessment of pure tone average (dB) and speech discrimination scores in pre- and post-treatment audiograms, no patient with acoustic neuroma (median follow-up: 19 months) showed a decrease in their hearing post-treatment. Two patients (20%) noticed improved hearing, and these two patients had a decrease in tumor volume. While all other patients with acoustic neuromas had stable Gardner Robertson scores, these two patients with improved hearing had a 1-point decrease in their scores. Overall, 5 of 26 patients (20%) showed a decrease in tumor volume.
Toxicity
Most patients developed some fatigue in the week following HSRT, but did not require intervention (Grade I). Seven patients (28%) reported persistent headaches, unsteadiness or tinnitus requiring an increase in steroid medication (Grade II). Two patients with acoustic neuroma described transient worsening of hearing, which improved during subsequent follow-up. One patient had a transient decrease in unilateral visual acuity from treatment-related edema. There was no acute or long-term Grade III or IV toxicity.
Discussion
Hypofractionated Stereotactic Radiotherapy (HSRT) is a strategy for quick and effective treatment of benign skull base tumors with maximal neurological function preservation. Patients with benign skull base meningiomas and sporadic acoustic neuromas have long life expectancies, and while most benign tumors grow at a very slow rate, many eventually progress to cause clinical neurological symptoms. It is desirable to preserve the patient's cranial nerve function and quality of life while achieving good control of the tumor. The available treatment options include surveillance, surgical resection and radiation therapy. While radiation therapy is not invasive, it is mostly useful in achieving stability of growth and symptoms. On the other hand, surgery can be curative in a single procedure and may obviate the need for radiation and its potential long-term toxicity, including secondary tumors.
Unfortunately, surgical resection, while achieving good tumor control rates, can also lead to significant, and possibly permanent, neurological morbidity. In one of the largest series to date, de Jesús et al. reported a CSF leak rate of up to 21% when aggressive surgical resection was performed in skull base meningiomas Citation[4]. In two other large series, Sindou et al. Citation[6] and Knosp et al. Citation[7] reported a trigeminal and visual cranial nerve deficit incidence of between 14 and 58%. Similarly, even with microsurgical resection with intra-operative monitoring, facial nerve dysfunction occurs in up to 7% of patients Citation[26–29] and hearing loss is universally observed in most acoustic neuromas exceeding 2–3 cm in diameter Citation[30–32]. Hence, radiation therapy has been a preferred choice for such tumors with preserved neurological function. Treatment decisions are usually based on multidisciplinary education of the patient Citation[33]. Large single-institution retrospective series Citation[34] and, more recently, two prospective series seem to indicate better neurological outcomes for acoustic neuromas with radiosurgery compared to surgery Citation[35], Citation[36] and probably equivalent long-term control for meningiomas Citation[37].
Stereotactic radiosurgery (SRS) is very effective in long-term control of meningiomas Citation[14], Citation[37] and acoustic neuromas Citation[12]; however, in cases of tumors located near or in the skull base, cranial nerve damage from radiation toxicity is of concern Citation[12], Citation[38]. Risk factors for neurological damage from SRS have been studied Citation[39]: neurofibromatosis type 2 (NF2), tumor size, peripheral dose, and prior surgical resection all correlate with risk of nerve injury in long-term follow-up.
Assessment of the radiosensitivity of cranial nerve VIII is highly problematic, since it is almost always irradiated in the context of acoustic neuroma treatment. As such, it is impossible to distinguish between nerve damage that is intrinsic to the disease process, and that which is induced by the radiation treatment, or some combination thereof. Unfortunately, most radiotherapy and radiosurgery series fail to document detailed audiologic assessments prior to and following treatment. The probability of cranial nerve VIII dysfunction (as measured by objective changes in pure tone audiometry) after radiosurgery for acoustic neuroma is as high as 60%. Ito et al. reported that 69% of patients who underwent radiosurgery (median dose: 16.8 Gy) for acoustic neuroma exhibited pure tone average (PTA) elevation of >20 decibels (dB), suggesting hearing loss after treatment Citation[16]. Similarly, Paek et al. reported that 16/25 patients (64%) with serviceable hearing pre-treatment suffered hearing loss of >20 dB after radiosurgery (12 Gy at the 50% isodose) Citation[17].
Fractionated radiotherapy exploits the radiobiological advantage of neurological function preservation due to small fraction size Citation[40]. Excellent tumor control (90–100%) and preservation of cranial nerve function has been reported with daily radiation of 1.8–2.0 Gy per fraction to doses of 50–60 Gy over 5–7 weeks Citation[18], Citation[19], Citation[41–49].
The fundamental radiobiological advantage of fractionation lies in the protection of normal tissue with a lower Biologically Equivalent Dose (BED) due to the lesser alpha/beta ratio of normal tissue (e.g., cranial nerves) when compared to tumors, while maintaining equivalent BED for tumor control Citation[50], Citation[51]. BEDs for benign skull base tumors and other tumors with similar alpha/beta ratios for treatments ranging from 1, 3 or 5 up to 33 fractions have been calculated with an assumption of an alpha/beta of around 2.5 Gy Citation[22]. This has been reproduced in clinical experience with meningiomas Citation[52] and acoustic neuromas Citation[53], Citation[54]. Because most acoustic neuromas abut the brain stem or involve the internal acoustic meatus, we used a previously employed Citation[53] 5 Gy × 5 schedule so as not to exceed brain stem tolerance. Due to the relatively larger size and more favorable locations, a 6 Gy × 5 schedule was used for most menigiomas, which is biologically equivalent to other stereotactic radiosurgery and radiotherapy schedules for tumor control Citation[52]. compares our experience with other published series using a comparable HSRT treatment paradigm for benign skull base tumors. The biologically equivalent doses are similar. Compared to surgery or SRS, the radiological and neurological control appears to be excellent in our series and in all these series over this relatively short-term follow-up period Citation[52], Citation[55–57].
Table II. Reported series of Hypofractionated Stereotactic Radiotherapy for benign skull base tumors.
While the radiological and neurological stabilization seems to be excellent in our series, the tumor shrinkage and neurological improvement rates appear less impressive. For acoustic neuromas, the rates of tumor shrinkage for single-fraction SRS approach 75% at 5 years Citation[12]. For daily fractionated radiation employing 50–60 Gy, tumor shrinkage rates of 40–45% have been reported Citation[47], Citation[49]. The shrinkage rate of 20% in our series is thus inferior by comparison; however, it is consistent with other HSRT series where relatively low tumor shrinkage rates have been reported (see ). While it is conventional to assume an alpha/beta ratio of 3 Gy for normal neurological tissue, the alpha/beta ratios for meningiomas and acoustic neuromas are not clearly known. Even though the ratio is assumed to be 10 Gy for “tumor tissue”, it is more likely to be closer to 3 Gy for normal neurological tissue. The BEDs for normal neurological tissues (BED3) are more favorable for an HSRT regime than for SRS, and this could explain the better neurological toxicity profile. However, the BED with alpha/beta of >3 Gy for the tumors would be more favorable for FSRT, which could explain the better shrinkage rates with FSRT.
Conclusion
It seems reasonable, safe and effective to deliver stereotactic radiotherapy in a hypofractionated regime (HSRT), exploiting the benefits of both quick and effective treatment while maximizing neurological function preservation. Our experience with a 5-fraction schedule for benign skull base tumors is consistent with that published by others and appears to validate this approach in the short term. Long-term follow-up and prospective studies are necessary to substantiate this initial assessment.
References
- Toussaint LG, Link MJ. Primary tumors of the cranial nerves and skull base. Principles of Neuro-Oncology., 1st edn, DS Schiff, BP O’Neill. McGraw-Hill, New York 2005; 447–479
- Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG. Descriptive epidemiology of primary brain and CNS tumors: Results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro-Oncology 1999; 1(1)14–25
- Rockhill J, Mrugala M, Chamberlain MC. Intracranial meningiomas: An overview of diagnosis and treatment. Neurosurg Focus 2007; 23(4)E1
- de Jesús O, Sekhar LN, Parikh HK, Wright DC, Wagner DP. Long-term follow-up of patients with meningiomas involving the cavernous sinus: Recurrence, progression, and quality of life. Neurosurgery 1996; 39(5)915–919, discussion 919–920
- DeMonte F, Smith HK, al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg 1994; 81(2)245–251
- Sindou M, Wydh E, Jouanneau E, Nebbal M, Lieutaud T. Long-term follow-up of meningiomas of the cavernous sinus after surgical treatment alone. J Neurosurg 2007; 107(5)937–944
- Knosp E, Perneczky A, Koos WT, Fries G, Matula C. Meningiomas of the space of the cavernous sinus. Neurosurgery 1996; 38(3)434–442, discussion 442–444
- Harner SG, Laws ER. Clinical findings in patients with acoustic neurinoma. Mayo Clin Proc 1983; 58(11)721–728
- Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg 2005; 103(1)59–63
- Sanna M, Taibah A, Russo A, Falcioni M, Agarwal M. Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol 2004; 25(3)379–386
- Minniti G, Amichetti M, Enrici M. Radiotherapy and radiosurgery for benign skull base meningiomas. Radiat Oncol 2009; 4(1)42
- Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 1998; 339(20)1426–1433
- Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: Summary of experience in 829 cases. J Neurosurg 2005; 102(Suppl)195–199
- Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, Niranjan A, Flickinger JC. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery 2008; 62(1)53–58, discussion 58–60
- Kurita H, Sasaki T, Kawamoto S, Taniguchi M, Terahara A, Tago M, Kirino T. Role of radiosurgery in the management of cavernous sinus meningiomas. Acta Neurol Scand 1997; 96(5)297–304
- Ito K, Shin M, Matsuzaki M, Sugasawa K, Sasaki T. Risk factors for neurological complications after acoustic neurinoma radiosurgery: Refinement from further experiences. Int J Radiat Oncol Biol Phys 2000; 48(1)75–80
- Paek SH, Chung HT, Jeong SS, Park CK, Kim CY, Kim JE, Kim DG, Jung HW. Hearing preservation after gamma knife stereotactic radiosurgery of vestibular schwannoma. Cancer 2005; 104(3)580–590
- Koh ES, Millar BA, Ménard C, Michaels H, Heydarian M, Ladak S, McKinnon S, Rutka JA, Guha A, Pond GR, Laperriere NJ. Fractionated stereotactic radiotherapy for acoustic neuroma: Single-institution experience at The Princess Margaret Hospital. Cancer 2007; 109(6)1203–1210
- McClelland S, 3rd, Gerbi BJ, Higgins PD, Orner JB, Hall WA. Safety and efficacy of fractionated stereotactic radiotherapy for acoustic neuromas. J Neurooncol 2008; 86(2)191–194
- Chan AW, Black P, Ojemann RG, Barker FG, 2nd, Kooy HM, Lopes VV, McKenna MJ, Shrieve DC, Martuza RL, Loeffler JS. Stereotactic radiotherapy for vestibular schwannomas: Favorable outcome with minimal toxicity. Neurosurgery 2005; 57(1)60–70, discussion 60–70
- Andrews DW, Werner-Wasik M, Den RB, Paek SH, Downes-Phillips B, Willcox TO, Bednarz G, Maltenfort M, Evans JJ, Curran WJ, Jr. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas: Comparison of two dose cohorts. Int J Radiat Oncol Biol Phys 2009; 74(2)419–426
- Flickinger JC. What is the optimal dose and fractionation for stereotactic irradiation of acoustic neuromas?. Int J Radiat Oncol Biol Phys 2002; 54(2)311–312
- Hara W, Soltys SG, Gibbs IC. CyberKnife robotic radiosurgery system for tumor treatment. Expert Rev Anticancer Ther 2007; 7(11)1507–1515
- Bondiau PY, Bénézery K, Beckendorf V, Peiffert D, Gérard JP, Mirabel X, Noël A, Marchesi V, Lacornerie T, Dubus F, et al. [CyberKnife robotic stereotactic radiotherapy: technical aspects and medical indications] [in French]. Cancer Radiother 2007; 11(6–7)338–344
- Sakamoto GT, Blevins N, Gibbs IC. CyberKnife radiotherapy for vestibular schwannoma. Otolaryngol Clin North Am 2009; 42(4)665–675
- Ebersold MJ, Harner SG, Beatty CW, Harper CM, Quast LM. Current results of the retrosigmoid approach to acoustic neurinoma. J Neurosurg 1992; 76(6)901–909
- Darrouzet V, Martel J, Enée V, Bébéar J, Guérin J. Vestibular schwannoma surgery outcomes: Our multidisciplinary experience in 400 cases over 17 years. Laryngoscope 2004; 114(4)681–688
- Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): The facial nerve-preservation and restitution of function. Neurosurgery 1997; 40(4)684–694, discussion 694–695
- Sampath P, Holliday MJ, Brem H, Niparko JK, Long DM. Facial nerve injury in acoustic neuroma (vestibular schwannoma) surgery: Etiology and prevention. J Neurosurg 1997; 87(1)60–66
- Glasscock ME, III, Kveton JF, Jackson CG, Levine SC, McKennan KX. A systematic approach to the surgical management of acoustic neuroma. Laryngoscope 1986; 96(10)1088–1094
- Yates PD, Jackler RK, Satar B, Pitts LH, Oghalai JS. Is it worthwhile to attempt hearing preservation in larger acoustic neuromas?. Otol Neurotol 2003; 24(3)460–464
- Tonn JC, Schlake HP, Goldbrunner R, Milewski C, Helms J, Roosen K. Acoustic neuroma surgery as an interdisciplinary approach: A neurosurgical series of 508 patients. J Neurol Neurosurg Psychiatry 2000; 69(2)161–166
- Rutherford SA, King AT. Vestibular schwannoma management: What is the ‘best’ option?. Br J Neurosurg 2005; 19(4)309–316
- Myrseth E, Møller P, Pedersen PH, Vassbotn FS, Wentzel-Larsen T, Lund-Johansen M. Vestibular schwannomas: Clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery 2005; 56(5)927–935, discussion 927–935
- Myrseth E, Møller P, Pedersen P, Lund-Johansen M. Vestibular schwannoma: Surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery 2009; 64(4)654–661, discussion 661–663
- Pollock BE, Driscoll CL, Foote RL, Link MJ, Gorman DA, Bauch CD, Mandrekar JN, Krecke KN, Johnson CH. Patient outcomes after vestibular schwannoma management: A prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery 2006; 59(1)77–85, discussion 77–85
- Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys 2003; 55(4)1000–1005
- Flickinger JC, Kondziolka D, Lunsford LD. Dose and diameter relationships for facial, trigeminal, and acoustic neuropathies following acoustic neuroma radiosurgery. Radiother Oncol 1996; 41(3)215–219
- Chihara Y, Ito K, Sugasawa K, Shin M. Neurological complications after acoustic neurinoma radiosurgery: Revised risk factors based on long-term follow-up. Acta Otolaryngol Suppl 2007, 559: 65–70
- Buatti JM, Friedman WA, Meeks SL, Bova FJ. The radiobiology of radiosurgery and stereotactic radiotherapy. Med Dosim 1998; 23(3)201–207
- Alheit H, Saran FH, Warrington AP, Rosenberg I, Perks J, Jalali R, Shepherd S, Beardmore C, Baumert B, Brada M. Stereotactically guided conformal radiotherapy for meningiomas. Radiother Oncol 1999; 50(2)145–150
- Maguire PD, Clough R, Friedman AH, Halperin EC. Fractionated external-beam radiation therapy for meningiomas of the cavernous sinus. Int J Radiat Oncol Biol Phys 1999; 44(1)75–79
- Debus J, Wuendrich M, Pirzkall A, Hoess A, Schlegel W, Zuna I, Engenhart-Cabillic R, Wannenmacher M. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: Long-term results. J Clin Oncol 2001; 19(15)3547–3553
- Jalali R, Loughrey C, Baumert B, Perks J, Warrington AP, Traish D, Ashley S, Brada M. High precision focused irradiation in the form of fractionated stereotactic conformal radiotherapy (SCRT) for benign meningiomas predominantly in the skull base location. Clin Oncol (R Coll Radiol) 2002; 14(2)103–109
- Selch MT, Ahn E, Laskari A, Lee SP, Agazaryan N, Solberg TD, Cabatan-Awang C, Frighetto L, Desalles AA. Stereotactic radiotherapy for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 2004; 59(1)101–111
- Milker-Zabel S, Zabel-du Bois A, Huber P, Schlegel W, Debus J. Fractionated stereotactic radiation therapy in the management of benign cavernous sinus meningiomas: Long-term experience and review of the literature. Strahlenther Onkol 2006; 182(11)635–640
- Henzel M, Hamm K, Sitter H, Gross MW, Surber G, Kleinert G, Engenhart-Cabillic R. Comparison of stereotactic radiosurgery and fractionated stereotactic radiotherapy of acoustic neurinomas according to 3-D tumor volume shrinkage and quality of life. Strahlenther Onkol 2009; 185(9)567–573
- Combs SE, Welzel T, Schulz-Ertner D, Huber PE, Debus J. Differences in clinical results after LINAC-based single-dose radiosurgery versus fractionated stereotactic radiotherapy for patients with vestibular schwannomas. Int J Radiat Oncol Biol Phys 2010; 76(1)193–200
- Fuss M, Debus J, Lohr F, Huber P, Rhein B, Engenhart-Cabillic R, Wannenmacher M. Conventionally fractionated stereotactic radiotherapy (FSRT) for acoustic neuromas. Int J Radiat Oncol Biol Phys 2000; 48(5)1381–1387
- Lo YC, Ling CC, Larson DA. The effect of setup uncertainties on the radiobiological advantage of fractionation in stereotaxic radiotherapy. Int J Radiat Oncol Biol Phys 1996; 34(5)1113–1119
- Hall EJ, Brenner DJ. The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys 1993; 25(2)381–385
- Adler JR, Jr, Gibbs IC, Puataweepong P, Chang SD. Visual field preservation after multisession CyberKnife radiosurgery for perioptic lesions. Neurosurgery 2006; 59(2)244–254
- Williams JA. Fractionated stereotactic radiotherapy for acoustic neuromas. Stereotact Funct Neurosurg 2002; 78(1)17–28
- Williams JA. Fractionated stereotactic radiotherapy for acoustic neuromas. Acta Neurochir (Wien) 2002; 144(12)1249–1254, discussion 1254
- Killory BD, Kresl JJ, Wait SD, Ponce FA, Porter R, White WL. Hypofractionated CyberKnife radiosurgery for perichiasmatic pituitary adenomas: Early results. Neurosurgery 2009; 64(2 Suppl)A19–A25
- Chang SD, Gibbs IC, Sakamoto GT, Lee E, Ovelese A, Adler JR, Jr. Staged stereotactic irradiation for acoustic neuroma. Neurosurgery 2005; 56(6)1254–1261, discussion 1261–1263
- Williams JA. Fractionated stereotactic radiotherapy for acoustic neuromas. Int J Radiat Oncol Biol Phys 2002; 54(2)500–504