Background
In intensive care units (ICUs), physicians and nursing auxiliary are confronted with a broad range of severe medical conditions, such as cardiac and respiratory disorders, viral and bacterial infections, severe bleedings, trauma and as a result of aforementioned situations (multiple-) organ dysfunction. CitationGoldhill and Sumner (1998) reported that 23.8% of 12,762 patients admitted to 15 British ICUs died. Similar figures (26.6%) were reported from a prospective multicenter trial which included 873 ICU patients (CitationNfor et al. 2006). Moreover, many patients died after discharge from ICU. Post-ICU (in-hospital) mortality has been reported to be 7.3% as well as 17.9 or 27% (CitationGoldhill & Sumner 1998, CitationLatour et al. 1990, CitationRowan et al. 1993). Despite the tremendous progress achieved in the care for patients requiring intensive care over the last decades, such as by modern antibiotic therapy and mechanical ventilation, the overall mortality of ICU patients improved surprisingly little (CitationResche-Rigon et al. 2006). One reason for this phenomena may be a lack of differentiation with an oversimplifying approach applying the current “gold standard therapy” for all patients with a given diagnosis. Indeed, it is most likely that we are not fully recognizing the existing heterogeneity with regard to the current status within one disease and thus ignoring potential individual needs in a given situation. It may be postulated that a more personalized therapeutic approach should lead to better medical care and as a result to better prognosis of the critically ill patient. A prerequisites for such an individualized rational decision making are objective parameters and criteria enabling meaningful differentiation, with biomarkers being the key candidate for such strategies.
The potential of biomarkers
In 2001, the term “biomarker” was defined by a working group of the National Institutes of Health as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” (CitationBiomarkers Definitions Working Group 2001). Several categories of biomarkers can be distinguished, including (i) disease biomarkers reflecting severity and status of disease, (ii) efficacy and pharmacodynamic biomarkers mirroring an effect of a given treatment/compound, (iii) safety biomarkers which indicate toxic and side effects of a therapy as soon as possible, (iv) stratification/prediction biomarker which helps to select patients most likely to benefit from treatment and (v) surrogate biomarkers regarded as valid substitute for a clinical outcomes measure (CitationKroll 2008). Different biomarker types and the respective field of application including examples are displayed in .
Table 1. Different biomarker types, the respective field of application and examples.
Biomarkers are of increasing importance for both, preclinical researchers and physicians in many areas including intensive care medicine. The assessment of biomarkers is a valuable tool in therapy monitoring, contributes to the prediction of outcome and is already indispensible in patient stratification today and it is most likely that biomarkers will have even stronger impact tomorrow. Even if the determination of a number of biomarkers in the intensive care units (ICUs) generates additional initial costs, it could be the least expensive alternative in the long run because biomarkers could help to characterize the status of the patient more precisely and enable selection of the most appropriate treatment regimen with the higher probability of success in this special situation. Such strategy adds up-front cost, which, however, is offset by a decrease in the mean residence time of patients in the ICU due to optimized care. Biomarkers are well established in oncology already, and are likely to change the clinical situation dramatically over the upcoming years. The most prominent examples for the use of biomarkers in order to optimize medical care in this indication is the patient stratification for therapy with trastuzumab (Herceptin®) and dasatinib (Sprycel®). Trastuzumab, a cytostatic humanized monoclonal antibody against human epidermal growth factor receptor 2 (HER2), is approved for the treatment of metastatic breast cancer and gastric or gastro-esophageal junction cancer only in patients with proven HER2 overexpression in tumor tissue (CitationBang et al. 2010, CitationSlamon et al. 2001, CitationDowsett et al. 2009). The tyrosine kinase inhibitor dasatinib is indicated for the treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia and adults with chronic myeloid leukemia with resistance to prior therapies. As dasatinib is still efficacious in patients displaying mutations of the Philadelphia chromosome (exception: mutation T315I), patients can be stratified for or excluded from dasatinib treatment based on therapy resistance and mutation analysis (CitationO’Hare et al. 2005, CitationHochhaus et al. 2007, CitationShah et al. 2008). These two examples underline the contribution of biomarkers to medical innovation and show the relevance of biomarker research for the development process of new chemical/biological entities in the pharmaceutical industry. Introduction of already established as well as newly identified and validated biomarkers in clinical trials is expected to increase the speed of development and to enhance the chances for success. In light of the exploding research and development costs, this seems urgently needed (CitationPharmaceutical Industry Profile 2010, CitationHughes 2008, CitationDickson and Gagnon 2004). Furthermore, biomarkers have the potential to speed up the development process. Beyond drug approval, biomarkers can represent a unique selling point of an innovative drug and thereby increase the likeliness of reimbursement by the public health systems. In summary, biomarkers can help to bring innovative medicine faster to the patient.
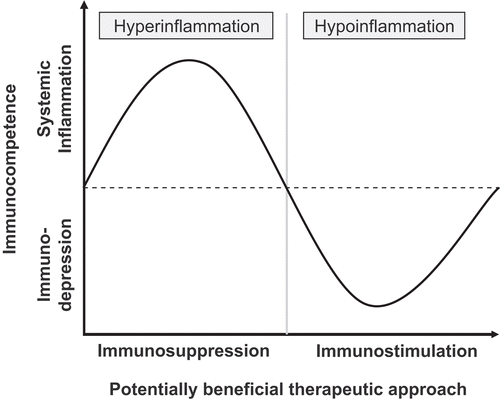
Like in oncology, in intensive care medicine, biomarkers have the potential to pave the way to personalized medicine. In the future, medical decisions based on biomarkers may become a matter of life and death in these seriously ill patients as well. Indeed, there is a trend to differentiate intensive care patients which previously were summarized in single diagnostic entities, with sepsis being the most obvious example. About three decades ago, sepsis was just seen as a life-threatening infection triggering systemic hyperinflammation. In the meanwhile, it became clear that patients differ tremendously and undergo different phases. Just from the immunological point of view, we can now differentiate a systemic hyperinflammatory response which may be flowed by a phase of immunodepression, e.g. compensatory anti-inflammatory response syndrome (). Whereas immunosuppression should be beneficial in the first phase, it could be detrimental in the second phase of the disease. Indeed, immunostimulation may be more appropriate in septic patients with immunodepression where immunosuppression may even be detrimental (CitationAsadullah et al. 1995, CitationVolk et al. 1996, CitationDöcke et al. 1997). As the majority of sepsis trials had a negative outcome (CitationNatanson et al. 1998, CitationZeni et al. 1997), it may be speculated that the failure of the anti-tumor necrosis factor α (anti-TNFα) trials in this indication, showing a trend towards an even increased mortality, is a result of the lacking stratification based on the immune status. Indeed, the anti-TNFα antibody fragment afelimomab for example reduced the 28-day all-cause mortality significantly in patients who were stratified based on serum interleukin-6 (IL-6) levels above 1000 pg/mL. Patients with IL-6 concentrations below the threshold of 1000 pg/mL did not benefit significantly from afelimomab (CitationPanacek et al. 2004). In consequence, it is essential to detect whether a septic patient is either in a hyper- or hypoinflammatoric phase in order to make the appropriate therapeutic decision () or to define the appropriate patient population (stratification) for meaningful clinical studies to develop new drugs.
Beside sepsis, a huge number of life-threatening conditions are commonly found in the ICUs like e.g. patients requiring high dose immunosuppressive therapy after organ transplantation, patients suffering from renal failure, acute lung injury/respiratory distress syndrome, acute decompensated heart failure (HF) or multiple trauma and shock. In a few indications, biomarkers are already used e.g. to guide immunosuppressive dosing in transplant patients. This includes CD3 cell count for the dosing of anti-thymocyte globulin therapy or measurement of human leukocyte antigen–DR expression on monocytes to guide immunosuppressive therapies (CitationAsadullah et al. 1995, CitationVolk et al. 1996, CitationDöcke et al. 1997). Furthermore, well-accepted biomarkers like e.g. bilirubin and creatinine are already an integral part of scoring systems like the Sequential Organ Failure Assessment Score, which is routinely applied to assess severity of disease, monitor therapy response and predict outcome (CitationVincent et al. 1996, Citation1998, CitationFerreira et al. 2001). However, biomarkers need to be applied in a broader range to dramatically change the situation by building the basis for therapeutic decisions, e.g. choice of the drug. This requires a new mindset of both clinicians and scientists in the public health sector as well as in the pharmaceutical industry.
Although biomarker application and even more biomarker-based decision making for intensive care health professionals are still in their infancy and we certainly will observe (more) failures, we are convinced that biomarkers will fundamentally change the fate of many ICU patients, even with the therapeutic options existing today. Moreover, it will pave the way for developing better drugs suited for well-defined subgroups. These positive statements does not ignore the fact that based on the severity of the diseases and the complexity of the underlying conditions, e.g. in severe poly-trauma, multi-morbid patients, mortality will always be high and development of new drugs in these patients still challenging and risky.
About this special issue
Within this special issue, the use of already well-established biomarkers as well as the status quo of the research for novel and unprecedented biomarkers in diseases requiring intensive care will be reviewed. This will include endothelial dysfunction and coagulation disorders in systemic inflammatory syndroms and sepsis (CitationPaulus et al., 2011), organ transplantation (CitationSawitzki et al., 2011), acute kidney injury (AKI) (CitationUrbschat et al., 2011), respiratory disease (CitationAmen et al., 2011) as well as HF and artificial heart transplantation (CitationKramer et al., 2011). The first review of the present special issue written by CitationPaulus et al. (2011) summarize the clinically used coagulation biomarkers with regard to their pathophysiological background. CitationSawitzki et al. (2011) review recently described biomarkers which might enable identification of patients suitable for partial or complete weaning of immunosuppression after organ transplantation. CitationUrbschat et al. (2011) highlight established and novel urine and serum biomarkers of AKI, which have progressed to clinical phase with regard to their diagnostic and prognostic value. CitationAmen et al. (2011) demonstrate that V/Q matching is a powerful biomarker to assess therapy response and prognosis in pulmonary disease. And last but not least, CitationKramer et al. (2011) summarize the current knowledge regarding remodeling biomarker in terminal HF and in patients pre- and postimplantation of left ventricular assist devices or total artificial hearts. Authors contributing to this special issue were recruited from both, leading academic and industrial institutions. This seems important to us, because modern biomarker discovery requires close interactions from scientist representing research and clinical development in both, pharmaceutical industry and academia. With this compilation of reviews on biomarkers and biomarker research in intensive care medicine, we aim to summarize the most relevant scientific knowledge accumulated in this field so far and thereby building a basis for further research.
Declaration of interest
K.A. and F.K. are full time employees of Bayer HealthCare AG, Germany.
References
- Amen EM, Becker EM, Truebel H. (2011). Analysis of V/Q–matching – a safety “biomarker“ in pulmonary drug development? Biomarkers 16 (Supplement 1):S5–S10.
- Asadullah K, Woiciechowsky C, Döcke WD, Egerer K, Kox WJ, Vogel S, Sterry W, Volk HD. (1995). Very low monocytic HLA-DR expression indicates high risk of infection–immunomonitoring for patients after neurosurgery and patients during high dose steroid therapy. Eur J Emerg Med 2:184–190.
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. (2010). Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697.
- Biomarkers Definitions Working Group. (2001). Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95.
- Dickson M, Gagnon JP. (2004). Key factors in the rising cost of new drug discovery and development. Nat Rev Drug Discov 3:417–429.
- Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. (1997). Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 3:678–681.
- Dowsett M, Procter M, McCaskill-Stevens W, de Azambuja E, Dafni U, Rueschoff J, Jordan B, Dolci S, Abramovitz M, Stoss O, Viale G, Gelber RD, Piccart-Gebhart M, Leyland-Jones B. (2009). Disease-free survival according to degree of HER2 amplification for patients treated with adjuvant chemotherapy with or without 1 year of trastuzumab: the HERA Trial. J Clin Oncol 27:2962–2969.
- Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. (2001). Serial evaluation of the SOFA score to predict outcome in critically ill patients. Jama 286:1754–1758.
- Goldhill DR, Sumner A. (1998). Outcome of intensive care patients in a group of British intensive care units. Crit Care Med 26:1337–1345.
- Hochhaus A, Kantarjian HM, Baccarani M, Lipton JH, Apperley JF, Druker BJ, Facon T, Goldberg SL, Cervantes F, Niederwieser D, Silver RT, Stone RM, Hughes TP, Muller MC, Ezzeddine R, Countouriotis AM, Shah NP. (2007). Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood 109:2303–2309.
- Hughes B. (2008). 2007 FDA drug approvals: a year of flux. Nat Rev Drug Discov 7:107–109.
- Kramer F, Milting H. (2011). Novel biomarkers in human terminal heart failure and under mechanical circulatory support. Biomarkers 16 (Supplement 1):S31–S41.
- Kroll W. (2008). Biomarkers− predictors, surrogate parameters− a concept definition. In: Schmitz G, Endres S, Götte D, eds. Biomarker. Stuttgart: Schattauer, 1–14.
- Latour J, Lopez-Camps V, Rodriguez-Serra M, Giner JS, Nolasco A, Alvarez-Dardet C. (1990). Predictors of death following ICU discharge. Intensive Care Med 16:125–127.
- Natanson C, Esposito CJ, Banks SM. (1998). The sirens’ songs of confirmatory sepsis trials: selection bias and sampling error. Crit Care Med 26:1927–1931.
- Nfor TK, Walsh TS, Prescott RJ. (2006). The impact of organ failures and their relationship with outcome in intensive care: analysis of a prospective multicentre database of adult admissions. Anaesthesia 61:731–738.
- O’Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, Cowan-Jacob SW, Lee FY, Heinrich MC, Deininger MW, Druker BJ. (2005). In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res 65:4500–4505.
- Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M, Teoh L, Van Meter L, Daum L, Lemeshow S, Hicklin G, Doig C; Monoclonal Anti-TNF: a Randomized Controlled Sepsis Study Investigators. (2004). Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med 32:2173–2182.
- Paulus P, Zacharowski K.. (2011). Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis?. Biomarkers 16 (supplement 1): S11–S21.
- Pharmaceutical Industry Profile. (2010). The Pharmaceutical Research & Manufacturers of America, PhRMA.
- Resche-Rigon M, Azoulay E, Chevret S. (2006). Evaluating mortality in intensive care units: contribution of competing risks analyses. Crit Care 10:R5.
- Rowan KM, Kerr JH, Major E, McPherson K, Short A, Vessey MP. (1993). Intensive Care Society’s APACHE II study in Britain and Ireland–II: Outcome comparisons of intensive care units after adjustment for case mix by the American APACHE II method. Bmj 307:977–981.
- Sawitzki B, Schlickeiser S, Reinke P, Volk HD. (2011). Monitoring tolerance and rejection in organ transplant recipients. Biomarkers 16 (Supplement 1):S42–S50.
- Shah NP, Kantarjian HM, Kim DW, Réa D, Dorlhiac-Llacer PE, Milone JH, Vela-Ojeda J, Silver RT, Khoury HJ, Charbonnier A, Khoroshko N, Paquette RL, Deininger M, Collins RH, Otero I, Hughes T, Bleickardt E, Strauss L, Francis S, Hochhaus A. (2008). Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol 26:3204–3212.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792.
- Urbschat A, Obermüller N, Haferkamp A. (2011). Biomarker of Kidney Injury. Biomarkers 16 (Supplement 1):S22–S30.
- Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. (1998). Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800.
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. (1996). The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710.
- Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Müller JM, Döcke WD, Kox WJ. (1996). Monocyte deactivation–rationale for a new therapeutic strategy in sepsis. Intensive Care Med 22 Suppl 4:S474–S481.
- Zeni F, Freeman B, Natanson C. (1997). Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 25:1095–1100.