Abstract
This review summarizes recent findings on novel biochemical plasma biomarkers in terminal heart failure patients, which might predict an advanced mortality risk or even recovery. Moreover, we discussed the regulation of these heart failure-related biomarkers under mechanical circulatory support.
Introduction
Heart failure (HF) is a deleterious condition with an estimated prevalence of about 1–2% in the western world and with an incidence of 0.2–2.2% in dependency of the age of the population. About 1–2% of the entire health care expenditures in industrialized countries are accounted for chronic HF. In addition, patients with New York Heart Association (NYHA) HF classification IV represent 10% of all HF procedures but cause 70% of costs.
On the other side, live expectancy in HF is considerably reduced and comparable with some forms of cancer. Patients suffering from end-stage HF are ultimately treated by orthotopic heart transplantation (HTx). However, the availability of donor organs is limited leading to death on the waiting list in a number of cases. The gap between availability and demand of donor hearts was increasing, that is, in Germany during the last decade. It is expected that due to the demographic development in the western world this lack will become even more dramatic in the coming years.
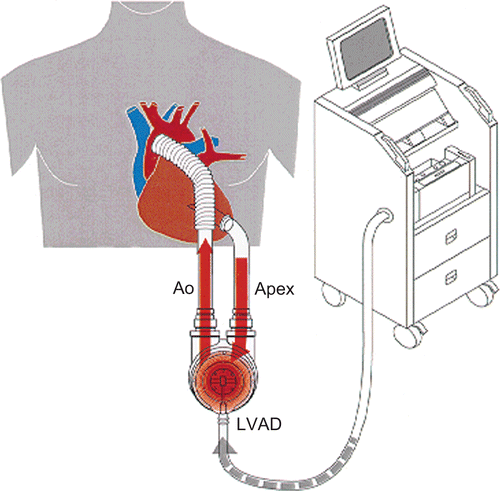
An alternative therapeutic option for patients with terminal HF might be the implantation of ventricular assist devices (VAD) or even total artificial hearts (TAH). Mechanical circulatory support (MCS) devices are currently used as a bridge to transplant (BTT) (), as destination therapy (CitationSlaughter, 2010), or in rare cases as a bridge to recovery (BTR) (CitationMaybaum et al., 2008). Indeed, during the last decade VADs have been considerably improved (CitationSlaughter et al., 2009) and might be used for destination therapy with acceptable quality of life (CitationCoyle et al., 2008) in coming years.
Of note, in the recent past a small group of patients was transiently supported by VAD without the need of HTx leading to the concept of BTR (CitationMaybaum et al., 2008). However, in clinical practice prediction of recovery under VAD support is challenging. CitationDandel et al. (2005) reported on 31 patients weaned from the device and concluded that off-pump echo data might be reliable for the prediction of sustainable recovery of VAD patients. These data, however, represent a single center experience. Currently, there are no commonly accepted biomarkers available, which reliably predict recovery of these patients at the time of device insertion.
In addition, a considerable number of patients do not survive BTT. The timing of VAD implantation is obviously of relevance for the survival and may also be of impact for recovery. Up to now reliable biomarkers are also still lacking, which predict the success rate of BTT.
Therefore, this review summarizes recent findings on novel biochemical plasma biomarkers in terminal HF patients, which might predict an advanced mortality risk or even recovery. Moreover, we discussed the regulation of these HF-related biomarkers under MCS.
Brain natriuretic peptide
Brain natriuretic peptide (BNP) is a peptide hormone released from cardiomyocytes upon mechanical stretch. The primary transcript pre-proBNP codes for 134 amino acids (AA). After cleavage of the leader sequence, the resulting 108 AA proBNP is cleaved again in the N-terminal peptide NT-proBNP (76 AA) and the C-terminal hormone BNP (32 AA). The mature hormone BNP finally contains a disulfide bridge leading to a cyclic peptide hormone. BNP is transcribed within the ventricular myocardium and has compensatory diuretic activity during HF. In HF, BNP is found in the plasma in considerable concentrations. NT-proBNP has in comparison with the hormone BNP a larger half-life of about 25–125 min (CitationKroll et al., 2007), which makes it more stable within the plasma and has no known hormone activity. BNP clearance is mediated by endopeptidases leading to a half-life of about 5–10 min (CitationPemberton et al., 2000) and is regarded to be a cardiac-specific hormone (CitationBruggink et al., 2006), reflecting mechanical stress on the myocardium. However, recently it was shown in 15 patients that even after replacement of the terminal failing ventricles by an artificial heart (TAH), plasma BNP levels did not reach control values of healthy blood donors even after 30 days post-TAH implantation (CitationMilting et al., 2008). Thus, BNP might be secreted by other extra-cardiac organs within the human body or even by the atrial remains in the patient after implantation of the TAH.
Since mechanical stress is regarded as the major trigger for hormone release, BNP and NT-proBNP were measured in several studies for risk assessment or diagnosis leading to guidelines for HF testing (CitationTang et al., 2007). Measurement of BNP has proven to be of clinical impact in the evaluation of dyspnea or risk stratification for cardiac death (CitationHarrison et al., 2002), respectively. Recently, CitationDoust et al. (2005) analyzed in a meta-analysis the risk assessment for cardiac death analyzed by plasma BNP measurements. They evaluated 32 studies including 6878 patients and concluded that the relative risk of death is increasing per 100 pg/mL BNP by 35–37%. Unfortunately, the risk of death and cardiovascular events is increasing especially with low concentrations of BNP. For instance CitationMcDonagh et al. (2001) identified in an urban population of 1640 individuals a significant rise of all-cause mortality, if the BNP was >17.9 pg/mL.
Natriuretic peptides were also measured in myocardial samples of patients awaiting cardiac transplantation and supported by VAD. CitationJames et al. (1995) analyzed in 13 VAD patients plasma concentrations of atrial natriuretic peptide (ANP), which is structurally related to BNP. They found that the plasma concentrations fell on average by −49% during VAD support. Myocardial immunoreactivity was also measured pre- and post-VAD in 8 BTT patients (CitationAltemose et al., 1997). The authors also found that the myocardial immunoreactivity was reduced from 28% to 4% for BNP. A comparative study on different devices was published by CitationMilting et al. (2001), who analyzed the plasma BNP profiles pre- and post-VAD implantation of four different pulsatile devices. The authors found that the Novacor (CitationKörfer et al., 1995) supported patients revealed the strongest down-regulation of plasma BNP, which could be described by a nonlinear regression model leading to the idea that the mode of mechanical unloading might be reflected by the BNP profile. In addition, in that study BNP was reduced after VAD implantation but did not reach control values of blood donors. CitationSodian et al. (2001) published in the same year another study on BNP in non-ischemic VAD patients stratified according to their clinical outcome: (1) patients experiencing death while on device (n = 9), (2) patients successfully bridged to transplantation (n = 8), and (3) patients weaned from the device due to myocardial recovery (n = 4). The authors found that BNP was significantly reduced after VAD implantation and decreased faster in those patients, who could be weaned from the device. The authors concluded that serial measurement of the BNP plasma concentration might be predictive for myocardial recovery under VAD support. The predictive value of BNP was also investigated in a Japanese retrospective study on 38 VAD patients (CitationMano et al., 2008). The authors found that patients who could be weaned from the VAD without HTx had lower fibrosis at the time of VAD implantation and lower BNP (66 pg/mL) 3 months after VAD implantation compared with those who did not recover (264.5 pg/mL). However, in contrast to these observations in a case report CitationReiss et al. (2005) presented data on the BNP profile pre- and post-VAD implantation of a patient with acute myocarditis. The authors found that the BNP plasma values transiently decreased within the first 3 days and re-increased to values >1000 pg/mL for 30 days under VAD support. The patient could finally be weaned from the device without need for cardiac transplantation. Comparable results were found by CitationKemperman et al. (2004), who could also not reproduce the predictive value of BNP for recovery of VAD patients. They measured in parallel BNP and NT-proBNP in 15 patients receiving VAD implantation (CitationKemperman et al., 2004). The authors found that plasma BNP and NT-proBNP was stabilized 1 week to 1 month after VAD implantation. They also found that the pre-VAD BNP values were as twice as high and NT-proBNP was even 10 times higher than reported for NYHA IV patients. CitationBruggink et al. (2006) found that plasma BNP and its myocardial mRNA was correlated. Both decreased after mechanical unloading. They also identified immunoreactivity against BNP in cardiomyocytes, endothelial cells, and infiltrating T cells and macrophages. Thus, the authors presented evidence that the expression of BNP is not restricted to cardiomyocytes within the heart. This supports the observation that even after radical removal of the failing ventricles BNP plasma values remain above normal controls (CitationMilting et al., 2008). Even though that BNP is reduced by mechanical unloading of the failing ventricles, in a recent report the authors of this review found evidence in 40 VAD-supported patients that within 30 days after implantation of the device the plasma BNP was not reduced to non-failing controls (CitationMilting et al., 2008). The authors also could not identify a predictive role for death of the patients while under VAD support, which is in agreement with the data of CitationSodian et al. (2001), who also were not able to identify differences between BTT patients and those who died under MCS within 5 months. However, in a recent study CitationKhan et al. (2009) reported that NT-proBNP and growth differentiation factor (GDF-15) (see below) are in combination predictive for the mortality in HF patients. It remains to be investigated whether this can also be found in MCS-supported patients.
In summary, currently there is limited data available on deteriorating HF patients supported by mechanical assist devices, due to the low number of patients receiving this therapy. Currently, measurement of BNP for the prediction of recovery or death under MCS is equivocal and therefore of limited value. BNP measurements before and after VAD implantation appear to be non-predictive for an increased risk of death under MCS.
Galectin-3
Galectin-3 (also known as Mac-2, CBP-35, or LBP) belongs to β-galactoside-binding proteins with a preference for lactose and N-acetyl lactosamine (CitationOchieng et al., 2004). Galectin-3 binds to a wide array of extracellular matrix (ECM) proteins such as tenascin, fibronectin, and laminin due to its carbohydrate recognition domain (CRD) and collagen-like protein domains (CitationOchieng et al., 2004). Galectin-3 is lacking a signal sequence, which is essential for the classical secretory pathway. It is therefore a cytoplasmic protein, which is also found within the nucleus. However, galectin-3 can be secreted via non-classical pathways (CitationHughes, 1999). There are a number of cell types that express galectin-3 such as neutrophils, macrophages, mast cells, lung, stomach, colon, uterus, and ovary (CitationKim et al., 2007). In the myocardium, the galectin-3 expression level is almost undetectable in cardiomyocytes, whereas cardiac fibroblasts express higher levels of this lectin (CitationSharma et al., 2004). Recently, CitationSchroen et al. (2004) identified strong up-regulation of galectin-3 mRNA in a rat model of renin-dependent hypertension proceeding later to HF. In further studies, CitationSharma et al. (2004) found that myocardial galectin-3 was increased in those rats, which progress to HF. They also found that infusion of galectin-3 into the pericardium-induced myocardial collagen deposition and remodeling. A profibrotic effect of galectin-3 was also identified for hepatic fibrosis and myofibroblast differentiation (CitationHenderson et al., 2006) and in a rat model treated by the naturally occurring antifibrotic peptide N-acetyl SDKP (CitationLiu et al., 2009). Thus there is experimental evidence that galectin-3 might be a culprit biomarker, which is involved in fibrosis induction and myocardial remodeling (Citationde Boer et al., 2010).
In the clinical setting, galectin-3 was found to be increased in the plasma of HF patients presenting in the emergency department. In a recent report, Citationvan Kimmenade et al. (2006) measured in a previously reported cohort of the PRIDE study (CitationJanuzzi et al., 2005) comparative data on NT-proBNP and galectin-3. Of note, galectin-3 was not correlated to the NYHA classification of 599 patients and it was also of lower specificity and sensitivity to identify HF within the cohort in comparison with NT-proBNP (ROC analysis for NT-proBNP and galectin-3: 0.94, P < 0.0001 and 0.72, P < 0.0001). The optimal cutoff for 80% specificity and 52% sensitivity was 6.88 ng galectin-3/mL. The AUC difference between NT-proBNP and galectin-3 was highly significant (P < 0.0001). However, the ROC for the 60 days mortality or recurrent HF was for galectin-3 AUC 0.74 (P < 0.0001) versus AUC 0.67 (P < 0.009) for NT-proBNP. The adjusted multivariate analysis revealed that galectin-3 was superior to NT-proBNP. The rates of death or recurrent HF were highest in patients with combined elevation of galectin-3 and NT-proBNP (Citationvan Kimmenade et al., 2006). Thus, although NT-proBNP was in this study superior to diagnose HF, galectin-3 was a stronger predictor of short-term mortality.
The predictive value of galectin-3 was also analyzed in a recent Dutch study of Lok et al., who analyzed 232 patients with chronic HF (NYHA III–IV) (CitationLok et al., 2010). The authors found by ROC analysis for the mortality within the study population an AUC of 0.612 (P = 0.004) for galectin-3, which was comparable with NT-proBNP (AUC 0.611). The highest product of sensitivity and specificity was found with 17.72 ng galectin-3/mL.
CitationShah et al. (2010) recently published data from 115 acutely decompensated HF patients. Of note, galectin-3 was the strongest predictor for the 4 years mortality, when compared by multivariate analysis including echocardiographic indices. Patients with galectin-3 levels >14.97 ng/mL had a hazard ratio of 5.5 (CitationShah et al., 2010).
Response to treatment was investigated in terminal HF patients with need for MCS. In 55 patients with deteriorating HF, galectin-3 was measured pre- and post-device implantation versus blood donor controls (mean 4.07 ng/mL). Galectin-3 was significantly increased in HF patients (mean 11 ng/mL). Although there is ample experimental evidence that galectin-3 is involved in fibrosis development (CitationSharma et al., 2004; CitationHenderson et al., 2006; CitationLiu et al., 2009), mechanical unloading (n = 40) or removal of the failing ventricles (n = 15) did not reduce the plasma levels of galectin-3. However, patients not surviving VAD support had significantly elevated plasma concentrations of galectin-3 at the time of VAD implantation compared with those patients who could be bridged to transplantation (CitationMilting et al., 2008). These data could recently be confirmed in a larger cohort of 151 VAD patients (CitationErkilet et al., 2010). The ROC analysis for death under VAD support provided comparable data to previous reports with an AUC for galectin-3 of 0.64 (P = 0.009). These data deserve further investigation, since galectin-3 might be a useful biomarker for prediction of mortality in severe HF patients even under MCS.
ST2
The interleukin-1 receptor family member ST2 is expressed as a transmembrane (ST2L) and soluble isoform (sST2) (CitationArend et al., 2008). Expression of the isoforms is regulated by two different promoters (CitationIwahana et al., 1999). Plasma levels of sST2 were described to be elevated in inflammatory diseases such as chronic obstructive pulmonary disease (COPD), pneumonia, and sepsis, as well as in cardiac and renal diseases (CitationBrunner et al., 2004; CitationShimpo et al., 2004; CitationMueller et al., 2008; CitationRehman et al., 2008; CitationSabatine et al., 2008; CitationDieplinger et al., 2009). Plasma ST2 level has been investigated in a number of different cardiovascular disease entities. In a multicenter trial including 810 acute myocardial infarction (AMI) patients, serum ST2 concentrations at baseline predicted mortality and development of new congestive HF by 30 days (CitationShimpo et al., 2004). These data were supported by a clinical trial investigating circulating ST2 levels in 1239 ST elevation myocardial infarction (STEMI) patients (CitationSabatine et al., 2008). In this cohort, ST2 was a significant predictor of cardiovascular death and HF independently of baseline characteristics. Furthermore, the investigators postulated that the combination of ST2 and NT-proBNP significantly improves risk stratification in STEMI patients. In HF, expression of ST2 mRNA and protein is markedly up-regulated in the myocardium and the soluble receptor isoform becomes detectable in serum (CitationWeinberg, 2003). In addition, it has been shown in a cohort of 139 HF patients that the change of serum ST2 levels is an independent significant (P = 0.048) predictor of subsequent mortality or transplantation in severe chronic HF (NYHA class III to IV) (CitationWeinberg et al., 2003). A trial investigating serum biomarker concentrations in acute HF demonstrated a significant correlation between ST2 and disease severity (P < 0.001), left ventricular ejection fraction (r = −0.134; P = 0.014), the pressure overload biomarker BNP (r = 0.293; p<0.001), and the biomarker of inflammation C-reactive protein (CRP) (r = 0.429; P < 0.001) (CitationRehman et al., 2008). Furthermore, the investigators found higher ST2 levels at presentation among patients who died by 1 year. A recently published trial demonstrated that ST2 is only moderately elevated in HF patients, which display no or only weak signs of inflammation assessed as serum IL-6 and CRP levels (CitationDieplinger et al., 2009). In this investigation, ST2 correlated better with plasma IL-6 and CRP concentrations than with BNP. The authors therefore postulated that ST2 is not up-regulated in HF due to pressure overload alone but rather due to inflammatory processes. Unfortunately, the impact of these observations is limited by the small cohort size of HF patients (N = 15) investigated in this study. Nevertheless, the association between ST2 elevation and the inflammatory status of the patient has been shown before, for example in the trial of CitationRehman et al. (2008). In contrast to the aforementioned trials, which investigated ST2 in acute HF, CitationKy et al. (2010) assessed the use of ST2 as a biomarker in 1141 chronic HF patients. Over the mean follow-up period of 2.8 years, 160 patients died and 107 underwent HTx. Increased ST2 levels were associated with a significantly increased risk of death or HTx (P < 0.0001). Individuals in the highest ST2 tertile displayed an increased risk of adverse outcomes compared with the lowest tertile (HR 3.2; 95% CI: 2.2–4.7; P < 0.0001).
To the best of our knowledge, there is no data available describing the impact of serum ST2 levels pre- and post-VAD support or TAH transplantation, respectively, for the survival of MCS so far. Whether ST2 has the potential to be the basis for therapeutic decisions in daily routine in order to improve medical care and thereby prognosis of the patient has to be demonstrated in further trials and clinical practice.
Matrix metalloproteinases
Matrix metalloproteinases (MMP) constitute a group of zinc- and calcium-dependent endopeptidases, which are able to degrade ECM components and modulate the function of a variety of endogenous proinflammatory and vasoactive molecules (CitationChow et al., 2007; CitationPage-McCaw et al., 2007; CitationManicone and McGuire, 2008). Besides the regulation of normal physiological processes (CitationGill et al., 2008), MMPs are also involved in pathomechanisms of cardiovascular, inflammatory, and oncological diseases (CitationRydlova et al., 2008). More than 20 MMPs were described so far, with MMP-2 (gelatinase A) and MMP-9 (gelatinase B) being the most relevant MMPs in cardiovascular disease. Transcription of MMP-2 and MMP-9 can be induced by a variety of stimuli, which leads to secretion of latent preforms (CitationVan Wart et al., 1990; CitationBirkedal-Hansen et al., 1993; CitationManicone and McGuire, 2008). The proteolytic activity of MMP-2 and MMP-9 is regulated by cleavage of the propeptide and through inhibition of the active enzyme by endogenous inhibitors of matrix metalloproteinases (tissue inhibitors of metalloproteinases [TIMPs]) or α2-macroglobulins (CitationClark et al., 2008; CitationOgata et al., 1992). Structural reshaping of the myocardium in HF involves collagen breakdown and resynthesis, which is mainly controlled by the activity of MMP-2 and MMP-9. Therefore, local and circulating concentrations of MMP-2 and MMP-9 might serve as biomarkers to assess status of tissue remodeling in HF.
MMP-2
The failing heart displays an increased expression of MMP-2 on mRNA and protein levels (CitationPolyakova et al., 2010; CitationGomez et al., 1997; CitationGreene et al., 1996). Prognosis of HF patients seems to be correlated with MMP-2 levels (George et al., 2005). In addition, a correlation between the severity of HF, as defined by NYHA class, and plasma levels of MMP-2 has been shown in human patients (CitationYamazaki et al., 2004; CitationAhmed et al., 2006). In previous surveys, the myocardial mRNA expression profile of MMPs and TIMPs of end-stage HF patients was examined revealing an increased MMP-2 mRNA content in patients with deteriorating HF and need for MCS in comparison with electively transplanted patients (CitationFelkin et al., 2009). Another study investigating MMP-2 gelatinolytic activity (zymography) in 14 dilated cardiomyopathy (DCM) and 16 ischemic cardiomyopathy (ICM) patients at the time of VAD implantation and again during HTx was published in 2001 (CitationLi et al., 2001). The investigators found that MMP-2 activity did not change during cardiac unloading by VAD. In contrast, the MMP-9 activity was decreased in 86.7% patients post-VAD compared with the baseline activity (p<0.05). In addition the pro-MMP-9 tissue content was significantly decreased by VAD (P < 0.05). A recent trial investigated plasma MMP-2 concentrations in terminal HF patients (n = 55) who required VAD, before and 30 days after implantation of VAD or TAH (CitationMilting et al., 2008). MMP-2 plasma concentrations at the time of VAD implantation were not significantly different from those of blood donors, whereas significantly higher MMP-2 concentrations were observed in those patients receiving a TAH. The implantation of VADs or TAHs did not influence MMP-2 plasma concentrations. In contrast, a more recent trial, which investigated whether reverse remodeling of the myocardium occurs upon VAD support, demonstrated an increased MMP-2 mRNA expression and increased MMP-2 activity after unloading of the heart compared with pre-VAD (CitationBruggink et al., 2007). These contradictory reports raise the question whether an increased MMP activity in the myocardium, induced by VAD support, is desired to remove excessive collagen, which accumulated in the course of the disease, or whether an increase of, for example, MMP-2 expression and activation leads to further deleterious remodeling?
Interestingly, a study of CitationMartos et al. (2009) suggests that MMP-2 can also be useful to identify patients with HF with preserved ejection fraction (HF-PEF) and diastolic dysfunction (DD). Based on observations in 85 hypertensive patients with HF-PEF and/or DD, the investigators found that serum MMP-2 concentrations are significant predictors of HF-PEF (ROC-AUC = 0.91, P < 0.01) and DD (ROC-AUC = 0.73, P < 0.05). Based on a serum MMP-2 cutoff of 1585 ng/mL, HF-PEF can be predicted with 91% sensitivity and 76% specificity. The authors concluded that serum MMP-2 may be more appropriate to identify patients with HF-PEF than BNP.
MMP-9
Numerous studies showing altered MMP-9 mRNA or protein expression in the diseased myocardium had been published so far (for a review, CitationSpinale, 2007). In contrast, only few investigators assessed circulating levels of MMP-9 or gelatinolytic activity of the endopeptidase. Nevertheless, CitationLi et al. (2001) studied MMP-9 activity (zymography) in 30 patients suffering from DCM or ICM at the time of VAD implantation and again at explantation of the device. The MMP-9 activity was decreased in 86.7% patients post-VAD compared with the baseline activity (P < 0.05). In addition, the pro-MMP-9 tissue content was significantly decreased by VAD (P < 0.05). This suggests that cardiac unloading by VAD leads to reduced ECM breakdown in the myocardium. A recent study investigated the prognostic value of plasma MMP-9 concentrations in patients with systolic HF due to ICM or non-ICM (CitationBuralli et al., 2010). In this study, multivariate analysis revealed that the ratio of mitral E peak velocity and averaged e′ velocity (E/e′), left ventricular ejection fraction (EF), and plasma MMP-9 are significant independent predictors of death (hazard ratio 1.11, P = 0.0028; 0.92, P = 0.017; 1.01, P = 0.027, respectively). Survival rate in patients displaying MMP-9 levels >89.9 ng/mL was significantly decreased (P < 0.0001). The authors concluded that assessment of plasma MMP-9 concentration can complement the prognostic value of echocardiographic parameters in patients with systolic HF.
Tissue inhibitors of metalloproteinases
The activity of MMPs is controlled by expression and secretion, by proteolytic activation of proenzymes and by the TIMPs. So far, the TIMP family consists of four members. TIMPs bind MMPs in a stoichiometric 1:1 ratio and thereby block access of substrates to the catalytic domain of the endopeptidases. TIMP-1, TIMP-2, and TIMP-4 are secreted proteins, whereas TIMP-3 as a membrane-bound TIMP is restricted to the ECM. In the context of secreted biomarkers in HF, TIMP-1 and TIMP-4 are the most frequently discussed TIMP family members.
TIMP-1
TIMP-1 is expressed in a broad variety of cell types (CitationGomez et al., 1997) and can be induced by proinflammatory cytokines and profibrotic stimuli, such as transforming growth factor β (TGF-β). Upon expression, TIMP-1 is able to promote cell proliferation and may have an antiapoptotic function (CitationBorden et al., 1997). Failing hearts display an increased expression of endogenous TIMP-1 on mRNA and protein levels (CitationSpinale et al., 2002; CitationPolyakova et al. 2004). In a rat model of pressure overload, induced HF plasma TIMP-1 concentrations reflected disease severity, assessed as left ventricular contractility (LVC), and mirrored response to treatment with the mineralocorticoid receptor antagonist spironolactone (CitationKramer et al., 2008). Results of different clinical trials suggest that plasma TIMP-1 concentrations can be useful to assess disease severity in patients suffering from hypertrophic cardiomyopathy or HF (CitationNoji et al., 2004). Furthermore, plasma TIMP-1 was related to indices of LV hypertrophy and systolic dysfunction (CitationSundstroem et al. 2004). In addition, a correlation between the severity of HF, as defined by NYHA class, and plasma levels TIMP-1 has been shown in human patients (CitationYamazaki et al.,2004). In contrast, CitationYang et al. (2010) recently found a negative correlation between plasma TIMP-1 concentrations and NYHA class with TIMP-1 levels being significantly lower in HF patients (n = 39) compared with healthy controls (n = 38). It is important to mention that plasma TIMP-1 levels are not influenced by hypertension as long as LVC is normal (CitationAhmed et al., 2006). A recent trial investigated plasma TIMP-1 concentrations in terminal HF patients (n = 55) who required VAD, before and 30 days after implantation of VAD or TAH (CitationMilting et al., 2008). The plasma level of TIMP-1 was higher in VAD patients at device implantation compared with healthy controls (n = 40), with no changes after unloading of the heart by the device. Interestingly, in TAH patients, TIMP-1 plasma levels were significantly increased at implantation and remained elevated after implantation of the TAH. The latter underlines that the myocardium is not the only source of circulating TIMP-1; otherwise, TIMP-1 plasma levels would have been decreased after substitution of the myocardium by the TAH. In general, this study demonstrated that plasma concentrations of TIMP-1 were not regulated during mechanical unloading, at least within the first 30 days. Most recently, TIMP-1 protein was quantified in various forms of HF (CitationPolyakova et al., 2010). Applying western blot, quantitative reverse transcriptase-polymerase chain reaction (RT-PCR), and quantitative immunofluorescent microscopy, the authors of this study investigated fibrosis and collagen metabolism as well as MMP, TIMP, and cytokine expression in dilated (n = 6), ischemic (n = 7), or inflammatory (n = 6) cardiomyopathies. Quantitative immunofluorescent microscopy revealed increased TIMP-1 protein in dilated and ischemic HF but not in inflammatory disease. The highest TIMP-1 levels were found in ICM (~6-fold up-regulation compared with control myocardium, P < 0.05).
TIMP-4
TIMP-4, also called cardiac inhibitor of metalloproteinases (CIMP), is the most recently cloned member of the TIMP family (CitationGreene et al., 1996) and has been found to be expressed predominantly in cardiovascular tissues (CitationGomez et al., 1997; CitationPage-McCaw et al., 2007; CitationRahkonen et al., 2002). TIMP-4 was increased in inflammatory cardiovascular disorders (CitationKoskivirta et al., 2006). Interestingly, it has been shown recently that Timp-4-deficient mice (Timp-4−/−) are more susceptible to myocardial infarction but not to pressure overload induced by aortic banding (CitationKoskivirta et al., 2010). In addition, (Timp-4−/−) mice suffer reduced cardiac function with aging. Most studies regarding TIMP-4 show gene expression data rather than plasma or serum concentration of the secreted protein. Nevertheless, there are a few publications describing serum/plasma concentrations of this biomarker in cardiovascular diseases. In contrast to most other studies that demonstrated increased TIMP-4 mRNA expression in stressed myocardium, CitationStroud et al. (2005) reported decreased plasma TIMP-4 levels after alcohol septal ablation in 16 patients suffering from hypertrophic obstructive cardiomyopathy. Furthermore, a recent trial showed that plasma TIMP-4 levels were highly significantly increased in 36 pulmonary hypertension (PH) patients compared with 44 healthy age and gender-matched volunteers (healthy: 1.35 ± 0.07 ng/mL, PH 2.32 ± 0.18 ng/mL, P < 0.0001) (CitationSchumann et al., 2010). Mean plasma TIMP-4 levels were significantly different between patients with higher NYHA classification (NYHA I–I I vs. NYHA III, P < 0.05) and in patients displaying more severe right ventricular (RV) hypertrophy (no and mild hypertrophy vs. moderate and severe hypertrophy, P < 0.001). ROC analysis revealed that PH patients exhibited more increased plasma TIMP-4 concentrations than 83% of the healthy controls (AUC: 0.83, P < 0.0001). Two studies by CitationFelkin et al. (2006, Citation2009) investigated the expression of TIMP-4 mRNA in the myocardium of DCM patients. DCM patients with deteriorating clinical status who required VAD support (n = 24) displayed 66% (±33%, P < 0.05) higher TIMP-4 mRNA expression than individuals with stable DCM undergoing HTx without prior VAD support (n = 7) (CitationFelkin et al., 2006). In a second trial, the same investigators compared myocardial TIMP-4 expression in advanced DCM patients who benefited from VAD unloading and pharmacotherapy (n = 11) with those patients who did not develop myocardial recovery (n = 5) (CitationFelkin et al., 2009). TIMP-4 expression was assessed by real-time RT-PCR at VAD implantation and again at explantation. Among 24 ECM genes, which were assessed in this study, only TIMP-4 mirrored the unloading of the left ventricle by being significantly lower expressed at explantation of the device (0.55 ± 0.25-fold down-regulated compared with pre-VAD, P < 0.001). However, in contrast, CitationLi et al. (2001) found TIMP-4 protein being unchanged during VAD support.
Taking into consideration the expression of the TIMP-4 gene, which is more or less restricted to cardiovascular tissues, plasma TIMP-4 seems to be predestined to serve as a biomarker to monitor tissue remodeling processes in HF. Nevertheless, due to the limited number of studies investigating plasma concentration of this biomarker, the potential of TIMP-4 cannot be assessed yet. Furthermore, additional studies investigating plasma TIMP-4 concentrations upon pharmacotherapy in less severe HF seems desirable.
GDF-15
GDF-15 (also known as PDF, PLAB, PTGFB, NAG-1, and MIC-1) is a member of the TGF-β superfamily (CitationBootcov et al., 1997). Expression of GDF-15 mRNA and protein is highly abundant in prostate and placenta, whereas GDF-15 expression is hardly detectable in the healthy myocardium (CitationTan et al., 2000). Expression of GDF-15 mRNA and subsequent secretion of the 25 kDa disulfide-linked dimeric protein is induced in cultured cardiomyocytes on experimental ischemia/reperfusion (I/R), upon nitrosative stress and upon stimulation with proinflammatory cytokines and interferon gamma (IFN-γ) (CitationKempf et al., 2006). In addition, GDF-15 becomes detectable in the human myocardium after AMI (CitationKhan et al., 2009). It is postulated that GDF-15, such as other members of the TGF-β family, signals via type I and type II receptors, both of which are essential for signal transduction (CitationWrana et al., 1994). Upon ligand binding, heterotetrameric complexes composed of type II and type I receptors are formed (CitationKirsch et al., 2000; CitationQin et al., 2002). Type I receptors represent actin receptor-like kinases (ALK). Complex formation leads to transphosphorylation of ALKs by the constitutively active type II receptor kinases and subsequent phosphorylation of downstream targets, such as Sma- and Mad-related proteins (SMAD) (CitationHeldin et al., 1997; CitationMiyazawa et al., 2002). After activation, SMADs form complexes, translocate into the nucleus, and regulate transcription of various target genes, such as type I collagen, junB, PAI-1, c-myc, and Id. Plasma GDF-15 concentrations has been already investigated in a broad range of cardiovascular disease entities in order to assess utility of this new biomarker to stratify patients, to monitor disease severity, and to predict prognosis. In a study recruiting 1142 post-AMI patients, GDF-15 did not only correlate with the pressure overload biomarker NT-proBNP but also provides prognostic information over and above clinical factors and NT-proBNP (CitationKhan et al., 2009). The multivariable Cox proportional hazard model revealed that besides NT-proBNP, age, Killip class >1, use of beta blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers the serum GDF-15 concentration was a significant independent predictor of death or HF. Furthermore, patients with NT-proBNP and GDF-15 levels above median displayed the highest event rate for death and HF. The authors therefore assumed that GDF-15 together with NT-proBNP can be used to identify high-risk patients in this indication.
In a smaller study with 158 patients Foley et al. investigated GDF-15 levels in NYHA class III and IV patients requiring cardiac resynchronization therapy (CRT) (CitationFoley et al., 2009). Similar to what has been described before for AMI patients, the combination of pre-CRT GDF-15 and NT-proBNP plasma concentrations were significant predictors for mortality and morbidity in these moderate to severe HF patients (HR 7.03). An early multicenter study of CitationKempf et al. (2007) assessed circulating levels of GDF-15 in 455 patients with chronic HF at various stages. Interestingly, in this study in which patients with myocardial infarction within the preceding 12 weeks of recruitment were excluded, GDF-15 was closely related to NYHA class (P < 0.001, Kruskal–Wallis test). Approximately 75% of patients presented with serum GDF-15 concentrations above the upper level in healthy elderly individuals (1200 ng/L). Mortality rate at 48 months ranged between 10% in the lowest GDF-15 quartile and 56.2% in the highest quartile, respectively. When data were adjusted for NT-proBNP, renal impairment, anemia, and hyperurecemia, serum GDF-15 remained an independent predictor of mortality (P < 0.001) and added prognostic value to the prognosis relevant parameters NYHA class, EF, and serum NT-proBNP. Furthermore, this study verified close correlation between NT-proBNP and GDF-15. Most recently, Wang et al. published a cross-sectional study designed to compare diagnostic utility of plasma GDF-15 concentrations with that of NT-proBNP in early-stage HF patients, which do not display symptoms of the disease (CitationWang et al., 2010). Based on the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines (CitationHunt et al., 2005), a total of 208 patients displaying stage A (hypertension and stable angina pectoris patients), B (old myocardial infarction and LV hypertrophy), and C (previously symptomatic but currently nonsymptomatic HF) of early HF were recruited. Taking into account that mortality in HF is still very high and screening strategies to detect patients at risk to develop HF are urgently needed, studies similar to these are of significant importance. GDF-15 plasma levels were significantly increased in stages A, B, and C patients compared with healthy controls. A strong correlation between GDF-15 and the left ventricular muscle index (LVMI) (P < 0.001) and EF (P < 0.01) was observed, respectively. In addition, multiple regression analyses revealed significant correlations between the biomarker and left atrial diameter, left atrial pressure, and left ventricular diastolic diameter, respectively (all P < 0.01). Taken together, the authors suggested that GDF-15 might have potential implications for detecting patients with cardiac structural abnormalities resulting from cardiac pathology such as previous AMI. Furthermore, the combination of GDF-15 and NT-proBNP might be useful to monitor patients with high risk to develop HF.
The aforementioned studies underline the potential of GDF-15 as a predictive biomarker or as a monitoring tool in the management of different heart diseases. Nevertheless, further, in particular longitudinal studies have to be enrolled in order to validate this new biomarker for the use in clinical practice and as potential tool to support clinical development of new pharmacotherapies. As far as we know, no trial has been published so far, which investigated GDF-15 response to therapy. Furthermore, factors besides cardiac disease that might affect differential expression and secretion of GDF-15 have to be investigated. Nevertheless, GDF-15 seems to be a promising biomarker candidate, which might help to better manage cardiovascular diseases in the future. Furthermore, data for deteriorating HF patients receiving VAD as a bridge to transplantation are currently completely lacking.
GDF-5
GDF-5, also known as bone morphogenetic protein (BMP) 14, is a secreted member of the TGF-β superfamily. GDF-5 exerts various functions including regulation of cell survival, apoptosis, differentiation, and migration (CitationNakahara et al., 2003; CitationZeng et al., 2007). Similar to GDF-15, GDF-5 signals via TGF-β receptors, but in contrast to the former factor, GDF-5 is postulated to signal via Smad 1/5/8 (CitationNakamura et al., 1999; CitationColeman and Tuan, 2003). Numerous publications describe role of GDF-5 in differentiation of ligament cells and regeneration/healing of ligament and periodontal injuries (CitationHogan et al., 2010; CitationKwon et al., 2010; CitationSaiga et al., 2010; CitationZhong et al., 2010).
Recently, GDF-5 has been described in the context of cardiovascular disease. In a mouse model of permanent ligation of the left anterior descending (LAD) coronary artery, GDF-5 mRNA was induced significantly in the infracted area of the left ventricle 7 days post-myocardial infarction (CitationZaidi et al., 2010). Myocardial GDF-5 protein levels were elevated 7, 21, and 40 days after LAD ligation. Unfortunately, plasma GDF-5 levels were not assessed in this model; therefore, the appropriateness of GDF-5 as circulating biomarker to assess, for example, dimension of the infracted area cannot be assessed based on this study. Furthermore, the investigators showed that GDF-5-knockout mice displayed increased infarct scar expansion, an increased LV dilation, and an impaired LV contractility compared with infarcted control animals. Therefore, an active involvement of GDF-5 in compensatory processes in the myocardium post-MI can be postulated. In regard to the potential use of secreted myocardium-derived GDF-5 as a biomarker to stratify or monitor MI or HF patients, further studies are required. In addition, GDF-5 data in patients experiencing deteriorating HF are completely lacking.
Conclusion
In conclusion, the use of novel biomarkers for the treatment decisions in end-stage HF patients, who frequently suffer from chronic heart disease, is at the very beginning. Currently, there is not a single blood biomarker available, which is suited for the prediction of the outcome of the most elaborative, for the patient incriminating and last but not least most expensive cardiovascular treatment. In our review, we focused on those biomarkers, which proved to be more than a biomarker candidate, since at least some clinical data are available from the literature. We showed that some of these biomarkers might be of interest for further evaluation in clinical trials (i.e. ST2, galectin-3).
However, for the introduction of biomarkers into clinical practice standardized methods and large samples sizes are needed (CitationPoste, 2011), which might be challenging especially in patients treated with MCS.
Declaration of interest
F.K. is full time employee of BayerHealthCare AG. H.M. and received research grants from Bayer HealthCare AG. F.K. and H.M. have filed a patent for the use of circulating TIMP-4 as biomarkers in cardiovascular and cardiopulmonary diseases.
References
- Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. (2006). Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 113:2089–2096.
- Altemose GT, Gritsus V, Jeevanandam V, Goldman B, Margulies KB. (1997). Altered myocardial phenotype after mechanical support in human beings with advanced cardiomyopathy. J Heart Lung Transplant 16:765–773.
- Arend WP, Palmer G, Gabay C. (2008). IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev 223:20–38.
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. (1993). Matrix metalloproteinases: A review. Crit Rev Oral Biol Med 4:197–250.
- Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. (1997). MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA 94:11514–11519.
- Borden P, Heller RA. (1997). Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukaryot Gene Expr 7:159–78.
- Bruggink AH, de Jonge N, van Oosterhout MF, Van Wichen DF, de Koning E, Lahpor JR, Kemperman H, Gmelig-Meyling FH, de Weger RA. (2006). Brain natriuretic peptide is produced both by cardiomyocytes and cells infiltrating the heart in patients with severe heart failure supported by a left ventricular assist device. J Heart Lung Transplant 25:174–180.
- Bruggink AH, van Oosterhout MF, de Jonge N, Cleutjens JP, van Wichen DF, van Kuik J, Tilanus MG, Gmelig-Meyling FH, van den Tweel JG, de Weger RA. (2007). Type IV collagen degradation in the myocardial basement membrane after unloading of the failing heart by a left ventricular assist device. Lab Invest 87:1125–1137.
- Brunner M, Krenn C, Roth G, Moser B, Dworschak M, Jensen-Jarolim E, Spittler A, Sautner T, Bonaros N, Wolner E, Boltz-Nitulescu G, Ankersmit HJ. (2004). Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med 30:1468–1473.
- Buralli S, Dini FL, Ballo P, Conti U, Fontanive P, Duranti E, Metelli MR, Marzilli M, Taddei S. (2010). Circulating matrix metalloproteinase-3 and metalloproteinase-9 and tissue Doppler measures of diastolic dysfunction to risk stratify patients with systolic heart failure. Am J Cardiol 105:853–856.
- Chow AK, Cena J, Schulz R. (2007). Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol 152:189–205.
- Clark IM, Swingler TE, Sampieri CL, Edwards DR. (2008). The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol 40:1362–78.
- Coleman CM, Tuan RS. (2003). Functional role of growth/differentiation factor 5 in chondrogenesis of limb mesenchymal cells. Mech Dev 120:823–836.
- Coyle LA, Martin MM, Kurien S, Graham JD, Gallagher C, Silver MA, Slaughter MS. (2008). Destination therapy: safety and feasibility of national and international travel. Asaio J 54:172–176.
- Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl HB, Hetzer R. (2005). Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation 112:I37–I45.
- de Boer RA, Yu L, van Veldhuisen DJ. (2010). Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep 7:1–8.
- Dieplinger B, Januzzi JL Jr, Steinmair M, Gabriel C, Poelz W, Haltmayer M, Mueller T. (2009). Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma—the Presage ST2 assay. Clin Chim Acta 409:33–40.
- Doust JA, Pietrzak E, Dobson A, Glasziou P. (2005). How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. Bmj 330:625.
- Erkilet G, Schulte-Eistrup S, Morshuis M, Bohms B, Roefe D, Gummert J, Milting H. (2010). Plasma galectin 3 is increased in terminal heart failure patients and is elevated in patients not surviving mechanical circulatory support. J Heart Lung Transplant 29:S65.
- Felkin LE, Birks EJ, George R, Wong S, Khaghani A, Yacoub MH, Barton PJ. (2006). A quantitative gene expression profile of matrix metalloproteinases (MMPS) and their inhibitors (TIMPS) in the myocardium of patients with deteriorating heart failure requiring left ventricular assist device support. J Heart Lung Transplant 25:1413–1419.
- Felkin LE, Lara-Pezzi E, George R, Yacoub MH, Birks EJ, Barton PJ. (2009). Expression of extracellular matrix genes during myocardial recovery from heart failure after left ventricular assist device support. J Heart Lung Transplant 28:117–122.
- Foley PW, Stegemann B, Ng K, Ramachandran S, Proudler A, Frenneaux MP, Ng LL, Leyva F. (2009). Growth differentiation factor-15 predicts mortality and morbidity after cardiac resynchronization therapy. Eur Heart J 30:2749–2757.
- Gill SE, Parks WC. (2008). Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 40:1334–47.
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. (1997). Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 74:111–122.
- Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. (1996). Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem 271:30375–30380.
- Harrison A, Morrison LK, Krishnaswamy P, Kazanegra R, Clopton P, Dao Q, Hlavin P, Maisel AS. (2002). B-type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea. Ann Emerg Med 39:131–138.
- Heldin CH, Miyazono K, ten Dijke P. (1997). TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390:465–471.
- Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. (2006). Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 103:5060–5065.
- Hogan M, Girish K, James R, Balian G, Hurwitz S, Chhabra AB. (2010). Growth differentiation factor-5 regulation of extracellular matrix gene expression in murine tendon fibroblasts. J Tissue Eng Regen Med 5:191–200.
- Hughes RC. (1999). Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta 1473:172–185.
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. (2005). ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 112:e154–e235.
- Iwahana H, Yanagisawa K, Ito-Kosaka A, Kuroiwa K, Tago K, Komatsu N, Katashima R, Itakura M, Tominaga S. (1999). Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem 264:397–406.
- James KB, McCarthy PM, Thomas JD, Vargo R, Hobbs RE, Sapp S, Bravo E. (1995). Effect of the implantable left ventricular assist device on neuroendocrine activation in heart failure. Circulation 92:II191–II195.
- Januzzi JL Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, Lloyd-Jones DM, Brown DF, Foran-Melanson S, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB. (2005). The N-terminal pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 95:948–954.
- Kemperman H, van den Berg M, Kirkels H, de Jonge N. (2004). B-type natriuretic peptide (BNP) and N-terminal proBNP in patients with end-stage heart failure supported by a left ventricular assist device. Clin Chem 50:1670–1672.
- Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. (2006). The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res 98:351–360.
- Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. (2007). Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol 50:1054–1060.
- Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, Davies JE, Ng LL. (2009). Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur Heart J 30:1057–1065.
- Kim H, Lee J, Hyun JW, Park JW, Joo HG, Shin T. (2007). Expression and immunohistochemical localization of galectin-3 in various mouse tissues. Cell Biol Int 31:655–662.
- Kirsch T, Sebald W, Dreyer MK. (2000). Crystal structure of the BMP-2-BRIA ectodomain complex. Nat Struct Biol 7:492–496.
- Körfer R, el-Banayosy A, Posival H, Minami K, Körner MM, Arusoglu L, Breymann T, Kizner L, Seifert D, Körtke H. (1995). Mechanical circulatory support: the Bad Oeynhausen experience. Ann Thorac Surg 59:S56–S62; discussion S63.
- Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kytö V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R. (2010). Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem 285:24487–24493.
- Koskivirta I, Rahkonen O, Mäyränpää M, Pakkanen S, Husheem M, Sainio A, Hakovirta H, Laine J, Jokinen E, Vuorio E, Kovanen P, Järveläinen H. (2006). Tissue inhibitor of metalloproteinases 4 (TIMP4) is involved in inflammatory processes of human cardiovascular pathology. Histochem Cell Biol 126:335–342.
- Kramer F, Sandner P, Klein M, Krahn T. (2008). Plasma concentrations of matrix metalloproteinase-2, tissue inhibitor of metalloproteinase-1 and osteopontin reflect severity of heart failure in DOCA-salt hypertensive rat. Biomarkers 13:270–281.
- Kroll MH, Twomey PJ, Srisawasdi P. (2007). Using the single-compartment ratio model to calculate half-life, NT-proBNP as an example. Clin Chim Acta 380:197–202.
- Kwon HR, Wikesjö UM, Park JC, Kim YT, Bastone P, Pippig SD, Kim CK. (2010). Growth/differentiation factor-5 significantly enhances periodontal wound healing/regeneration compared with platelet-derived growth factor-BB in dogs. J Clin Periodontol 37:739–746.
- Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries D, Tang WH, Wu AH, Fang JC, Boxer R, Sweitzer NK, Levy W, Goldberg L, Jessup M, Cappola TP. (2010). High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. (In Press).
- Li YY, Feng Y, McTiernan CF, Pei W, Moravec CS, Wang P, Rosenblum W, Kormos RL, Feldman AM. (2001). Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation 104:1147–1152.
- Liu YH, D’Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, André S, Gabius HJ, Carretero OA. (2009). N-Acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol 296:H404–H412.
- Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. (2010). Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin Res Cardiol 99:323–8.
- Manicone AM, McGuire JK. (2008). Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol 19:34–41.
- Mano A, Nakatani T, Oda N, Kato T, Niwaya K, Tagusari O, Nakajima H, Funatsu T, Hashimoto S, Komamura K, Hanatani A, Ueda IH, Kitakaze M, Kobayashi J, Yagihara T, Kitamura S. (2008). Which factors predict the recovery of natural heart function after insertion of a left ventricular assist system? J Heart Lung Transplant 27:869–874.
- Martos R, Baugh J, Ledwidge M, O’Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K. (2009). Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail 11:191–197.
- Maybaum S, Kamalakannan G, Murthy S. (2008). Cardiac recovery during mechanical assist device support. Semin Thorac Cardiovasc Surg 20:234–246.
- McDonagh TA, Cunningham AD, Morrison CE, McMurray JJ, Ford I, Morton JJ, Dargie HJ. (2001). Left ventricular dysfunction, natriuretic peptides, and mortality in an urban population. Heart 86:21–26.
- Milting H, El Banayosy A, Kassner A, Fey O, Sarnowski P, Arusoglu L, Thieleczek R, Brinkmann T, Kleesiek K, Körfer R. (2001). The time course of natriuretic hormones as plasma markers of myocardial recovery in heart transplant candidates during ventricular assist device support reveals differences among device types. J Heart Lung Transplant 20:949–955.
- Milting H, Ellinghaus P, Seewald M, Cakar H, Bohms B, Kassner A, Körfer R, Klein M, Krahn T, Kruska L, El Banayosy A, Kramer F. (2008). Plasma biomarkers of myocardial fibrosis and remodeling in terminal heart failure patients supported by mechanical circulatory support devices. J Heart Lung Transplant 27:589–596.
- Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. (2002). Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells 7:1191–1204.
- Mueller T, Dieplinger B, Gegenhuber A, Poelz W, Pacher R, Haltmayer M. (2008). Increased plasma concentrations of soluble ST2 are predictive for 1-year mortality in patients with acute destabilized heart failure. Clin Chem 54:752–756.
- Nakahara T, Tominaga K, Koseki T, Yamamoto M, Yamato K, Fukuda J, Nishihara T. (2003). Growth/differentiation factor-5 induces growth arrest and apoptosis in mouse B lineage cells with modulation by Smad. Cell Signal 15:181–187.
- Nakamura K, Shirai T, Morishita S, Uchida S, Saeki-Miura K, Makishima F. (1999). p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp Cell Res 250:351–363.
- Noji Y, Shimizu M, Ino H, Higashikata T, Yamaguchi M, Nohara A, Horita T, Shimizu K, Ito Y, Matsuda T, Namura M, Mabuchi H. (2004). Increased circulating matrix metalloproteinase-2 in patients with hypertrophic cardiomyopathy with systolic dysfunction. Circ J 68:355–360.
- Ochieng J, Furtak V, Lukyanov P. (2004). Extracellular functions of galectin-3. Glycoconj J 19:527–535.
- Ogata Y, Enghild JJ, Nagase H. (1992). Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem 25;267:3581–4.
- Page-McCaw A, Ewald AJ, Werb Z. (2007). Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8:221–233.
- Pemberton CJ, Johnson ML, Yandle TG, Espiner EA. (2000). Deconvolution analysis of cardiac natriuretic peptides during acute volume overload. Hypertension 36:355–359.
- Polyakova V, Hein S, Kostin S, Ziegelhoeffer T, Schaper J. (2004). Matrix metalloproteinases and their tissue inhibitors in pressure overloaded human myocardium during heart failure progression. Journal of the American College of Cardiology 44:1609–1618.
- Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, Dammer S, Risteli J, Schaper J, Kostin S. (2010). Fibrosis in endstage human heart failure: severe changes in collagen metabolism and MMP/TIMP profiles. Int J Cardiol. (In Press).
- Poste G. (2011). Bring on the biomarkers. Nature 469:156–157.
- Qin BY, Lam SS, Correia JJ, Lin K. (2002). Smad3 allostery links TGF-beta receptor kinase activation to transcriptional control. Genes Dev 16:1950–1963.
- Rahkonen OP, Koskivirta IM, Oksjoki SM, Jokinen E, Vuorio EI. (2002).Characterization of the murine Timp4 gene, localization within intron 5 of the synapsin 2 gene and tissue distribution of the mRNA. Biochim Biophys Acta 1577:45–52.
- Rehman SU, Mueller T, Januzzi JL Jr. (2008). Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol 52:1458–1465.
- Reiss N, El-Banayosy A, Arusoglu L, Milting H, Körfer R. (2005). Erholung der myokardialen Pumpfunktion bei fulminanter Myokarditis unter biventrikulärer Kreislaufunterstützung. J Cardiol 12:177–181.
- Rydlova M, Holubec L Jr, Ludvikova M Jr, Kalfert D, Franekova J, Povysil C, Ludvikova M. (2008) Biological activity and clinical implications of the matrix metalloproteinases. Anticancer Res 28:1389–97.
- Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, Rifai N, Cannon CP, Gerszten RE, Lee RT. (2008). Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation 117:1936–1944.
- Saiga K, Furumatsu T, Yoshida A, Masuda S, Takihira S, Abe N, Ozaki T. (2010). Combined use of bFGF and GDF-5 enhances the healing of medial collateral ligament injury. Biochem Biophys Res Commun 402:329–334.
- Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, Janssen BJ, Debets JJ, Smits JF, Daemen MJ, Crijns HJ, Bornstein P, Pinto YM. (2004). Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res 95:515–522.
- Schumann C, Lepper PM, Frank H, Schneiderbauer R, Wibmer T, Kropf C, Stoiber KM, Rüdiger S, Kruska L, Krahn T, Kramer F. (2010). Circulating biomarkers of tissue remodelling in pulmonary hypertension. Biomarkers 15:523–532.
- Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. (2010). Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail 12:826–832.
- Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, André S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. (2004). Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110:3121–3128.
- Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. (2004). Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation 109:2186–2190.
- Slaughter MS. (2010). Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Card Surg 25:490–494.
- Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH; HeartMate II Investigators. (2009). Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 361:2241–2251.
- Sodian R, Loebe M, Schmitt C, Potapov EV, Siniawski H, Müller J, Hausmann H, Zurbruegg HR, Weng Y, Hetzer R. (2001). Decreased plasma concentration of brain natriuretic peptide as a potential indicator of cardiac recovery in patients supported by mechanical circulatory assist systems. J Am Coll Cardiol 38:1942–1949.
- Spinale FG. (2002). Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circulation Research 90:520–530
- Spinale FG. (2007). Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev 87:1285–1342.
- Stroud RE, Deschamps AM, Lowry AS, Hardin AE, Mukherjee R, Lindsey ML, Ramamoorthy S, Zile MR, Spencer WH, Spinale FG. (2005). Plasma monitoring of the myocardial specific tissue inhibitor of metalloproteinase-4 after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. J Card Fail 11:124–130.
- Sundstroem J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Wilson PW, Vasan RS. (2004). Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: The Framingham heart study. European Heart Journal 25:1509–1516.
- Tan M, Wang Y, Guan K, Sun Y. (2000). PTGF-beta, a type beta transforming growth factor (TGF-beta) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-beta signaling pathway. Proc Natl Acad Sci USA 97:109–114.
- Tang WH, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, Storrow AB, Christenson RH, Apple FS, Ravkilde J, Wu AH; National Academy of Clinical Biochemistry Laboratory Medicine. (2007). National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: clinical utilization of cardiac biomarker testing in heart failure. Circulation 116:e99–e109.
- van Kimmenade RR, Januzzi JL Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM. (2006). Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol 48:1217–1224.
- Van Wart HE, Birkedal-Hansen H. (1990) The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A 87:5578–82.
- Wang F, Guo Y, Yu H, Zheng L, Mi L, Gao W. (2010). Growth differentiation factor 15 in different stages of heart failure: potential screening implications. Biomarkers 15:671–676.
- Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. (2003). Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation 107:721–726.
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. (1994). Mechanism of activation of the TGF-beta receptor. Nature 370:341–347.
- Yamazaki T, Lee JD, Shimizu H, Uzui H, Ueda T. (2004). Circulating matrix metalloproteinase-2 is elevated in patients with congestive heart failure. Eur J Heart Fail 6:41–45.
- Yang DC, Ma ST, Tan Y, Chen YH, Li D, Tang B, Chen JS, Su XH, Li G, Zhang X, Yang YJ. (2010). Imbalance of matrix metalloproteinases/tissue inhibitor of metalloproteinase-1 and loss of fibronectin expression in patients with congestive heart failure. Cardiology 116:133–141.
- Zaidi SH, Huang Q, Momen A, Riazi A, Husain M. (2010). Growth differentiation factor 5 regulates cardiac repair after myocardial infarction. J Am Coll Cardiol 55:135–143.
- Zeng Q, Li X, Beck G, Balian G, Shen FH. (2007). Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone 40:374–381.
- Zhong ZM, Chen JT, Zhang Y, Zha DS, Lin ZS, Zhao CY, Xu JC, Li T, Xu ZX. (2010). Growth/differentiation factor-5 induces osteogenic differentiation of human ligamentum flavum cells through activation of ERK1/2 and p38 MAPK. Cell Physiol Biochem 26:179–186.