Abstract
Recovery from acute kidney injury (AKI) is related to long-term prognosis. This study, involving 56 patients with AKI and 56 controls from a prospective cohort undergoing coronary artery bypass grafting (CABG), investigated the prognostic performance of serum levels of advanced oxidation protein products (AOPPs) for predicting non-recovered AKI and non-completely recovered AKI. AOPP levels increased significantly 7 days after surgery in patients with non-recovered or non-completely recovered AKI. Increased AOPP levels were associated with both types of poor recovery from AKI. Results from receiver-operating characteristic (ROC) curves demonstrated that AOPP levels had good prognostic value for predicting non-recovered and non-completely recovered AKI.
Introduction
Use of coronary artery bypass grafting (CABG) has been increasing rapidly over the last decade in China. Acute kidney injury (AKI) after CABG is common (CitationTuttle et al. 2003; CitationRosner & Okusa 2006). Epidemiologic evidences have suggested that not only the cardiac surgery-associated AKI (CSA-AKI) itself (CitationKangasniemi et al. 2008; CitationGoldberg & Dennen 2008; CitationCharytan et al. 2010), but also the lack of recovery from CSA-AKI are related to long-term adverse prognoses (CitationSpurgeon-Pechman et al. 2007; CitationSwaminathan et al. 2010; CitationSrisawat et al. 2010). However, there are no effective treatments to improve renal recovery. One important barrier to progress in this area has been the lack of a reliable biomarker to predict recovery in individual patients. The ability to predict poor renal recovery would be extremely valuable for clinical decisions and to guide future research (CitationSrisawat et al. 2011).
Prognostic biomarkers of AKI based on the physiology of renal recovery might also help to predict AKI recovery (CitationSrisawat et al. 2010). However, few studies have stressed this issue, except a recent investigation using a panel of urine biomarkers (CitationSrisawat et al. 2011). Cardiopulmonary bypass (CPB) during CABG is recognized as a cause of complex systemic inflammatory and oxidative stress responses which significantly contribute to CSA-AKI and non-recovery of renal function (CitationGerritsen et al. 2001; CitationBiglioli et al. 2003; CitationLiu & Brakeman 2008; CitationStafford-Smith et al. 2008; CitationSrisawat et al. 2010). Advanced oxidation protein products (AOPPs) are oxidized proteins and are used widely as biomarkers of inflammation and oxidative stress (CitationWitko-Sarsat et al. 1998; CitationChen et al. 2011). AOPPs can activate inflammatory cells (CitationWitko-Sarsat et al. 1998) and contribute to the progression of renal failure (CitationLi et al. 2007). Therefore, they are potential biomarkers for predicting renal recovery. In critically ill subjects, AOPP levels were shown to be higher in AKI patients compared with those in non-AKI patients, and AOPP levels were associated with AKI severity (CitationLentini et al. 2010).
The aim of the present study was to assess the prognostic performance of serum levels of AOPPs for the recovery of CSA-AKI after CABG.
Material and methods
This observational study complied with the tenets of the Helsinki Declaration. It was approved by the Research Ethics Committee of Guangdong General Hospital (Guangzhou, China). Written informed consent was obtained from each participant.
Study population
We retrospectively investigated all the subjects undergoing CABG between September 2007 and December 2008 from a prospective cohort in the Guangdong Cardiovascular Institute (which is one of the three major cardiovascular centers in China). In these patients, creatinine levels in serum were assessed every day for 7 days as a routine measurement for monitoring AKI. Exclusion criteria were: history of preoperative renal replacement therapy (RRT); estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2; previous cardiac surgery; emergency or salvage CABG; potential pulmonary, endocardial or urinary infections (according to local protocols; by clinical manifestations; blood and urine examination; X-ray and cardiac ultrasonography) before hospital admission or during hospital stay; death of the patient; or loss of the patient to follow-up <3 months after surgery.
Definitions
Data obtained at the closest time point before surgery were defined as “baseline data”. The stratification of cardiac risk was evaluated by the European System for Cardiac Operative Risk Evaluation (EuroSCORE) with slight modifications to the definition of neurological disease, recent myocardial infarction and emergency surgery. A close approximation of the modified edition had been validated (CitationZheng et al. 2009). Renal function was evaluated by the eGFR, which was calculated by the modified diet and renal disease (MDRD) formula for Chinese populations (CitationMa et al. 2006). AKI was diagnosed and graded by the Acute Dialysis Quality Initiative (ADQI) consensus of RIFLE criteria based on serum creatinine levels (CitationBellomo et al. 2004):
“Risk”: increase in creatinine of 1.5–2.0-fold from baseline.
“Injury”: increase in creatinine of 2–3-fold from baseline.
“Failure”: increase in serum creatinine of >3-fold from baseline.
“Lose”: need for RRT for >4 weeks.
“End-stage kidney disease (ESKD)”: need for dialysis for >3 months.
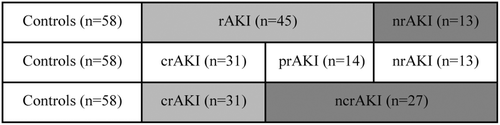
Recovered AKIs (rAKI) were included as completely recovered AKI (crAKI) and partially recovered AKI (prAKI) using RIFLE criteria at 3 months. crAKI was observed if patients returned to their baseline classification within the RIFLE criteria. prAKI was noted if there was a persistent change in RIFLE classification (R, I, or F). Non-recovered AKI (nrAKI) was observed if there was a persistent need for RRT (CitationBellomo et al. 2004). As a result, non-completely recovered AKI (ncrAKI) was composed of prAKI and nrAKI (). nrAKI and ncrAKI were defined as adverse outcomes of AKI recovery.
Laboratory measurements
Serial serum samples were obtained from patients with AKI at baseline, 2 days, 7 days, and 3 months, and from controls at baseline, 2 days, and 7 days, after surgery. Fresh serum samples were used for the measurement of serum creatinine, whereas samples for the measurement of AOPPs were stored at −80°C until use. Serum AOPP levels were determined as described previously (CitationWitko-Sarsat et al. 1998; CitationChen et al. 2011). Briefly, 200 µL of serum was diluted 1:5 in phosphate-buffered saline (PBS) and mixed with 20 µL of acetic acid. In the standard wells for creation of the standard curve, 10 µL of potassium iodide (Sigma–Aldrich, St Louis, MO, USA) was added to 200 µL of chloramine-T solution (Sigma–Aldrich) followed by 20 µL of acetic acid. The absorbance of the reaction mixture at 340 nm was analyzed in a microplate reader (Thermo Multiskan MK3, Vantaa, Finland) within 3 min of adding acetic acid. AOPP levels were expressed as µmol/L of chloramine-T equivalents.
Statistical analyses
Continuous variables are mean values ± SD or medians (25th and 75th percentile) and categorical data are percentages. Multiple groups were compared using the χ2 test for nominal variables. ANOVA (with Bonferroni correction) or the Kruskal–Wallis test was employed for numerical variables if appropriate. The AOPP level at baseline and after surgery was compared using the paired t-test. Spearman’s correlation coefficient (rs) was used to assess the correlation between two variables. Univariate and multivariate logistic regression analyses were done to assess the effect of AOPPs on the adverse outcomes of renal recovery. Odds ratios (ORs) and their associated 95% confidence intervals (95% CIs) were estimated. To compare the discriminatory value of AOPP levels for renal recovery, a receiver-operating characteristic (ROC) curve was plotted and the areas under the ROC curve (ROC AUC) calculated. Statistical procedures were undertaken using SPSS software (version 13.0; SPSS, Chicago, IL, USA). Two-tailed tests were carried out for all comparisons. p < 0.05 was considered significant.
Results
Patient characteristics and outcomes
A total of 185 patients fulfilled the inclusion criteria (including 58 patients with AKI). From 127 subjects without AKI, 58 controls were selected randomly. Baseline characteristics of the controls and patients with AKI are shown in . Patients with AKI were older and had a higher urine albumin–creatinine ratio and higher EuroSCORE than controls. There were 22 in RIFLE R, 17 in RIFLE I, and 19 in RIFLE F in the 58 patients with AKI. Among them, 4 patients with RIFLE I and all the 17 patients with RIFLE F received RRT, and 13 patients had nrAKI and 27 patients had ncrAKI 3 months after surgery. These patients with AKI were further divided into two groups according to renal recovery ( and ). Patients with nrAKI were more likely to be older and to have a longer CPB time compared with patients with rAKI. Patients with nrAKI were older and had a higher EuroSCORE compared with those with ncrAKI.
Table 1. Demographic, clinical characteristics and blood chemistry data at baseline.
Table 2. Clinical features between different groups with recovered AKI and non-recovered AKI.
Table 3. Clinical features between groups with completely recovered AKI and non-completely recovered AKI.
Kinetics of AOPPs
AOPP level at 7 days increased by ≈50 µmol/L in the nrAKI group and increased by 40 µmol/L in the ncrAKI group after surgery. Patients with nrAKI had a higher AOPP level 7 days after surgery than patients with rAKI and controls (121.2 ± 37.9 vs 88.3 ± 22.9 and 71.7 ± 28.0), as well as patients with ncrAKI than patients with crAKI and controls (115.5 ± 32.4 vs 78.4 ± 11.6 and 71.7 ± 28.0) (). Given that the levels of AOPPs at baseline and 2 days after surgery were comparable among groups, the results of the following two-time points are not shown. The AOPP level of all the patients with AKI and of controls 7 days after surgery was correlated to EuroSCORE values and CPB time, and had a mild inverse correlation with the baseline values of left ventricular ejection faction ().
Figure 2. Kinetics of AOPP level in different groups. AOPP levels at baseline, days 2 and days 7 after surgery in controls, patients with recovered AKI and patients with non-recovered AKI (a) or in controls, patients with completely recovered AKI and patients with non-completely recovered AKI (b). rAKI, recovered AKI; crAKI, complete-recovered AKI; nrAKI, non-recovered AKI; ncrAKI, non-completely recovered AKI. *p < .05 AOPP level between patients with nrAKI and controls. #p < .05 AOPP level between patients with nrAKI and patients with rAKI. †p < .05 AOPP level between the baseline and at 7 days in the patients with nrAKI. ‡p < .05 AOPP level between patients with ncrAKI and controls. §p < .05 AOPP level between patients with ncrAKI and patients with crAKI. ¶p < .05 AOPP level between the baseline and at 7 days in the patients with ncrAKI.
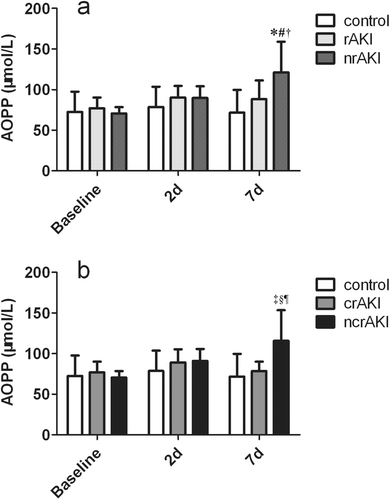
Table 4. Relationship between AOPP level at 7 days and clinical indices.
AOPP levels as prognostic risk factors for adverse outcomes of AKI recovery
To evaluate the independent effect of AOPP level at 7 days on nrAKI or ncrAKI, AOPP level and variables with p < 0.10 in the univariate analysis ( and ) were candidates for the multivariable logistic analysis. An increased level of AOPPs of 10 µmol/L was related to an increased prevalence of nrAKI and ncrAKI of 50.9% and 188.3%, respectively ().
Table 5. AOPP as risk factors for adverse outcomes of AKI recovery.
Prognostic performance of AOPP for adverse outcomes of AKI recovery
In the patients with AKI, the AOPP level at day 7 was a good biomarker for predicting nrAKI (nrAKI vs rAKI) and ncrAKI (ncrAKI vs crAKI) using ROC curves analyses. The prognostic strength of AOPP levels for adverse outcomes of AKI recovery is shown in .
Table 6. Predictive performances of serum AOPP at day 7 for adverse outcomes of AKI recovery.
Discussion
Patients with poor recovery from AKI had a higher AOPP level, and an increased AOPP level was associated with nrAKI and ncrAKI. These findings demonstrating that the AOPP level was a potential candidate for predicting adverse recovery from AKI. ROC curve analyses confirmed that the AOPP level had good prognostic value for predicting adverse outcomes after AKI.
Pre-existing proteinuria is associated with CSA-AKI (CitationHuang et al. 2011). However, the urine albumin–creatinine ratio was not associated with renal recovery in the present study. Given that tubular injury, renal hemodynamics and intestinal inflammation have key roles in renal recovery, albuminuria (a classical biomarker of glomerular injury) might not be a marker for recovery from AKI. No authors have demonstrated that the level of albuminuria is a risk factor for renal recovery. At the very least, the present study suggested that albuminuria was not a strong prognostic factor for renal recovery.
The lifespan of oxidized albumin (the main component of AOPPs) is shorter than that of native albumin (CitationIwao et al. 2006), but the kinetics of AOPP in CABG patients has not been investigated. We studied AOPP levels at 2 days and 7 days after CABG. The AOPP level at day 7 but not at day 2 had good prognostic value for renal recovery, but an optimized time point for measurement of the AOPP level needs to be investigated.
There were some limitations to our study. Results were from a relatively small number of patients in a single center. Thus, the results will need to be validated in a larger population. In addition, our study focused only on the AKI recovery after CABG. As a result, the serum level of AOPPs in controls undergoing CABG in the present study was higher than that of health controls in the same center (CitationChen et al. 2011; data not shown). We were not able to generalize our results to all cases of AKI. However, this homogenous study is possibly a better model for the prediction of adverse AKI recovery in such a population with a higher baseline level of AOPP. Finally, renal outcomes were followed up for only 3 months based on the suggestion of ADQI consensus. Late recovery of AKI after 3 months in patients requiring RRT is also recognized, but it is uncommon (CitationSiddiqui et al. 2008). Therefore, the value of AOPP levels for the long-term recovery from AKI remains to be validated.
In conclusion, increased AOPP levels 7 days after CABG were associated with adverse outcomes of AKI recovery (nrAKI and ncrAKI) in CABG patients in a Chinese population. AOPP levels had good prognostic value for predicting adverse renal recovery.
Declaration of interest
This work was supported by the National Natural Science Foundation of China (81170683) and the grant of Science and Technique Project of Guangdong Province, China (2010B031600157).
References
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. (2004). Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212.
- Biglioli P, Cannata A, Alamanni F, Naliato M, Porqueddu M, Zanobini M, Tremoli E, Parolari A. (2003). Biological effects of off-pump vs. on-pump coronary artery surgery: Focus on inflammation, hemostasis and oxidative stress. Eur J Cardiothorac Surg 24:260–269.
- Charytan DM, Yang SS, McGurk S, Rawn J. (2010). Long and short-term outcomes following coronary artery bypass grafting in patients with and without chronic kidney disease. Nephrol Dial Transplant 25:3654–3663.
- Chen YH, Shi W, Liang XL, Liang YZ, Fu X. (2011). Effect of blood sample type on the measurement of advanced oxidation protein products as a biomarker of inflammation and oxidative stress in hemodialysis patients. Biomarkers 16:129–135.
- Gerritsen WB, van Boven WJ, Driessen AH, Haas FJ, Aarts LP. (2001). Off-pump versus on-pump coronary artery bypass grafting: Oxidative stress and renal function. Eur J Cardiothorac Surg 20:923–929.
- Goldberg R, Dennen P. (2008). Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis 15:297–307.
- Huang TM, Wu VC, Young GH, Lin YF, Shiao CC, Wu PC, Li WY, Yu HY, Hu FC, Lin JW, Chen YS, Lin YH, Wang SS, Hsu RB, Chang FC, Chou NK, Chu TS, Yeh YC, Tsai PR, Huang JW, Lin SL, Chen YM, Ko WJ, Wu KD; National Taiwan University Hospital Study Group of Acute Renal Failure. (2011). Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol 22:156–163.
- Iwao Y, Anraku M, Hiraike M, Kawai K, Nakajou K, Kai T, Suenaga A, Otagiri M. (2006). The structural and pharmacokinetic properties of oxidized human serum albumin, advanced oxidation protein products (AOPP). Drug Metab Pharmacokinet 21:140–146.
- Kangasniemi OP, Mahar MA, Rasinaho E, Satomaa A, Tiozzo V, Lepojärvi M, Biancari F. (2008). Impact of estimated glomerular filtration rate on the 15-year outcome after coronary artery bypass surgery. Eur J Cardiothorac Surg 33:198–202.
- Lentini P, de Cal M, Cruz D, Chronopoulos A, Soni S, Nalesso F, Zanella M, Garzotto F, Brendolan A, Piccinni P, Ronco C. (2010). The role of advanced oxidation protein products in intensive care unit patients with acute kidney injury. J Crit Care 25:605–609.
- Li HY, Hou FF, Zhang X, Chen PY, Liu SX, Feng JX, Liu ZQ, Shan YX, Wang GB, Zhou ZM, Tian JW, Xie D. (2007). Advanced oxidation protein products accelerate renal fibrosis in a remnant kidney model. J Am Soc Nephrol 18:528–538.
- Liu KD, Brakeman PR. (2008). Renal repair and recovery. Crit Care Med 36:S187–S192.
- Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. (2006). Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17:2937–2944.
- Rosner MH, Okusa MD. (2006). Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1:19–32.
- Siddiqui S, Norbury M, Robertson S, Almond A, Isles C. (2008). Recovery of renal function after 90 d on dialysis: Implications for transplantation in patients with potentially reversible causes of renal failure. Clin Transplant 22:136–140.
- Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. (2007). Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293:F269–F278.
- Srisawat N, Murugan R, Wen X, Singbartl K, Clermont G, Eiam-Ong S, Kellum JA. (2010). Recovery from acute kidney injury: Determinants and predictors. Contrib Nephrol 165:284–291.
- Srisawat N, Wen X, Lee M, Kong L, Elder M, Carter M, Unruh M, Finkel K, Vijayan A, Ramkumar M, Paganini E, Singbartl K, Palevsky PM, Kellum JA. (2011). Urinary biomarkers and renal recovery in critically ill patients with renal support. Clin J Am Soc Nephrol 6:1815–1823.
- Stafford-Smith M, Patel UD, Phillips-Bute BG, Shaw AD, Swaminathan M. (2008). Acute kidney injury and chronic kidney disease after cardiac surgery. Adv Chronic Kidney Dis 15:257–277.
- Swaminathan M, Hudson CC, Phillips-Bute BG, Patel UD, Mathew JP, Newman MF, Milano CA, Shaw AD, Stafford-Smith M. (2010). Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thorac Surg 89:1098–1104.
- Tuttle KR, Worrall NK, Dahlstrom LR, Nandagopal R, Kausz AT, Davis CL. (2003). Predictors of ARF after cardiac surgical procedures. Am J Kidney Dis 41:76–83.
- Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drüeke T, Descamps-Latscha B. (1998). Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol 161:2524–2532.
- Zheng Z, Li Y, Zhang S, Hu S; Chinese CABG Registry Study. (2009). The Chinese coronary artery bypass grafting registry study: How well does the EuroSCORE predict operative risk for Chinese population? Eur J Cardiothorac Surg 35:54–58.