Abstract
Context: Inflammatory, endothelial and neurohormonal biomarkers are involved in heart failure (HF) and pulmonary hypertension (PH) pathogenesis.
Objective: To study these biomarkers in PH due to advanced HF.
Materials and methods: Thirty adults with HF were included. Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), high-sensitivity C-reactive protein (hsCRP), endothelin-1 and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) were measured in peripheral vein and pulmonary artery during right heart catheterisation.
Results: IL-6, TNF-α, hsCRP and NT-proBNP correlated with pulmonary pressures independent of ventricular function, HF etiology and vascular bed. IL-6 was independent predictor of systolic pulmonary artery pressure (sPAP).
Discussion and conclusion: Inflammatory biomarkers correlate to PH severity. IL-6 predicts sPAP in advanced HF.
Introduction
Heart failure (HF) is the most common cause of pulmonary hypertension (PH) (Haddad et al., Citation2011). In patients with HF, PH is associated with increased short- and long-term mortality (Guglin & Khan, Citation2010). In pathogenesis of PH in HF, there are two major components. In all cases, there is a passive component associated with increased left atrial filling pressure. In some patients, a superimposed active component caused by pulmonary arterial vasoconstriction and vascular remodeling may lead to a further increase in pulmonary artery pressure (Guglin & Khan, Citation2010; Haddad et al., Citation2011). Although there is a large amount of data supporting the role of chronic inflammation, endothelial dysfunction and neurohormone activation in pathogenesis of HF (Araujo et al., Citation2009; Aubert, Citation2005; Banerjee et al., Citation2010; Bogaard et al., Citation2009; Bozkurt et al., Citation2010; Deswal et al., Citation2001; Gullestad et al., Citation2012; Hocher et al., Citation1997; Joppa et al., Citation2006; Kawanabe & Nauli, Citation2011; Koller-Strametz et al., Citation1998; Pan et al., Citation2004; Petersen & Felker, Citation2006; Potter, Citation2011; Rafeq et al., Citation2009; Von Haehling et al., Citation2009; Yin et al., Citation2004) and several classes of PH (Aubert, Citation2005; Collados et al., Citation2003; Cracowski & Leuchte, Citation2012; Heresi, Citation2011; Joppa et al., Citation2006; Kawanabe & Nauli, Citation2011; Mathew, Citation2010; Pullamsetti & Schermuly, Citation2009; Savale et al., Citation2009; Selimovic et al., Citation2009; Shao et al., Citation2011; Soon et al., Citation2010), the role of these processes in PH due to left HF is less well understood.
Tumor necrosis factor α (TNF-α) plays an important role in inflammatory response in numerous different conditions. It is also strong interleukin 6 (IL-6) and C-reactive protein (CRP) excretion inducer (Von Haehling et al., Citation2009). IL-6 is known to be the strongest CRP inducer in the liver (Araujo et al., Citation2009; Petersen & Felker, Citation2006; Savale et al., Citation2009; Yin et al., Citation2004). In turn, CRP stimulates endothelin 1 (ET-1) and TNF-α production by activating the complement system (Yin et al., Citation2004). TNF-α and IL-6 are cytokines acting proinflammatory on the vascular endothelium both in HF and PH patients. There is also growing amount of evidence supporting similar active role of CRP in HF pathogenesis by means of its direct modulatory influence on vascular endothelium (Joppa et al., Citation2006; Yin et al., Citation2004). All three biomarkers are therefore not only just passive markers of inflammation but also play active role in the disease. They are elevated in patients with HF (Bogaard et al., Citation2009) and are related to HF severity, prognosis and mortality (Araujo et al., Citation2009; Bozkurt et al., Citation2010; Deswal et al., Citation2001; Gullestad et al., Citation2012; Koller-Strametz et al., Citation1998; Pan et al., Citation2004; Petersen & Felker, Citation2006; Von Haehling et al., Citation2009; Yin et al., Citation2004). Inflammation also plays an important role in different types of PH, especially idiopathic PH (Heresi, Citation2011; Joppa et al., Citation2006; Mathew, Citation2010; Savale et al., Citation2009; Selimovic et al., Citation2009; Soon et al., Citation2010; Von Haehling et al., Citation2010). Elevated inflammatory biomarkers may predict mortality and worse outcome in PH (Mathew, Citation2010).
ET-1 is mainly excreted from endothelial cells and vascular smooth muscle cells after stimulation by various signaling molecules including TNF-α and CRP (Joppa et al., Citation2006; Kawanabe & Nauli, Citation2011; Shao et al., Citation2011). It is most potent vasoconstrictor (Aubert, Citation2005; Bozkurt et al., Citation2010; Collados et al., Citation2003; Hocher et al., Citation1997) with cytokine- and hormone-like activity (Kawanabe & Nauli, Citation2011) known also for its fibrogenic, mitogenic and angiogenic properties (Shao et al., Citation2011). ET-1 is therefore a marker of endothelial dysfunction (Rafeq et al., Citation2009) and its elevated levels in HF and PH patients (Hocher et al., Citation1997; Kawanabe & Nauli, Citation2011) suggest its important role in disease pathogenesis. ET-1 is related to hemodynamic parameters and predicts prognosis in HF patients (Hocher et al., Citation1997). Elevated levels of ET-1 are also being described in various types of PH, including PH due to left heart disease (Guglin & Khan, Citation2010; Haddad et al., Citation2011).
Brain natriuretic peptide (BNP) is a neurohormone excreted predominantly by the failing ventricles, both right (Bogaard et al., Citation2009) and especially left (Aubert, Citation2005; Banerjee et al., Citation2010; Potter, Citation2011; Rafeq et al., Citation2009). BNP and its inactive prehormone cleavage product N-terminal prohormone of brain natriuretic peptide (NT-proBNP) are elevated in patients with depressed systolic and diastolic left ventricular (LV) function (Potter, Citation2011). Elevated NT-proBNP in various forms of PH (Aubert, Citation2005; Khush et al., Citation2009; Von Haehling et al., Citation2010) reflects right ventricular (RV) dysfunction (Banerjee et al., Citation2010; Rafeq et al., Citation2009) and also relates to pulmonary haemodynamics (Cracowski & Leuchte, Citation2012), New York Heart Association (NYHA) functional class and six-minute walk test distance (Aubert, Citation2005). NT-proBNP and BNP are the only biomarkers included in the current guidelines for assessment of PH prognosis (Galiè et al., Citation2009).
To our knowledge, similar findings in PH due to left heart disease are scarce. Therefore, the aim of our study was to explore the role of systemic and pulmonary vascular bed inflammation, endothelial dysfunction and neurohormonal activation in patients with PH due to advanced HF.
Methods
Patients
From March 2010 to May 2012, we prospectively included 30 adult patients (>18 years) with moderate to severe LV systolic failure (LV ejection fraction <45%). All patients had advanced HF (NYHA III–IV) and were included in heart transplantation evaluation protocol. Patients with valvular HF etiology were excluded from the study. All patients gave informed consent to the study. Furthermore, the study was approved by the National Medical Ethics Committee.
Methods
Demographic, clinical and echocardiographic data
Demographical and clinical data of the patients were collected and a standard six-minute walk test was performed. All patients had conventional transthoracic echocardiographic examination performed by the same investigator using Aloka SSD-α10 ultrasound system (Aloka Co. Ltd., Tokyo, Japan). Measurements were performed according to the American Society of Echocardiography guidelines (Rudski et al., Citation2010).
Hemodynamic data
In the setting of PH reversibility testing, a 7.5 French flow-directed Swan-Ganz catheter (Edwards Lifesciences LLC, Irvine, CA) was inserted percutaneously under local anesthesia into a subclavian vein. Resting pulmonary systolic, diastolic and mean pulmonary arterial pressure and pulmonary capillary wedge pressure were measured and transpulmonary pressure gradient was calculated (mean pulmonary artery pressure – pulmonary capillary wedge pressure).
Laboratory data
Simultaneously, blood samples were collected on a single occasion after a resting period from the antecubital vein and from the Swan-Ganz catheter tip placed in the pulmonary artery. All patients were clinically stable at the time of blood sampling while receiving continuous therapy for HF. For IL-6 and TNF-α assays, 4.5 mL of blood was collected in Vacutainer vacuum tubes (Laboratorijska tehnika Burnik, Skaručna, Slovenia) containing 0.11 M sodium citrate, thoroughly mixed with the anticoagulant, placed immediately in ice water and centrifuged within four hours of collection at 4 °C and 2000g for 30 min to obtain platelet-poor plasma. Aliquots of platelet-poor plasma were then frozen in liquid nitrogen and stored at −70 °C until analyzed. Commercially available enzyme-linked immunosorbent assay kits were used for measuring IL-6 and TNF-α levels (R&D Systems, Minneapolis, MN). For high-sensitivity C-reactive protein (hsCRP) assay, 3 mL of blood was collected in Vacutube vacuum tubes (Laboratorijska tehnika Burnik) containing clot activator, placed immediately in ice water and centrifuged within four hours of collection at 20 °C and 2000g for 15 min to obtain serum. Serum aliquots for hsCRP assay were frozen and stored in the same way as plasma samples until analyzed. hsCRP levels were measured using particle-enhanced immunonephelometry system (Siemens, Marburg, Germany). Samples for ET-1 assay were collected in 2 mL Vacutube vacuum tubes (Laboratorijska tehnika Burnik) containing ethylenediaminetetraacetic acid (EDTA) to obtain EDTA plasma and were processed and frozen in the same way as sodium citrate samples. Commercially available enzyme-linked immunosorbent assay kit was used for measuring ET-1 levels (Biomedica Medicinprodukte, Wien, Austria). For NT-proBNP assay, blood samples were obtained and prepared in the same way as for the hsCRP measurements. NT-proBNP assay was performed immediately. NT-proBNP levels were measured using radial partition immunoassay (Siemens).
Statistical analysis
The variables showing a normal distribution, as determined by the Kolmogorov–Smirnov test, were expressed as means with standard deviations. Other variables were described as median with range. Differences between groups were tested for significance by Student’s t-test for unpaired data for normally distributed variables, and Mann–Whitney U-test for non-normally distributed variables. For correlation analysis, Pearson’s correlation coefficient was calculated for normally distributed variables and Spearman’s rank-correlation coefficient for non-normally distributed variables. Multiple regression analysis was carried out to find independent determinants for the variations in univariate analysis. A p value of less than 0.05 was considered to be statistically significant. Statistical analysis was performed by the Statistica software, version 7.1 (StatSoft Inc. 2005, Tulsa, OK).
Results
Basic patients characteristics
Baseline clinical, echocardiographic and hemodynamic characteristics of study group are summarized in and . Eighty-three percent of patients were male, 63% had non-ischemic HF etiology. Great majority of patients were receiving standard HF medical therapy, and most of the study group had enlarged both ventricles with reduced systolic function. All but one patient had postcapillary PH (mean pulmonary artery pressure ≥25 mmHg, pulmonary capillary wedge pressure ≥15 mmHg).
Table 1. Baseline clinical characteristics.
Table 2. Baseline echocardiographic and hemodynamic characteristics.
Univariate analysis
There were no significant differences in measured levels of biomarkers in peripheral venous and pulmonary artery samples (). There were also no significant differences in measured levels of biomarkers regarding HF etiology ().
Table 3. Differences in measured levels of biomarkers in peripheral vein and pulmonary artery samples.
Table 4. Differences in measured levels of biomarkers in peripheral vein and pulmonary artery regarding heart failure etiology.
In univariate correlation analysis, we analyzed the correlations between pulmonary pressures and selected biomarkers (). IL-6 and hsCRP correlated positively with systolic, diastolic and mean pulmonary artery pressure and pulmonary capillary wedge pressure, while NT-proBNP correlated positively with diastolic and mean pulmonary artery pressure and pulmonary capillary wedge pressure. The correlations were significant in both peripheral vein and pulmonary artery samples. TNF-α correlated with pulmonary capillary wedge pressure in both samples, while the correlation with mean pulmonary artery pressure was significant only in peripheral vein sample. ET-1 showed no significant correlations with pulmonary hemodynamics. Furthermore, none of the analyzed biomarkers correlated significantly with transpulmonary pressure gradient.
Table 5. Correlation coefficients in univariate analysis of pulmonary pressures and biomarker levels.
There were no significant differences in echocardiographic LV and RV function parameters and PH severity.
Multivariate analysis
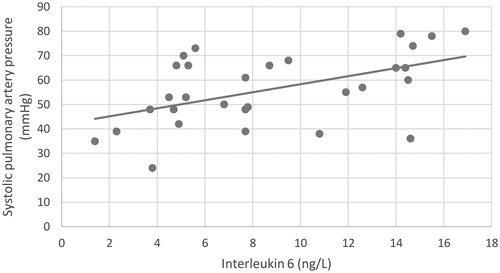
In multiple linear regression analysis with systolic pulmonary artery pressure (sPAP) as the dependent variable, and IL-6, hsCRP and NT-proBNP in both peripheral venous and pulmonary artery samples as the independent variables, IL-6 in pulmonary artery sample was independent predictor of sPAP (; ).
Table 6. Summary of multiple linear regression analysis.
Discussion
Our study suggests that PH in patients with advanced HF is associated with chronic inflammation, since in multivariate regression analysis, increased level of IL-6 in pulmonary artery was independent predictor of sPAP.
Vascular bed, inflammation and neurohormonal activation
Another original aspect of our study was to analyze the differences in levels of selected biomarkers between systemic and pulmonary vascular bed in order to see whether inflammatory and neurohormonal activation in PH due to HF is systemic or is it more related to local expression and/or degradation of observed biomarkers in RV and great pulmonary arteries. No such differences were observed in our study suggesting that both processes are systemic. However, in a study performed by Selimovic et al. (Citation2009), IL-6 levels were significantly higher in radial than in pulmonary artery suggesting increased local production and/or decreased degradation in pulmonary microvascular bed. ET-1 is also being actively degraded in pulmonary circulation by endopeptidases and hence systemic versus pulmonary levels could be different (Aubert, Citation2005). Furthermore, we could expect NT-proBNP levels to be higher in pulmonary artery samples since it is mainly excreted from the stressed ventricles (Aubert, Citation2005; Banerjee et al., Citation2010; Potter, Citation2011; Rafeq et al., Citation2009). Measured levels were similar in both sampling sites, probably because both RV and LV dysfunction were present in the majority of patients masking the predominant site of neurohormone excretion.
HF etiology and inflammation
Since chronic low-grade inflammation was first described in atherosclerosis-related conditions, such as coronary artery disease (Deswal et al., Citation2001; Yin et al., Citation2004), we could expect inflammatory markers to be higher in ischemic than in non-ischemic HF patients. No such difference was found in our group suggesting HF etiology does not play an important role in inflammation of end-stage HF. Our observation is similar to some more recently published studies where no difference was found in IL-6, TNF-α and hsCRP levels in ischemic and non-ischemic HF patients (Araujo et al., Citation2009; Bozkurt et al., Citation2010; Pan et al., Citation2004).
PH and inflammation
In our study, IL-6 and hsCRP correlated with all three measured pulmonary pressures and pulmonary capillary wedge pressure in both sampling sites. In multiple linear regression analysis, IL-6 in pulmonary artery sample was independent predictor of sPAP supporting the importance of inflammation in PH due to left heart disease. TNF-α related only with pulmonary capillary wedge pressure from both sampling sites and mean pulmonary artery pressure in peripheral vein sample. Elevated levels of IL-6, CRP and TNF-α have been reported in patients with idiopathic and familial PH (Heresi, Citation2011; Mathew, Citation2010; Soon et al., Citation2010), PH in chronic obstructive pulmonary disease, connective tissue disease-related PH, chronic thromboembolic PH (Von Haehling et al., Citation2010) and PH in Gaucher disease (Joppa et al., Citation2006; Savale et al., Citation2009; Selimovic et al., Citation2009). To our knowledge, no such data exist in patients with PH due to HF. We also excluded the influence of HF on inflammation by demonstrating there was no difference in LV and RV function regarding PH severity, similar to observations published by Von Haehling et al. (Citation2010). These findings suggest that inflammation in patients with PH due to advanced HF is less HF-dependent and directly reflects PH severity, which seems to be a common characteristic of different classes of PH.
PH and endothelial dysfunction
Unfortunately, our study did not demonstrate correlation of elevated ET-1 levels with pulmonary hemodynamics. Elevated levels of ET-1 are being described in various types of PH, including idiopathic PH (Aubert, Citation2005; Collados et al., Citation2003; Shao et al., Citation2011) and PH due to left heart disease (Guglin & Khan, Citation2010; Haddad et al., Citation2011). In idiopathic PH, elevated ET-1 is related to pulmonary hemodynamic parameters (Kawanabe & Nauli, Citation2011; Shao et al., Citation2011) and predicts degree and prognosis of PH (Pullamsetti & Schermuly, Citation2009; Rafeq et al., Citation2009; Shao et al., Citation2011). ET-1 relation to pulmonary hemodynamics is also being reported in PH due to left heart disease (Guglin & Khan, Citation2010). We assume suppressed ET-1 excretion in our group is a consequence of long-term therapy with beta blockers, statins and angiotensin-converting enzyme inhibitors, which are all well known downregulators of ET-1 expression (Cracowski & Leuchte, Citation2012; Rafeq et al., Citation2009). Other possible explanation of suppressed ET-1 response in our study group is that endothelial dysfunction becomes less expressed in advanced stage of HF.
PH and neurohormonal activation
According to our analysis, NT-proBNP levels were not sampling-site-dependent and correlated with diastolic and mean pulmonary artery pressure and pulmonary capillary wedge pressure in both peripheral vein and pulmonary artery. Elevated BNP and NT-proBNP levels are well documented in RV (Bogaard et al., Citation2009) and especially LV systolic (Aubert, Citation2005; Banerjee et al., Citation2010; Potter, Citation2011; Rafeq et al., Citation2009) and diastolic failure (Potter, Citation2011). Elevated NT-proBNP in various forms of PH (Aubert, Citation2005; Khush et al., Citation2009; Von Haehling et al., Citation2010) reflects RV dysfunction (Banerjee et al., Citation2010; Rafeq et al., Citation2009) and also relates to pulmonary hemodynamics (Cracowski & Leuchte, Citation2012). Therefore, elevated NT-proBNP in PH due to left heart disease probably reflects both LV and RV dysfunction. On the other hand, in a study of idiopathic and chronic thromboembolic PH, NT-proBNP levels correlated with pulmonary hemodynamics only when patients with reduced LV ejection fraction were excluded from analysis (Goto et al., Citation2010). However, our findings suggest that NT-proBNP originates from both failing ventricles probably as a consequence of depressed RV systolic function and elevated LV filling pressure.
Study limitations
Our prospective exploratory study included relatively small number of patients with advanced HF and secondary PH. Due to low number of cases, no confirmatory conclusions can be drawn. Uneven sex and heart rhythm distribution and mixed HF etiology are other limitations in results interpretation. Since right heart catheterization is an invasive procedure, it was not justified in HF patients with no PH on echocardiography and would not be approved by the National Medical Ethics Committee. That is why we could not evaluate a control group in our study.
Conclusions
In a small prospective study of PH in patients with advanced HF, we observed correlation of elevated inflammatory biomarkers with pulmonary hemodynamic parameters independent of LV and RV function. IL-6 was independent predictor of sPAP and hence a predictor of PH in advanced HF. ET-1 levels did not relate to PH severity in our study group. Elevated NT-proBNP correlated with pulmonary hemodynamics as well, probably reflecting LV and RV dysfunction rather than PH itself. Inflammatory and neurohormonal activation seems to be systemic since we found no differences in levels of biomarkers in samples from systemic and pulmonary circulation. HF etiology had no influence on measured levels of observed biomarkers in our study. Further larger studies on more homogenous patient population should be performed to confirm our findings, which are not confirmatory due to low number of cases.
Declaration of interest
The authors report no declarations of interest.
References
- Araujo JP, Lourenco P, Azevedo A, et al. (2009). Prognostic value of high-sensitivity C-reactive protein in heart failure: a systematic review. J Cardiac Fail 15:256–66
- Aubert JD . (2005). Biochemical markers in the management of pulmonary hypertension. Swiss Med Wkly 135:43–9
- Banerjee D, Haddad F, Zamanian RT, Nagendran J . (2010). Right ventricular failure: a novel era of targeted therapy. Curr Heart Fail Rep 7:202–11
- Bogaard HJ, Abe K, Noordegraaf AV, Voelkel NF . (2009). The right ventricle under pressure. Chest 135:794–804
- Bozkurt B, Mann DL, Deswal A . (2010). Biomarkers of inflammation in heart failure. Heart Fail Rev 15:331–41
- Collados MT, Velazques B, Borbolla JR, et al. (2003). Endothelin-1 and functional tissue factor: a possible relationship with severity in primary pulmonary hypertension. Heart Vessels 18:12–17
- Cracowski JL, Leuchte HH . (2012). The potential of biomarkers in pulmonary arterial hypertension. Am J Cardiol 110:32S–8S
- Deswal A, Petersen NJ, Feldman AM, et al. (2001). Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 103:2055–9
- Galiè N, Hoeper MM, Humbert M, et al. (2009). Guidelines for the diagnosis and treatment of pulmonaryhypertension: the Task Force for the Diagnosis and Treatment of PulmonaryHypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30:2493–537
- Goto K, Arai M, Watanabe A, et al. (2010). Utility of echocardiography versus BNP level for the prediction of pulmonary arterial pressure in patients with pulmonary arterial hypertension. Int Heart J 51:343–7
- Guglin M, Khan H . (2010). Pulmonary hypertension in heart failure. J Card Fail 16:461–74
- Gullestad L, Ueland T, Vinge LE, et al. (2012). Inflammatory cytokines in heart failure: mediators and markers. Cardiology 122:23–35
- Haddad F, Kudelko K, Mercier O, et al. (2011). Pulmonary hypertension associated with left heart disease: characteristics, emerging concepts, and treatment strategies. Prog Cardiovasc Dis 54:154–67
- Heresi GA . (2011). Clinical perspective: biomarkers in pulmonary arterial hypertension. Int J Clin Pract 65:5–7
- Hocher B, Thone-Reineke C, Bauer C, et al. (1997). The paracrine endothelin system: pathophysiology and implications in clinical medicine. Eur J Clin Chem Biochem 35:175–89
- Joppa P, Petrasova D, Stancak B, Tkacova R . (2006). Systemic inflammation in patients with COPD and pulmonary hypertension. Chest 130:326–33
- Kawanabe Y, Nauli SM . (2011). Endothelin. Cell Mol Life Sci 68:195–203
- Khush KK, Tasissa G, Butler J, et al. (2009). Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the evaluation study of congestive heart failure and pulmonary artery catheterisation effectiveness (ESCAPE) database. Am Heart J 157:1026–34
- Koller-Strametz J, Pacher R, Frey B, et al. (1998). Circulating tumor necrosis factor-α levels in chronic heart failure: relation to its soluble receptor II, interleukin-6, and neurohumoral variables. J Heat Lung Transplant 17:356–62
- Mathew R . (2010). Inflammation and pulmonary hypertension. Cardiol Rev 18:67–72
- Pan JP, Liu TY, Chiang SC, et al. (2004). The value of plasma levels of tumor necrosis factor-α and interleukin-6 in predicting the severity and prognosis in patients with congestive heart failure. J Chin Med Assoc 67:222–8
- Petersen JW, Felker M . (2006). Inflammatory biomarkers in heart failure. Congest Heart Fail 12:324–8
- Potter LR . (2011). Natruretic peptide metabolism, clearance and degradation. FEBS J 278:1808–17
- Pullamsetti SS, Schermuly RT . (2009). Endothelin receptor antagonists in preclinical models of pulmonary hypertension. Eur J Clin Invest 39:3–13
- Rafeq S, Shah M, Preston IR . (2009). Biomarkers in pulmonary arterial hypertension. Int J Clin Pract 63:36–41
- Rudski LG, Lai WW, Afilalo J, et al. (2010). Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713
- Savale L, Tu L, Rideau D, et al. (2009). Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res 10:6–19
- Selimovic N, Bergh CH, Andersson B, et al. (2009). Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 34:662–8
- Shao D, Park JES, Wort SJ . (2011). The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res 63:504–11
- Soon E, Holmes AM, Treacy CM, et al. (2010). Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122:920–7
- Von Haehling S, Schefold JC, Lainscak M, et al. (2009). Inflammatory biomarkers in heart failure revisited: Much more than innocent bystanders. Heart Failure Clin 5:549–60
- Von Haehling S, von Bardeleben RS, Kramm T, et al. (2010). Inflammation in right ventricular dysfunction due to thromboembolic pulmonary hypertension. Int J Cardiol 144:206–11
- Yin WH, Chen JW, Jen HL, et al. (2004). Independent prognostic value of elevated high-sensitivity C-reactive protein in chronic heart failure. Am Heart J 147:931–8