Abstract
Although osteoporosis in men is an increasing health problem, studies on osteoporosis in males are still scarce. The aim of our study was to determine the characteristics of bone tissue and bone turnover in men with idiopathic osteoporosis. Transiliac crest bone samples were histomorphometrically analyzed after double tetracycline labeling in 32 men aged 37–65 years who were diagnosed with idiopathic osteoporosis by densitometry of the lumbar spine and hip. Bone volume, osteoid surface, osteoblast surface, eroded surface, osteoid thickness, trabecular thickness, trabecular number, trabecular separation, and mineral apposition rate (MAR) were determined in all trabecular bone specimens. Bone volume and structural parameters indicated trabecular bone loss in most patients. Cellular parameters and MAR indicated variations in bone cell actions. No age-related decrease in histomorphometric parameters was found. After the patients were grouped according to MAR values, osteoblast and eroded surfaces were found to be lower in the group with decreased MAR values and elevated in the group of patients with increased MAR parameter. Trabecular thickness was greater in patients with lower than normal MAR, due to reduced resorption and probably loss of very thin trabeculae. Our results suggest that idiopathic osteoporosis in man resembles many characteristics of postmenopausal osteoporosis in women resulting in impaired trabecular structure due to unbalanced cellular activity and bone turnover rate.
Introduction
Osteoporosis in men is an increasing health problem. The estimated prevalence of hip osteoporosis in men older than 50 years of age is 3–6% [Citation1]. One-third of all hip fractures occur in men [Citation2]. However, the consequences of osteoporosis seem more severe in men than in women, as the mortality rate after hip fracture in men is twice that in women. The lower frequency of osteoporotic fractures in men in comparison with women is explained by a more balanced relationship between load and bone strength and more appropriate bone geometry despite the reduced bone size and bone mineral density (BMD) [Citation3–6].
As opposed to extensive research and accumulation of data on osteoporosis in women, especially postmenopausal women, investigation of osteoporosis in males still needs to elucidate many aspects of the mechanism, diagnosis, and treatment of this disorder. Current knowledge on osteoporosis in males largely depends on the experience and data derived from investigations of osteoporosis in women and diagnosis based on densitometry. The World Health Organization's definition of osteoporosis is based on BMD T-score <2.5. Initially developed for use in white postmenopausal women, it is now being applied to men, although no validation has been carried out. Similarity or dissimilarity of etiological mechanisms of osteoporosis between men and women remains unknown.
It seems that the risk of osteoporosis-related fracture in men, as compared with that in women, is influenced by lifestyle and other factors, such as recreational activity, alcohol intake, and body weight, which are on average greater in men. Nevertheless, studies in larger population groups are necessary to identify other risk factors of osteoporosis-related fractures in men [Citation2].
Imbalanced bone turnover underlying bone loss and leading to osteoporosis in women is characterized by increased bone resorption surface and osteoclast count; however, these characteristics have not been observed in men [Citation7–11]. Studies on bone markers show that increased bone turnover is associated with fracture susceptibility [Citation12]. This finding is in agreement with increased bone resorption observed in bone biopsy studies. Rehman et al. [Citation9] investigated histomorphometric changes in healthy British men and women and found that bone volume decreased with age. The authors explained this finding by decreased osteoblast activity and increased osteoclastic activity, which was more pronounced in women than men. It was presumed that the combined effect was responsible for the reduction in bone mass. Also, age-related decrease in osteoid thickness, surface and volume were observed [Citation9]. Imbalance between bone resorption and bone formation was found in men with osteoporosis, as well as decreased bone volume and osteoblast function [Citation11,Citation13]. In another study, Rehman et al. [Citation14] described five distinct groups of histomorphometric changes in cell function in women with postmenopausal osteoporosis, which indicated histological heterogeneity of this disorder. Comprehensive studies in bone histomorphometry in men, particularly in men with osteoporosis, are still scarce and the results are either inconclusive or insufficient for thorough understanding of bone remodeling that leads to osteoporosis in males.
The aim of our study was to identify and describe different histomorphometric types of idiopathic osteoporosis in men on the basis of histomorphometric analysis of bone samples from 32 male patients with osteoporosis.
Material and methods
Patients
Study participants were selected from the patient population referred to our Clinic for evaluation of osteoporosis. Diagnostic criteria for idiopathic osteoporosis included densitometry T-score <−2.5 at the left hip or lumbar spine and absence of clinical evidence of secondary osteoporosis. Patients with secondary osteoporosis or metabolic bone diseases were excluded, as well as those with Mb. Paget and hyperparathyrodism. The study sample included 32 men aged between 37 and 65 years (mean age, 49.7 ± 8.8 years) with no history of osteoporotic or pathologic fractures. Although the skeleton was not systematically searched for fractures, standard chest X-ray revealed no spinal fractures. The mean BMD of the lumbar spine was 0.78 ± 0.11 g/cm2 (range, 0.60–0.99 g/cm2) and the mean T-score was −3.01 ± 0.97 (range, −4.80 to −1.21). The mean total hip BMD was 0.94 ± 1.45 g/cm2 (range, 0.37–8.87 g/cm2) and the mean T-score was −2.16 ± 0.93 (range, −2.20 to −4.28). Bone biopsy was indicated for further clinical evaluation. All patients signed an informed consent and agreed to the bone biopsy procedure with double tetracycline labeling. The Hospital Ethics Committee approved this study.
Method
Tetracycline double-labeling protocol consisted of oral tetracycline for 3 days (3 × 250 mg/day), no agent for 10 days, and oral tetracycline (3 × 250 mg/day) for another 3 days, followed by biopsy 3 days later. The transiliac crest bone biopsy was performed under local anesthesia or general analgesia. The obtained bone specimens were embedded in resin and stained with Goldner trichrome and toluidine blue. Histomorphometry was performed at 200× magnification using a grid over the entire trabecular bone area. The standard histomorphometric parameters that were measured or calculated included bone volume (BV/TV, %,), osteoid surface (OS/BS, %), osteoblast surface (Ob.S/BS, %), eroded surface (ES/BS, %), osteoid thickness (O.Th, μm), trabecular thickness (Tb.Th, μm), trabecular number (Tb.N, mm−1), trabecular separation (Tb.S, μm), and mineral apposition rate (MAR, μm/day) [Citation15]. Structural parameters including trabecular thickness, number and separation were derived from the measurements of trabecular area and perimeter [Citation15]. The obtained results were compared with the reference data for histomorphometric parameters using the grid method according to Malluche and Faugere [Citation16].
Statistical analysis
Data are presented descriptively as means with standard deviation (±SD) and range. Differences among groups were tested with Kruskal–Wallis test, and differences between groups were evaluated using Mann–Whitney test or chi-square test, as appropriate. The level of statistical significance was set at <0.05. All statistical analyses were performed with Statistica 6.0 software (StatSoft, Tulsa, OK).
Results
Histomorphometric parameters were measured in all 32 patients (). Most patients had normal or decreased bone volume (). Osteoid and osteoblast surfaces were increased in 50% of patients, but osteoid thickness was mostly reduced or normal. Increased osteoid surface and slightly increased osteoid thickness were found in only two patients (). Greater osteoid and osteoblast surfaces were not considered indicative of osteomalacia, because osteoid thickness was not increased and none of the patients had any clinical or laboratory evidence of disease. Trabecular thickness and trabecular separation were increased, whereas trabecular number was decreased in most patients. For erosion surface and MAR, approximately similar proportions of decreased, normal, and increased results were found. Patients were further divided into three groups according to the MAR results (decreased, normal, or increased) and compared using Kruskal–Wallis test.
Table I. Histomorphometric parameters measured in transiliac bone samples from 32 men with idiopathic osteoporosis.
Table II. Number of patients with idiopathic osteoporosis divided into groups with decreased, normal, or increased measured histomorphometric parameters with respect to reference values (presented in Table I).
Statistically significant differences between MAR groups were found for osteoblast surface (Ob.S/BS, p < 0.003), eroded surface (ES/BS, p < 0.020), and trabecular thickness (Tb.Th, p < 0.04) (). The three MAR groups did not differ in age. The mean age of eight patients with decreased MAR, nine patients with normal MAR and 15 patients with increased MAR was 52.6 ± 9.2, 51.1 ± 10.9, and 47.3 ± 6.8 years, respectively (chi-square = 1.92, p = 0.300).
Figure 1. Box-plot graphs for bone volume (BV/TV, %), osteoid surface (OS/BS, %), osteoid thickness (O.Th, μm), osteoblast surface (Ob.S/BS, %) eroded surface (ES/BS, %), trabecular thickness (Tb.Th, μm); trabecular number (Tb.N, mm−1); trabecular separation (Tb.S, μm); according to reduced, normal and increased mineral apposition rate (MAR, μm/day) in 32 male patients with idiopathic osteoporosis are presented as medians and quartiles, with indicated outliers (O) and extreme values (*). Horizontal lines indicate reference range (1 SD).
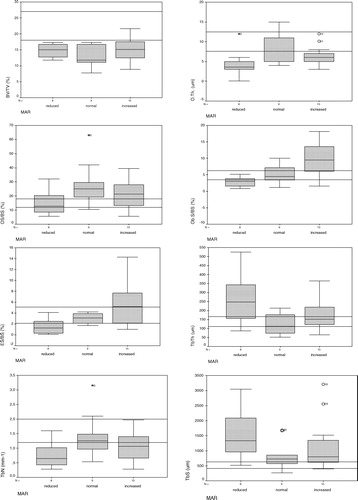
The group with increased MAR had significantly higher values (Mann–Whitney test) for osteoblast and eroded surface parameters than the group with decreased MAR and significantly higher Ob.S/BS values than the group with normal MAR (). The group with decreased MAR had significantly lower eroded surface and significantly greater trabecular thickness than the group with normal MAR (). Trabecular separation was greater and trabecular number was lower in the group with decreased MAR than in the other two groups, although the differences were not statistically significant. Osteoid surface was normal in the group with decreased MAR and increased in the groups with normal or increased MAR, but there was no significant difference between the groups.
Table III. Comparison of patients with decreased, normal, and increased mineral apposition rate (MAR) for osteoblast surface (ObS/BS), eroded surface (ES/BS), and trabecular thickness (Tb.Th).
On average, bone volume was decreased in all three groups, and osteoid thickness was mostly decreased or normal. Osteoblast and eroded surface results corresponded to MAR in each group, i.e. they were decreased in the decreased MAR group and increased in the increased MAR group. Trabecular thickness was greater in the group with decreased MAR than in other two groups ().
Discussion
The histomorphometric analysis of transiliac crest bone samples from 32 men with idiopathic osteoporosis allowed us identify three different histomorphometric groups – those with decreased, normal, and increased MAR values. In patients with increased MAR, osteoblast surface was greater than in patients with normal or decreased MAR, whereas the eroded surface was greater than in patients with normal MAR. Osteoporotic patients with decreased MAR showed lower eroded and osteoblast surface and also greater trabecular thickness in comparison with the group with normal MAR. These results showed heterogeneity of bone turnover in osteoporosis in males, which was characterized by increased, normal, or decreased bone cell activity. Reduced trabecular meshwork in patients with decreased turnover was distinguished by a greater trabecular thickness than that in patients with normal turnover.
Age-related bone loss in men is characterized by increased bone resorption and steady bone formation after the age of 60 [Citation17–19] due to the changes in levels of sex steroid hormones, e.g. estrogens, and changes in levels of growth hormone, insulin-like growth factor-1, and parathyroid hormone [Citation19–21]. The type of bone loss differs between men and women – trabecular thinning is predominant in men, whereas connectivity loss is more prevalent in women [Citation8,Citation22]. Connectivity loss is also found in men with osteoporosis and vertebral fractures [Citation23,Citation24]. These structural abnormalities are considered to originate from the growth and ageing processes [Citation3]. Increased bone resorption is a predominant feature of osteoporosis in both men and women [Citation22,Citation25–28], although it has not been demonstrated in all studies [Citation13].
Osteoblastic activity and proliferation [Citation29] in men with osteoporosis either remain unchanged [Citation30] or decrease [Citation13,Citation26,Citation27]; they are considered responsible for reduced bone formation. MAR also seems to be unchanged [Citation11,Citation13]. Reduced osteocyte density in osteoporotic bone has been related to decreased osteoblast availability for matrix embedding [Citation22].
Trabecular bone volume was mostly reduced in our male patients with idiopathic osteoporosis, which is in agreement with their bone densitometry results and previous research [Citation13]. Trabecular structure parameters indicated reduced trabecular meshwork, i.e. decreased trabecular number and increased trabecular separation. Osteoid thickness was reduced or normal, trabecular thickness was increased or normal, while the results for other histomorphometric parameters showed greater variation.
Our histomorphometric findings, mostly regarding osteoblast-related parameters in men with osteoporosis, differ from those reported by Ciria et al. [Citation13], who found decreased osteoid and osteoblast surfaces, modest increase in osteoid thickness and unchanged eroded surface and MAR. In our patients, osteoid thickness was low or normal, osteoid surface values were higher than reference data and there was a wide variation in other histomorphometric parameters (i.e. decreased, normal, and increased values), such as osteoblast surface, eroded surface, and MAR. When patients were grouped according to MAR results, a pattern of decreased, normal, and increased bone turnover was revealed. Osteoblast and eroded surface parameters corresponded to MAR values, i.e. they were decreased in the decreased MAR group and increased in the increased MAR group. Age-related trabecular bone loss in men is consistent with the decreased wall thickness and trabecular thinning [Citation31,Citation32] although some investigators reported a variation in wall thickness in osteoporosis in males [Citation33]. In our patients, trabecular thickness was mostly normal, except for the decreased MAR group.
Trabecular thickness was greater in the group with decreased MAR than the group with normal MAR. In this group, trabecular number was lower and trabecular separation was higher, although there was no statistically significant difference in comparison with groups with normal or increased cell activity. Similar observation was explained by reduced erosion depth and subsequent increase in wall thickness [Citation32,Citation34], but also with disappearance of thinner trabeculae [Citation31]. Our study results indicate heterogeneity of bone cell actions in clinically manifest osteoporosis in men.
In other studies, osteoporosis in men was attributed to the decreased osteoblast function and decreased bone turnover [Citation5,Citation22]. Osteoblast activity was also reduced in the subgroup of our patients with decreased MAR; similar histomorphometric data were reported by Ciria et al. [Citation13] and Mullender et al. [Citation22]. Trabecular thickness, which also differed between MAR groups in our study, mostly declines with age [Citation9] and has lower values in men with osteoporosis than healthy men [Citation22]. In women with osteoporosis, trabecular thickness was neither found to be different from that in healthy women [Citation22] nor recognized as a parameter distinguishing between different histomorphometric types of postmenopausal osteoporosis [Citation14]. The diversity of histomorphometric parameters observed in our male patients with osteoporosis does not contradict the existing data and probably arises from the sample size, which was larger than samples in other similar studies [Citation13]. Nevertheless, the subgroups of patients in our study were small. For the time being, it is uncertain whether a greater number of subjects would contribute to resolving the controversy of bone tissue in osteoporosis in men.
Our results are in agreement with the reported diversity of bone turnover in women with postmenopausal osteoporosis, which includes other combinations of bone cell actions [Citation14] that were not observed in our study. Whether the reduced, normal, or increased bone turnover in men leads to osteoporosis can only be speculated.
Our study has several limitations. First, as there are no appropriate reference values either for osteoporosis in men or for histomorphometric method applied in our study, we used published reference values, assuming that it would not affect our evaluation of idiopathic osteoporosis in men. Second, comparison with age-matched healthy individuals from identical population pool would have been ideal, but it could not be performed due to the need for double tetracycline labeling. Nevertheless, even if population differences were considerable, the observed deviations would be relative. This limitation should have no influence on the differences in bone turnover between our patient subgroups. The third limitation of our study was a relatively small sample size; therefore, caution is required in the interpretation of our results. On the other hand, our sample was still larger than the samples in the studies by Ciria et al. [Citation13] and Mullender et al. [Citation22]. Fractures in this patient group were not specifically ruled out, although based on patient history and standard chest X-ray none was found.
Although histomorphometric analysis of bone biopsy is performed only at specialized laboratories, our results could contribute to the knowledge of microscopic bone features in osteoporosis in males.
In conclusion, the present study has shown that idiopathic osteoporosis in men has features of decreased, normal, or increased bone turnover. Structural properties of trabecular bone loss were demonstrated between decreased and normal MAR for trabecular thickness. These results indicate the diversity of bone cell actions in osteoporosis in males, similar to that in postmenopausal osteoporosis in women.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Olszynski WP, Shawn Davison K, Adachi JD, Brown JP, Cummings SR, Hanley DA, Harris SP, Hodsman AB, Kendler D, McClung MR, et al Osteoporosis in men: epidemiology, diagnosis, prevention and treatment. Clin Ther 2004;26:15–28.
- Johnell O, Kanis J, Gullberg G. Mortality, morbidity, and assessment of fracture risk in male osteoporosis. Calcif Tissue Int 2001;69:182–184.
- Seeman E. The growth and age-related origins of bone fragility in men. Calcif Tissue Int 2004;75:100–109.
- Seeman E, Bianchi G, Khosla S, Kanis JA, Orwoll E. Bone fragility in men – where are we? Osteoporosis Int 2006;17:1577–1583.
- Seeman E. During ageing, men lose less bone than women because they gain more periosteal bone, not because they resorb less endosteal bone. Calcif Tissue Int 2001;69:205–208.
- Rochira V, Balestrieri A, Madeo B, Zirilli L, Granata ARM, Carani C. Osteoporosis and male age-related hypogonadism: role of sex steroids on bone (patho)physiology. Eur J Endocrinol 2006;154:175–184.
- Meunier P, Courpron P, Edouard C, Bernard J, Bringuier J, Vignon G. Physiological senile involution and pathological rarefaction of bone. Quantitative and comparative histological data. Clin Endocrinol Metab 1973;2:239–256.
- Aaron JE, Makins NB, Sagreiy K. The microanatomy of trabecular bone loss in normal ageing men and women. Clin Orthop Rel Res 1987;215:260–271.
- Rehman MTA, Hoyland JA, Denton J, Freemont AJ. Age-related histomorphometric changes in bone in normal British men and women. J Clin Pathol 1994;47:529–534.
- Ericksen EF, Mosekilde L, Melsen F. Trabecular bone resorption depth decreases with age. Differences between normal males and females. Bone 1985;6:141–146.
- Chavassieux P, Meunier J. Histomorphometric approach of bone loss in men. Calcif Tissue Int 2001;69:209–213.
- Bonick SL, Shulman L. Monitoring osteoporosis therapy: bone mineral density, bone turnover markers, or both? Am J Med 2006;119:255–315.
- Ciria-Recasens M, Perez-Edo L, Blanch-Rubio J, Marinoso ML, Benito-Ruuiz P, Serrano S, Carbonell-Abello J. Bone histomorphometry in 22 male patients with normocalciuric idiopathic osteoporosis. Bone 2005;36:926–930.
- Rehman MTA, Hoyland JA, Denton J, Freemont AJ. Histomorphometric classification of postmenopausal osteoporosis: implications for the management of osteoporosis. J Clin Pathol 1995;48:229–235.
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardisation of nomenclature, symbols, and units. J Bone Min Res 1987;2:595–610.
- Malluche HH, Faugere MC. Mineralized bone histology of metabolic bone diseases. Atlas of mineralized bone histology.New York, Basel: Karger; 1986; pp 50–118.
- Khosla S, Melton LJ, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 1998;83:2266–2274.
- Szulc P, Garner P, Munoz F, Marchand F, Delmas PD. Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res 2001;16:1642–1650.
- Meier C, Liue PY, Handelsman DJ. Endocrine regulation of bone turnover in men. Clin Endocrinol 2005;63:603–616.
- Khosla S. Role of hormonal changes in the pathogenesis of osteoporosis in men. Calcif Tissue Int 2004;75:110–113.
- Gallagher JC, Kinyamu HK, Fowler SE, Dawson-Hughes B, Dalsky GP, Sherman SS. Calciotropic hormones and bone markers in the elderly. J Bone Miner Res 1998;13:475–482.
- Mullender MG, Tan SD, Vico L. Differences in osteocyte density and bone histomorphometry between men and women and between healthy and osteoporotic subjects. Calcif Tissue Int 2005;77:291–296.
- Hordon LD, Raisi M, Aaron JE. Trabecular architecture in women and men of similar bone mass with and without vertebral fracture: two-dimensional histology. Bone 2001;27:271–276.
- Legrand E, Chappard D, Pascaretti C. Trabecular bone microarchitecture, bone mineral density and vertebral bone microarchitecture, bone mineral density and vertebral fractures in male osteoporosis. J Bone Min Res 2000;15:13–19.
- Perry HM, Fallon MD, Bergfeld M. Osteoporosis in young men. Arch Intern Med 1982;142:1295–1298.
- Delichatsios HK, Lane JM, Rivlin RS. Bone histomorphometry in men with spinal osteoporosis. Calcif Tissue Int 1995;56:359–363.
- Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med 1995;123:452–460.
- Chavasieux P, Meunier PJ. Histomorphometric approach of bone loss in men. Calcif Tissue Int 2001;69:209–213.
- Marie PJ, de Vernejoul MC, Connes D, Hott M. Decreased DNA synthesis by cultured osteoblastic cells in eugonadal osteoporotic men with defective bone formation. J Clin Invest 1991;88:1167–1172.
- Francis RM, Peacock M, Aaron JE, Selby PL, Taylor GA, Thompson J, Marshall DH, Horsman A. Osteoporosis in hypogonadal men: role of decreased plasma 1,25-dyhdroxyvitamin D, calcium malabsorption and low bone formation. Bone 1986;7:261–268.
- Birkenhager-Frenkel DH, Nigg AL, Hens CJ, Birkenhager JC. Changes of interstitial bone thickness with age in men and women. Bone 1993;14:211–216.
- Szulc P. Pathophysiology and diagnosis of osteoporosis in aging men. IBMS BoneKEy 2008;5:370–380.
- Kelepouris N, Harper KD, GAnnon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med 1995;123:452–460.
- Croucher PI, Mellish RWE, Vedi S, Garrahan NJ, Compston JE. The relationship between resorption depth and mean interstitial bone thickness: age-related changes in man. Calcif Tissue Int 1989;45:15–19.
