Abstract
Background. Prostate cancer incidence varies significantly among different ethnic groups. However, the report concerning the clinical outcome after radical prostatectomy (RP) in the low incidence Asian population is still limited. We aimed to compare the clinical outcome in patient treated with RP among different ethnic groups and to identify significant prognostic factors in Taiwanese patients.
Methods. A total of 341 patients with clinical localized prostate cancer undergoing curative RP in three medical centers in Taiwan were included in this study. Ethnic group comparison was performed using the CaPSURE, SEARCH databases from United States (US) and one large European series. The Kaplan–Meier analysis and Cox proportional hazard model were used to identify significant predictors for prostate-specific antigen (PSA) recurrence.
Results. Compared to the Caucasian white population in the US and Europe studies, the Taiwanese population have higher age at surgery and higher pre-operative PSA level. With mean and median follow-up of 39.1 months and 31.0 months (range 5–120 months), 127 men (37.2%) had PSA recurrence which was significant higher than the Western series. Significant predictors for PSA recurrence identified in the post-operative overall model were PSA level, pathological Gleason Score, pathological tumor stage and lymph node metastasis.
Conclusions. The clinical outcome of Taiwanese male with prostate cancer post-RP appears inferior to the Western country, which is largely due to delay surgery at higher PSA level. Earlier diagnosis and treatment may improve the cancer control of RP.
Introduction
Prostate cancer is the most common cancer diagnosed and the second common cause of cancer-related death in American men, however, its incidence is relatively lower in the Asian countries [Citation1]. In Taiwan, the incidence of prostate cancer has been rapidly increasing in the past years. It became the 7th most common cause of cancer deaths, resulting in 1003 deaths in 2007 [Citation2]. Prostate cancer has become an important issue for male health and further studies are needed to clarify clinical characteristics and identify the prognosis factors.
With the widespread use of prostate-specific antigen (PSA) as a screening tool, more and more newly diagnosed prostate cancers are clinically localized and are candidate for curative treatment. Radical prostatectomy (RP) is one of the principal treatment modalities for men with clinically localized prostate cancer. Although, approximately two thirds of patients treated with curative surgery will remain disease-free for more than 10 years following RP, a substantial portion of these patients experience early PSA recurrence [Citation3,Citation4] and are prone to develop metastatic lesions with significant mortality [Citation5–7]. Several clinico-pathologic characteristics and the commonly used pre-operative risk grouping models are reported as predictors or for stratifying the risk groups for PSA recurrence [Citation3,Citation8–14]; since an accurate prediction for these high risk patients is of paramount importance because they may benefit from more intensive follow-up or aggressive adjuvant therapy.
Although the prostate cancer incidence varies significantly among the ethnic groups, whether the clinical outcome differs is still unclear [Citation15]. Regarding the long-term clinical outcome after RP in the low-incidence Asian population, the number of reported and the number of patient studied are still limited [Citation16–18]. Thus, in this study, we aimed to compare the clinical outcome in patient treated with RP among different ethnic groups and to identify significant prognostic factors in Taiwanese patients.
Materials and methods
Study population
The study subjects were expanded from our hospital-based case–control study that has previously been described. Briefly, patients with diagnosed and pathologically confirmed prostate cancer were actively recruited from the Kaohsiung Medical University Hospital (KMUH), Kaohsiung Veterans General Hospital (KVGH), and National Taiwan University Hospital (NTUH), three medical centers in Taiwan. In this study, we followed-up a subset of these patients, those diagnosed with clinical localized prostate cancer and had received RP, to investigate the significant prognostic roles of clinico-pathological characteristics in the progression of prostate cancer (defined by the recurrence of PSA). The decision of RP was based on the clinical stage, patient's age and general health status and patients' preference after full discussion with their doctors about the benefit and risk of RP compared to other treatment modalities. PSA recurrence was defined as two consecutive PSA measurements of >0.2 ng/mL at an interval of >3 months [Citation19], and the PSA level of >0.2 ng/mL at the first follow-up was considered the date of recurrence. We excluded those who received neo-adjuvant/adjuvant hormone therapy or radiotherapy and those without sufficient follow-up data, leaving 341 cases into final analysis. Among the 341 cases, 204 received retropubic RP and 137 received laparoscopic RP; 159 cases were from NTUH, 118 cases were from KVGH and 64 cases were from KMUH.
Disease stage was determined by pathology findings, pelvic computed tomography or magnetic resonance image, and radio-nucleotide bone scans, according to the criteria of the American Joint Committee on Cancer tumor-node-metastasis classification system [Citation20]. Pathologic grading was recorded as the Gleason Score [Citation21] and was further classified into three groups: Gleason Score 2–7, Gleason 7 and Gleason 8–10 to assess the prognostic value after RP. Pathology analyses were performed on the whole specimen with step sections (2–3 mm). Positive surgical margin was defined as tumor cells present at the inked margin.
Risk grouping analysis was also investigated using the commonly used risk grouping system (D'amico risk classification): low risk (PSA <10 ng/mL, biopsy Gleason Score of 6 or less and cT1c/T2a), intermediate risk (PSA 10–20 ng/mL, biopsy Gleason Score 7 or T2b) and high risk (PSA >20 ng/mL, biopsy Gleason Score of 8 or greater) [Citation13].
For ethnic groups comparison, we choose the Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE), Shared Equal Access Regional Access Regional Cancer Hospital (SEARCH) databases (mainly Caucasian White and African American in United States) and one large series from Europe () [Citation15,Citation22,Citation23] to compare the difference of the clinical outcome and pathological features of prostate cancer patients undergoing RP between Taiwanese men and Western populations.
Table II. Clinico-pathological characteristics of prostate cancer patients undergoing radical prostatectomy: ethnic group comparison.
Statistical analysis
Contingency tables were constructed to examine the difference between groups with χ2 test. t-test was used to compare mean variables. The significance of clinico-pathologic factors as predictors for PSA recurrence-free survival after RP was determined using the Kaplan–Meier analysis and log-rank test. Actuarial estimates for survival were calculated using life table methods. The Cox proportional hazard regression model was used for multivariate analysis. We separate these variables into pre-operative model (age, pre-operative PSA level, biopsy Gleason Score and clinical stage) and overall model (age, pre-operative PSA level, pathologic Gleason Score, pathological stage and surgical margin status) for further analysis.
The Statistical Package of the Social Sciences software version 13.0 (SPSS Inc., Chicago, IL) was used for statistical analyses. A two-sided p value < 0.05 was considered statistically significant.
Results
showed the clinico-pathological characteristics of the 341 prostate cancer patients undergoing RP. The mean and median diagnosed age was 65.7 ± 6.7 and 67-years old. The mean preoperative PSA level was 15.4 ± 13.0 ng/mL, of them, 138 (40.5%) patients had < 10 ng/mL, 114 (33.4) patients had PSA 10–20 ng/mL, and 76 patients had PSA > 20 ng/mL. The distributions of biopsy Gleason Score, clinical stages, pathologic Gleason Score, pathological stages were shown as details. Of them, 102 (29.9%) had positive surgical margin. The risk groups according to D'Amico classification were 27.0% in low risk, 41.3% in intermediate risk and 31.1% in high risk.
Table I. Clinico-pathological characteristics of the 341 prostate cancer patients undergoing radical prostatectomy.
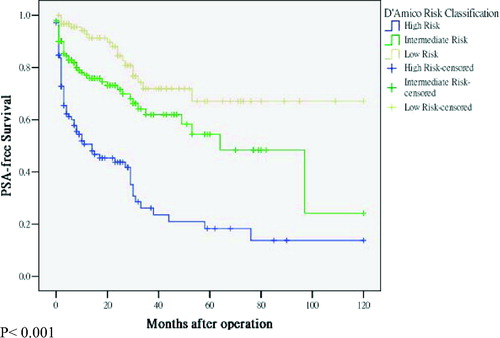
With mean and median follow-up of 39.1 months and 31.0 months (range 5–120 months), 127 men (37.2%) had biochemical recurrence. Most of the PSA recurrence 117 (92.1%) developed in the first 3-year periods (83 PSA recurrence events in the first year, 12 PSA recurrence events in the second year and 22 PSA recurrence events in the third year). The overall actuarial 5 and 10 year biochemical-free survival rates were 43% and 33%, respectively (). Subset analysis for those with clinical T1–T2 stages showed similar results ().
Figure 1. Overall PSA-free survival and life tables analyses. With mean and median follow-up of 39.1 months and 31.0 months (range 5–120 months), 127 men (37.2%) had PSA recurrence. Majority of the PSA recurrence 117 (92.1%) developed in the first 3-year period (83 PSA recurrence events in the first one year, 12 PSA recurrence events in the second year and 22 PSA recurrence events in the third year). Subset analysis of cT1–cT2 cases showed similar trend. Eighty-eight men (31.2%) had PSA recurrence. Majority of the PSA recurrence 78 (88.6%) developed in the first 3-year period.
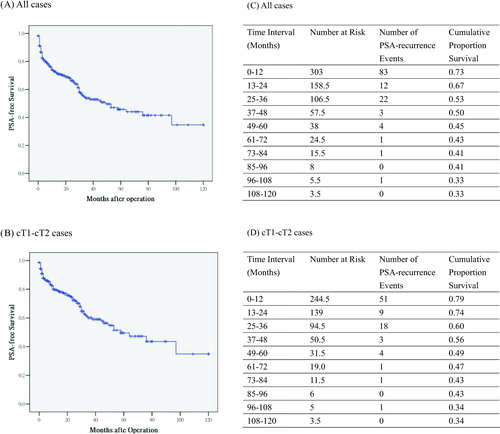
Kaplan–Meier analyses of PSA recurrence-free survival probability stratified according to main clinico-pathological variables were illustrated in . The PSA recurrence-free probability was not significantly different between the young age group (<67-year old) and old age group (≥67-year old) ().
Figure 2. PSA-free survival analyses according to clinico-pathological characteristics. The PSA recurrence free probability was not significant different between the young age group (<67-years old) and old age group (≥67-year old) (Figure 2a). The PSA-free survival probabilities were significant different between the per-operative PSA level (<10 ng/mL vs., 10–20 ng/mL vs. > 20 ng/mL), biopsy Gleason Score and pathological Gleason Score (2–6 vs. 7 vs. 8–10), clinical stages, pathological stages, surgical margin and lymph node metastasis. The log-rank tests comparing these variables were all statistically significant (all p < 0.001) (Figure 2b–h).
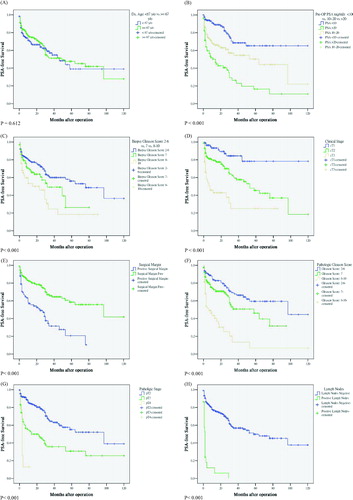
The PSA-free survival probabilities were significantly different between the per-operative PSA level (<10 ng/mL vs. 10–20 ng/mL vs. > 20 ng/mL), biopsy Gleason Score and pathological Gleason Score (2–6 vs. 7 vs. 8–10), clinical stages, pathological stages, surgical margin and lymph node metastasis. The log-rank tests comparing these variables were all statistically significant (all p < 0.001) (–h). showed risk stratification suggested by D'Amico et al. [Citation13]. The log-rank test across three strata was statistically different (p < 0.001) ().
Figure 3. PSA-free survival stratified according to D'Amico risk classification. The log-rank test across three strata (low risk group vs. intermediate risk group vs. high risk group) was statistically different (p < 0.001).
For ethnic group comparison of clinical outcome and pathological features of prostate cancer patients undergoing RP (), we found that one of the most striking difference between our series and the western population is our series that had higher proportion of cT3 stage (18.2% vs. 1–3%), thus, we separate our series into s subset of cT1–cT2 stage cases for further analysis. The mean age at surgery in our series was slightly higher compared to the Caucasian White and European men (65.7-year old vs. 63.8-year old vs. 62.2–year old). The pre-operative PSA level is significant higher in our series than the western populations (15.4 vs. 9.6 vs. 9.8 vs. 9.1, p < 0.001). The proportion of biopsy and pathologic Gleason Score 8–10 cases is slightly higher in the Taiwanese men than the CaPSUE and SEARCH databases, and lowest in the European series. Although the clinical T3 stages cases is significantly higher in the Taiwanese cases, however, the distribution of pathologic T3,T4, lymph node metastasis and positive surgical margin is rather similar compared to the western population.
Overall, the PSA recurrence rate was higher in the Taiwanese men (37.2%) compared to the American men (22%) and European patients (20.6%). This trend persisted when we restrict our series to clinical T1–T2 cases (31.5% vs. 22% vs. 20.6%, p < 0.001).
showed the pre- and post-operative univariate and multivariate Cox regression analyses predicting PSA recurrence after RP. In univariate analyses, all pre- and post-operative variables were significant predictors for PSA recurrence (p < 0.05), except for age (p = 0.922). In multivariate analyses, pre-operative PSA level, biopsy Gleason Score and clinical stage were independently associated with PSA recurrence. For the post-operative model, the pre-operative PSA level, pathologic Gleason Score, pathologic stage and lymph node metastasis were significant predictors for PSA recurrence ().
Table III. Univariate and multivariate Cox proportional hazards analyses of clinico-pathological factors predicting PSA recurrence after radical prostatectomy.
Discussion
Our series showed that 127 men (37.2%) had PSA recurrence with mean and median follow-up of 39.1 months and 31.0 months (range 5–120 months) for the Taiwanese men who underwent RP for clinical localized prostate cancer. The overall actuarial 5- and 10-year PSA-free survival rates were 43% and 33%, respectively. Significant predictors for PSA recurrence identified for the pre-operative model were PSA level, biopsy Gleason Score and clinical stage. Predictive factors identified for the post-operative overall model were PSA level, pathological Gleason Score, pathological tumor stage and lymph node metastasis. The D'Amico classification system could stratify the Taiwanese men into low, intermediate and high-risk groups with statistically significantly difference.
Although the prostate cancer incidence varies significantly among the ethnic groups, whether the clinical outcome differs is still unclear. Despite excellent long-term outcome control after RP has been reported, mainly data were reported from North American addressing on Caucasian White and African American [Citation3,Citation4,Citation24]. Regarding the long-term clinical outcome after RP in the low-incidence Asian population, the numbers of reported and the number of patient studied are still limited [Citation16–18]. To our knowledge, our present study showed the largest long-term follow-up series regarding the PSA recurrence after RP in the low-incidence Asian Taiwanese population.
Overall, we found that the PSA recurrence rate was significantly higher in the Taiwanese men (37.2%) compared to the American men (22%) and European patients (20.6%) (). When we restrict our series to clinical T1–T2 cases, the PSA recurrence rate remains higher than the American and European series (31.5% vs. 22% vs 20.6%) (). The high PSA recurrence rate (30%) for 70 pT2 Taiwanese prostate cancer patients who received RP also been reported by Wu et al. [Citation17]. Because the distribution of pT3–T4 and positive surgical margin was similar among groups, it is plausible that the higher PSA recurrence rate of Taiwanese is mainly due to higher pre-operative PSA level (Taiwanese vs. American white vs. European men: 15.4 ng/mL vs. 9.6 ng/mL vs 9.1 ng/mL, respectively) (), which may also reflect the delay diagnosis and surgery of prostate cancer among the Taiwanese men. We think this observation is true for the following reasons. First, large-scale PSA or prostate cancer screening has not been conducted in Taiwan owing to the low cost-effectiveness in such a low-incidence country. Second, prostate cancer awareness is low among the Taiwanese population and even among general practitioner. There is only about 3000 incident cases occurred, with an age-adjusted incidence rate of only 21.9 per 100,000 person per year, markedly lower than the high incidence of male cancer in Taiwan such as liver, lung, colorectal, gastric and oral cancers [Citation25]. Third, not all Taiwanese male with an abnormal PSA level undergo biopsy as a result of patient decision. Not surprising, the proportion of Taiwanese prostate cancer with metastatic disease at diagnosis is much greater than the American men (25.5–32.7% vs. 5%, respectively) [Citation26,Citation27].
It is also reasonable to argue that the higher PSA recurrence rate of Taiwanese male might simply due to more aggressive (high grade) prostate cancer in our series. However, we think this probability is relatively low because the pathological Gleason Score 8–10 cases is similar between Taiwanese and American cases (11.1% vs. 10%) (). Although one study hypothesized that prostate cancer in Chinese individuals has more aggressive biological behavior than in the white individuals [Citation28], however, more studies were needed to confirm this observation. Furthermore, the pathologic T3 stages distribution and positive surgical margin rate were similar (), these may exclude the possibility of inferior experience or surgical technique for urologic surgeon in Taiwan. Thus, we proposed that enhancing people's awareness of prostate cancer in Taiwan and treat prostate cancer earlier at lower PSA level may improve the clinical outcome of RP.
Regarding the risk classification system, the D'amico risk classification is well practiced in Caucasian population, however, how it performs in the Asian population was not tested. Our result showed the D'amico risk classification system can separate RP cases into different risk groups well in the low-incidence Asian population ().
Our study has some strength and limitation. Adequate follow-up period and detailed clinical information allows for the stratification of data by clinical features and PSA recurrence of the prostate cancer post-RP. Our shortage is that compared to other large Caucasian series (n > 1000), our series may need to recruit more cases for further validation. Thus, including more update series from other populations and using standard criteria for RP for more precise comparison will be helpful to clarify whether the clinical outcome differ between the ethnic groups. Furthermore, although pre-operative PSA level is highly associated with PSA recurrence after RP, whether PSA-based screening will reduce the rate of death from prostate cancer remains inconsistent [Citation29,Citation30].
Conclusion
The clinical outcome of Taiwanese male with prostate cancer post-RP appears inferior to the Western country, which is largely due to delay surgery at higher PSA level. Enhancing people's awareness of prostate cancer and earlier diagnosis and treatment may improve the cancer control of RP for the Taiwanese population.
Acknowledgments
This study was supported by grants from the Taiwan National Science Council (NSC 95-2314-B-037-053-MY2 and NSC 96-2314-B-037 -012 -MY3) and Kaohsiung Medical University Hospital (KMUH96-6G27 and KMUH96-6G28). We thank Ms. Chao-Shih Chen for her help on data analyses.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66.
- Annual Report, Bureau of Health Promotion, Department of Health (DOH), Taiwan 2007.
- Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin N Am 2001;28:555–565.
- Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol 2004;172:910–914.
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005;294:433–439.
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Partin AW. Time to prostate specific antigen recurrence after radical prostatectomy and risk of prostate cancer specific mortality. J Urol 2006;176(4 Part 1):1404–1408.
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591–1597.
- Ozcan F. Correlation of perineural invasion on radical prostatectomy specimens with other pathologic prognostic factors and PSA failure. Eur Urol 2001;40:308–312.
- Walsh PC, Partin AW, Epstein JI. Cancer control and quality of life following anatomical radical retropubic prostatectomy: results at 10 years. J Urol 1994;152(5 Part 2):1831–1836.
- Babaian RJ, Troncoso P, Bhadkamkar VA, Johnston DA. Analysis of clinicopathologic factors predicting outcome after radical prostatectomy. Cancer 2001;91:1414–1422.
- Pettus JA, Weight CJ, Thompson CJ, Middleton RG, Stephenson RA. Biochemical failure in men following radical retropubic prostatectomy: impact of surgical margin status and location. J Urol 2004;172:129–132.
- Moul JW, Connelly RR, Lubeck DP, Bauer JJ, Sun L, Flanders SC, Grossfeld GD, Carroll PR. Predicting risk of prostate specific antigen recurrence after radical prostatectomy with the Center for Prostate Disease Research and Cancer of the Prostate Strategic Urologic Research Endeavor databases. J Urol 2001;166:1322–1327.
- D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. JAMA 1998;280:969–974.
- Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998;90:766–771.
- Grossfeld GD, Latini DM, Downs T, Lubeck DP, Mehta SS, Carroll PR. Is ethnicity an independent predictor of prostate cancer recurrence after radical prostatectomy? J Urol 2002;168:2510–2515.
- Ou YC, Chen JT, Yang CR, Cheng CL, Ho HC, Ko JL, Hsieh YS. Predicting prostate specific antigen failure after radical retropubic prostatectomy for T1c prostate cancer. Jpn J Clin Oncol 2002;32:536–542.
- Wu TT, Hsu YS, Wang JS, Lee YH, Huang JK. The role of p53, bcl-2 and E-cadherin expression in predicting biochemical relapse for organ confined prostate cancer in Taiwan. J Urol 2003;170:78–81.
- Ho SF, Lao HF, Li K, Tse MK. Clinical results of radical prostatectomy for patients with prostate cancer in Macau. Chin Med J 2008;121:295–298.
- Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology 2003;61:365–369.
- American Joint Committee on Cancer Staging. American Joint Committee on Cancer Staging manual, 5th ed. Lippincott Raven Publishers; 1997.
- Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58–64.
- Freedland SJ, Amling CL, Dorey F, Kane CJ, Presti JC Jr, Terris MK, Aronson WJ. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology 2002;60:670–674.
- Chun FK, Graefen M, Zacharias M, Haese A, Steuber T, Schlomm T, Walz J, Karakiewicz PI, Huland H. Anatomic radical retropubic prostatectomy-long-term recurrence-free survival rates for localized prostate cancer. World J Urol 2006;24:273–280.
- Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol 2002;167(2 Part 1):528–534.
- National Cancer Registry. Annual Report of Taiwan Cancer Registry; National Cancer Registry; Department of Health, Taiwan. 2006.
- Chen CH, Tzai TS, Huang SP, Wu HC, Tai HC, Chang YH, Pu YS. Clinical outcome of Taiwanese men with metastatic prostate cancer compared with other ethnic groups. Urology 2008;72:1287–1292.
- Pu YS. Prostate cancer in Taiwan: epidemiology and risk factors. Int J Androl 2000;23(Suppl 2):34–36.
- Zhau H, Zhao LS, Chung LWK, Chen BQ, Troncoso P, Kao C, Kojima M, Fraser Symmans W, Zheng N, Palmer JL, Moul JW, Davis R, Ye MF, Xiao LS, Craig Hall M. Comparative studies of prostate cancers among United States, Chinese, and Japanese patients: characterization of histopathology, tumor angiogenesis, neuroendocrine factors, and p53 protein accumulations. Urol Oncol 1995;1:51–63.
- Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD. PLCO Project Team. N Engl J Med 2009;360:1310–1319.
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, et al Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320–1328.
