Abstract
Vitamin D is a group of lipophilic hormones with pleiotropic actions. It has been traditionally related to bone metabolism, although several studies in the last decade have suggested its role in muscle strength and falls, cardiovascular and neurological diseases, insulin-resistance and diabetes, malignancies, autoimmune diseases and infections. Vitamin D appears to be a hormone with several actions and is fundamental for many biological systems including bone, skeletal muscle, brain and heart.
The estimated worldwide prevalence of vitamin D deficiency of 50% in elderly subjects underlines the importance of vitamin D deficiency for public health.
In this review, we will describe changes in vitamin D levels with age in both sexes, cut off values to define Vitamin D status, the impact of vitamin D deficiency in age-related disease and finally different therapeutic options available to treat Vitamin D deficiency in older populations.
Keywords:
Introduction
Vitamin D is a group of lipophilic hormones with pleiotropic actions. It has been traditionally related to bone metabolism, although several studies in the last decade have suggested its role on cardiovascular diseases, diabetes, malignancies, autoimmune diseases and infections.
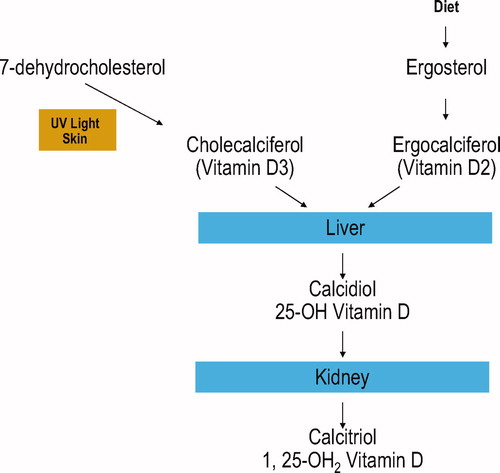
There are two active types of vitamin D: vitamin D3 (colecalciferol) derived by the irradiation in the skin of 7-dehydrocholesterol, the precursor of vitamin D3 and vitamin D2 (ergocalciferol) derived from irradiation in the skin of the ergosterol, which is the precursor of the vitamin D2 from plant origin.
Because vitamin D is fat soluble, it is readily taken up by fat cells. Then, vitamin D3 and vitamin D2 are hydroxylated to 25 (OH) vitamin D (or calcidiol or calciferol) by several tissue (mainly by the liver) and hydroxylated in the kidneys to the active form. 25(OH) vitamin D is further hydroxylated to the active form of vitamin D3 to 1,25 (OH)D (or calcitriol). 1,25(OH)2 Vitamin D produced by the kidneys enters into circulation and travels to its major target tissues such as the intestine and bone, where after interaction with its receptor enhances intestinal calcium adsorption and modulates the osteoclastic activity ().
Knowledge of the different ways of vitamin D synthesis is important to understand the available therapeutic options of vitamin D. For example, the pharmaceutical form of vitamin D in the United States is vitamin D2 (ergocalciferol), while in Canada, Europe, Japan and India, vitamin D3 (colecalciferol) is the principal pharmaceutical form.
Epidemiology of the vitamin-D deficiency
Older persons are prone to develop low vitamin D concentrations. This phenomenon is the effect of the reduced capacity of the skin to produce vitamin D. The reduced dermal synthesis of vitamin D is unlikely to be compensated by dietary intake of vitamin D in the elderly. The estimated worldwide prevalence of vitamin D deficiency among the elderly of about 50% underlines the importance of vitamin D deficiency for public health [Citation1].
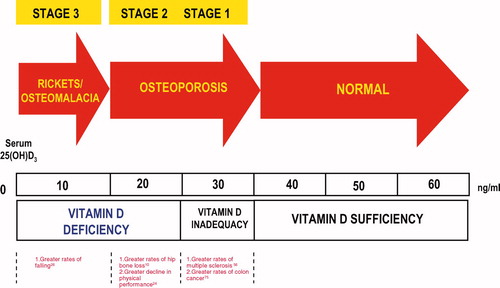
Much debate has taken place over the definition of vitamin D deficiency [Citation2]. There is evidence that 25(OH)D concentration <50 nmol/l, or 20 ng/ml, is an indicator of vitamin D deficiency, whereas a 25(OH)D concentration of 51–74 nmol/l, or 21–29 ng/ml indicate insufficiency; finally vitamin D serum levels >30 ng/l or 75 nmol/l suggest a status of vitamin D sufficiency [Citation3]. This evidence is based on intestinal calcium absorption that is maximised above 80 nmol/l, or 32 ng/ml, in postmenopausal women [Citation4]. On the other hand, parathyroid hormone (PTH) concentrations in adults continue to decline and reach their nadir at 75–100 nmol/l, or 30–40 ng/ml of vitamin D levels () [Citation4]. It has been assumed that children have the same demand of vitamin D of those of adults although no comparable studies have been carried out on intestinal calcium transport or PTH levels in this population [Citation4].
Independent of the cut-off used to define vitamin D deficiency, low levels of 25(OH)D are extremely common in older persons, and particularly in the oldest old and in the female sex. This is due to specific physiological and lifestyle factors linked to advanced age, such as impaired production of 7-dehydrocholesterol in the skin, insufficient exposure to sunlight (and/or excess clothing), poor dietary intake of vitamin D, as well as to chronic diseases, pharmacological treatments and disability [Citation5]. Although vitamin D deficiency has originally been reported more prevalent at higher latitudes, even free-living older Southern Europeans are at significant risk of developing vitamin D deficiency [Citation4].
In a recent population study performed in the Chianti area, known for its temperate climate and sunny countryside, a high prevalence was found of low 25(OH)D levels. Serum levels of vitamin D diminish with age in both sexes, but the decline starts substantially earlier and it is steeper in women from the perimenopausal period, while in men it becomes apparent 20 years later starting from 7th decade [Citation6]. In another study conducted in a U.S. cohort of older men in the United States, both vitamin D deficiency and insufficiency were common. Approximately one-fourth had 25(OH)D levels below the threshold of frank deficiency (<20 ng/ml), and the majority had vitamin D insufficiency (<30 ng/ml). Vitamin D deficiency was particularly common during the winter and spring time (especially in the northern communities) and in the oldest and more obese subjects. In fact, 86% of these subjects with multiple risk factors were vitamin D deficient [Citation7].
Causes of different decline in vitamin D levels in men and women
The distinctive pattern of age-related decline in 25(OH)D in men and women is unlikely to be explained by differences in the hormonal milieu between the two sexes. Although estrogens may modulate renal 1-alfa-hydoxylase activity [Citation6], 17-beta-estradiol is not recognised as a modulator of vitamin D or 25(OH)D production. Skin synthesis of 7-dehydrocholesterol is not influenced by estrogens, although skin thinning, an age-related factor capable of lowering serum 25(OH)D, does occur as a consequence of menopause [Citation6].
The crucial finding of the InCHIANTI study concerns the modulating effect of age on the PTH–25(OH)D relationship. Although a clear threshold in 25(OH)D levels below which calcium homeostasis is stressed and PTH increases was not identified, older participants need higher 25(OH)D levels to offset age-associated hyperparathyroidism, which inevitably determines bone loss and increases the risk of osteoporosis. Although the precise mechanisms explaining this phenomenon remain unclear, age-related changes in renal function (with the consequent decrease in production of 1,25(OH)2D) and resistance to suppression of PTH secretion mediated by 25(OH)D and 1,25(OH)2D, are possible causative factors [Citation6].
Mechanism of vitamin D on bone and other systems
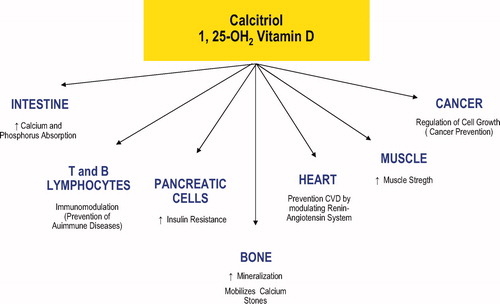
showed almost all sites of action of vitamin D. They include bone, muscle, heart, B and T lymphocytes, pancreatic cells and cell growth and differentiation. reports studies concerning the effects of vitamin D (associative and clinical trials) on various systems that is discussed below.
Table I. Studies of vitamin D (associative and clinical trials) on various systems.
Vitamin D and bone
Vitamin D deficiency has both direct and indirect consequences on bone cell function [Citation1]. Direct effects mainly concern reduced recruitment and differentiation of osteoclastic progenitors into mature osteoclasts, mostly mediated by osteoblast secretion of activating factors. There is also a diminished synthesis of specific collagenous and non-collagenous proteins in the osteoblasts. The main indirect consequences of vitamin D deficiency on bone cell function are defective mineralisation of osteoid seams, due to inadequate intestinal absorption of calcium and phosphate, and an age-related form of compensatory hyperparathyroidism, which drives an accelerated bone loss. Recently, it has been suggested that the minimal 25(OH)D serum concentrations needed to avoid compensatory hyperparathyroidism are significantly higher at older ages [Citation6]. In a prospective, observational study designed to analyse risk factors for fracture in an ambulatory population aged >55 years, 73 (88%) had evidence of osteopenia or osteoporosis (T-score <−1.5) and/or low 25VitD [Citation9]. Similar results were observed in patients enrolled in two Finnish hospitals for fracture during approximately 13 months with hip fracture, fresh of previous [Citation10].
Recent data from the Osteoporotic Fractures in Men (MrOS) study performed in healthy older men and focussing on osteoporosis showed that 25(OH)D level <20 ng/ml is associated with greater rates of hip bone loss, while rates of bone loss were similar among men with higher levels of total 25(OH)D [Citation9]. The association between 25(OH)D levels and bone loss was stronger among men 75 years and older [Citation8]. These findings suggest that low 25(OH)D levels are detrimental to bone mineral density (BMD) in older men [Citation8].
Bishoff-Ferrari et al. published a systematic review of English and non-English articles using MEDLINE and the Cochrane Controlled Trials Register (1960–2005), and EMBASE (1991–2005). They found that a vitamin D dose of 700–800 IU/day reduced the relative risk (RR) of hip fracture by 26% (three RCTs with 5572 persons; pooled RR, 0.74; 95% confidence interval [CI]: 0.61–0.88) and any non-vertebral fractures by 23% (five RCTs with 6098 persons; pooled RR, 0.77; 95% CI: 0.68–0.87) versus calcium or placebo. Oral vitamin D supplementation (700–800 IU/day) reduces the risk of hip and any non-vertebral fractures in ambulatory or institutionalised elderly persons [Citation34]. These data confirm previous results of two landmark randomised clinical trials [Citation35,Citation36].
By contrast, a recent review on the effect of vitamin D and vitamin D analogues for preventing fractures associated with involutional osteoporosis showed that institutionalised frail older people treated with vitamin D and calcium but not with Vitamin D alone may sustain fewer hip fractures [Citation33]. These results are also confirmed by an other study derived by pooled data seven major randomised trials of vitamin D with calcium or vitamin D alone, yielding a total of 68,517 participants [Citation47].
Vitamin D and skeletal muscle and falls
Muscle weakness has long been associated with vitamin D deficiency. A vitamin D receptor is present in skeletal muscle [Citation48], and vitamin D deficiency has been associated with proximal muscle weakness, increase in body sway and an increased risk of falling [Citation49].
Vitamin D deficiency in adults can also cause a skeletal mineralisation defect. The unmineralised osteoid provides little structural support for the periosteal covering. As a result, patients with osteomalacia often complain of isolated or global bone discomfort along with aches and pains in their joints and muscles [Citation50]. These patients may be misdiagnosed with fibromyalgia, dysthymia, degenerative joint disease, arthritis, chronic fatigue syndrome and other diseases [Citation51].
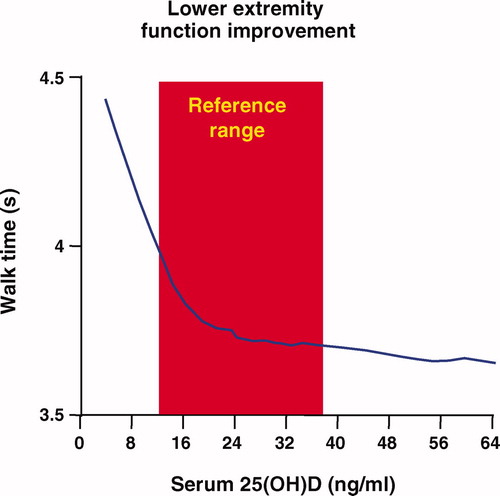
Speed performance and proximal muscle strength were markedly improved when 25-hydroxyvitamin D levels increased from 4 to 16 ng/ml (10–40 nmol/l) and continued to improve as the levels increased to more than 40 ng/ml (100 nmol/l) The relationship between walking speed and vitamin D serum levels are show in [Citation52]. Interestingly, persons with low (<25hairsp;nmol/l) baseline 25-OHD levels were 2.57 (95% confidence interval 1.40–4.70, based on grip strength) and 2.14 (0.73–6.33, based on muscle mass) times more likely to experience sarcopenia, compared with those with high (>50 nmol/l) levels [Citation13].
Low serum 25-hydroxyvitamin D levels have even been associated with impaired physical performance in a previous cross-sectional analysis conducted at baseline in the InCHIANTI study [Citation53,Citation54]. Low 25(OH)D may affect physical performance and frailty, defined as ‘a biologic syndrome of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems and causing vulnerability to adverse outcomes' [Citation53], via effects on muscle strength. Vitamin D receptors (VDRs) are located in skeletal muscle cells, and low 25(OH)D may result in decreased muscle strength from both decreased muscle synthesis and altered contractile properties of muscle. Muscle protein synthesis is initiated by binding 1,25-(OH)2D to its nuclear receptor. The influence of 1,25-(OH)2D on calcium homeostasis is believed to influence contractile properties of muscle cells via both a VDR-mediated genomic pathway and a non-genomic rapid mechanism. Thus, the association between low 25(OH)D and frailty may be explained by associations of insufficient 25(OH)D with sarcopenia and muscle weakness because both are a central role for the development of the frailty syndrome. In a cross-sectional study, 6-min walk distance was correlated with higher 25-hydroxyvitamin D (25OHD) level even in patients affected by chronic heart disease [Citation11]. In the Longitudinal Aging Study Amsterdam, an association between vitamin D and physical function has been described. Compared with individuals with serum 25-OHD levels above 30 ng/ml, physical performance was poorer in participants with serum 25-OHD less than 10 ng/ml [regression coefficient (B) = –1.69; 95% confidence interval (CI) = −2.28; −1.10], and with serum 25-OHD of 10–20 ng/ml (B = −0.46; 95% CI = −0.90; −0.03). After adjustment for confounding variables, participants with 25-OHD less than 10 ng/ml and 25-OHD between 10 and 20 ng/ml had significantly higher odds ratios (OR) for 3-year decline in physical performance (OR = 2.21; 95% CI = 1.00−4.87; and OR = 2.01; 95% CI = 1.06−3.81), compared with participants with 25-OHD of at least 30 ng/ml [Citation12].
In a meta-analysis recently published, this relationship was further investigated. Of the 102 selected studies, 16 met the selection criteria and were included in the final analysis. There were eight observational studies and eight intervention studies. The number of participants ranged from 24 to 33,067. A majority of studies examined community-dwelling older women. Five observational studies showed a significant positive association, whereas three studies did not. Four of the five studies and two of the three studies that tested the vitamin D supplementation effect, respectively on balance and gait, showed no significant effect. Four studies showed a significant effect on muscle strength, while this effect was not observed in three other studies. In addition, there was no significant association between vitamin D supplementation and an improvement of the sit-to-stand test in 50% of the studies. Authors concluded that the association between vitamin D and physical performance remains controversial. Observational studies and clinical trials yielded divergent results, which highlights the complex and to date still poorly understood association between serum vitamin D concentration or vitamin D supplementation and physical performance [Citation37].
Poor vitamin D status is independently associated with an increased risk of falling in the elderly, particularly in those aged 65–75 year. In a prospective cohort study of older persons enrolled in the Longitudinal Aging Study Amsterdam, low levels of 25(OH)D (<10 ng/ml) were associated with an increased risk of falling. After fully-adjustment, the odds ratios (95% confidence interval) were 1.78 (1.06–2.99) for subjects who experienced two falls or more as compared with those who did not fall or fell once for subjects who fell three or more times as compared with those who fell two times or less per years [Citation14].
A meta-analysis of five randomised clinical trials (with a total of 1237 subjects) revealed that increased vitamin D intake reduced the risk of falls by 22% (pooled corrected odds ratio, 0.78; 95% CI, 0.64−0.92) as compared with only calcium or placebo [Citation55]. The same meta-analysis examined the frequency of falls and suggested that 400 IU of vitamin D3 per day is not effective in preventing falls, whereas 800 IU of vitamin D3 per day plus calcium reduces the risk of falls (corrected pooled odds ratio, 0.65; 95% CI, 0.4−1.0) [Citation56]. In a randomised controlled trial conducted over a 5-month period, nursing home residents receiving 800 IU of vitamin D2 per day plus calcium had a 72% reduction in the risk of falls as compared with the placebo group (adjusted rate ratio, 0.28%; 95% CI, 0.11–0.75) [Citation57].
Recently, the same authors published a new meta-analysis reporting results from eight randomised controlled trials (n = 2426) of supplemental vitamin D and risk for falling [Citation38]. They found that high dose supplemental vitamin D (700–1000 IU/day vs. 200–600 IU/day) reduced risk of falling by 19% (pooled relative risk (RR): 0.81, 95% CI: 0.71–0.92; n = 1921 from seven trials), whereas achieved serum 25(OH)D concentrations of 60 nmol/L or more resulted in a 23% fall reduction (pooled RR: 0.77, 95% CI: 0.65–0.90). However, a recent review published by the Cochrane Library on randomised trials of interventions to reduce falls in community-dwelling older people, showed that only exercise interventions reduce risk and rate of falls. Authors concluded that research is needed to confirm the contexts in which multifactorial assessment and intervention, home safety interventions, vitamin D supplementation and other interventions are effective [Citation39].
Vitamin D and insulin resistance/diabetes
Hypovitaminosis D has long been suspected as a risk factor for glucose intolerance. The 25(OH)D concentration is lower in patients with type 2 diabetes than in the non-diabetic control subjects [Citation58]. A higher prevalence of hypovitaminosis D was noted in women affected by type 2 diabetes [Citation59]. The 25(OH)D concentrations were lower in patients at risk for diabetes than in the control group [Citation60]. Furthermore, hypovitaminosis D is associated with impaired insulin secretion in a population at high risk for diabetes [Citation60]. Hyper-responsive insulin secretion after a glucose challenge has been found in older men with hypovitaminosis D. Recent data show that, in glucose-tolerant subjects, 25(OH)D concentration has a positive relation with insulin sensitivity and a positive effect on ß cell function. These relations are independent of confounding factors [Citation61].
Several observations have linked vitamin D deficiency to alterations in circulating glucose and insulin levels and, possibly, insulin sensitivity [Citation19,Citation20]. Human studies suggest that increased vitamin D intake early in life may reduce the subsequent risk of type 1 diabetes. In one study, infants who received dietary supplementation with cod liver oil, a rich source of vitamin D, during their first year of life were found to have a reduced risk of type 1 diabetes [Citation62]. In the nationwide Diabetes Incidence Study in Sweden (DISS), the plasma 25OHD level is lower at diagnosis of autoimmune type 1 diabetes than in control subjects and may have a role in the development of type 1 diabetes [Citation21]. Similarly, the European Community sponsored Concerted Action on the Epidemiology and Prevention of Diabetes study found a 33% reduction in the risk of developing childhood-onset type 1 diabetes in children who received vitamin D supplementation compared with non-supplemented children (combined odds ratio: 0.67, 95% confidence interval: 0.53–0.85) [Citation63]. Moreover, a study in Finland found an association between dietary vitamin D supplementation in the first year of life and a reduced risk of type 1 diabetes mellitus, even after adjustment for social confounders [Citation64].
Studies in adults have also suggested that reduced vitamin D intake and circulating vitamin D concentrations are associated with reduced insulin sensitivity and an increased risk of developing the metabolic syndrome and type 2 diabetes mellitus. In the NHANES III cross-sectional survey of American adults 40–74 years of age, for example, serum 25-hydroxyvitamin D levels were inversely related to the presence of type 2 diabetes and to increased insulin resistance, with odds ratios for diabetes of 0.25 (95% confidence interval: 0.11–0.6) in non-Hispanic whites and 0.17 (95% confidence interval: 0.08–0.37) in Mexican Americans with 25-hydroxyvitamin D levels ≥81 nmol/l compared with those with levels ≤43.9 nmol/l [Citation65]. An inverse relation has also been observed between serum 25-hydroxyvitamin D levels and the prevalence of the metabolic syndrome in American adults [Citation66] and with approximately twice the rate (27.5% vs. 13.5%) in those with 25-hydroxyvitamin D levels ≤48.4 nmol/l compared with those with levels ≥96.4 nmol/l [Citation67].
The mechanisms of action of vitamin D on glucose and insulin metabolism are probably all mediated by its receptors. There is evidence that vitamin D may stimulate pancreatic insulin secretion directly. Vitamin D exerts its effects through nuclear vitamin D receptors [Citation68], which are found in a wide variety of tissues, including the pancreatic islet β-cells [Citation69]. However, the stimulatory effects of vitamin D on insulin secretion may be manifest only when calcium levels are adequate. Glucose-stimulated insulin secretion is lower in vitamin D-deficient rats when concurrent hypocalcaemia is not corrected than when it is [Citation70], whereas in vitro glucose-stimulated insulin release from pancreatic islet cells is stimulated by 1,25-dihydroxyvitamin D3 treatment in the presence but not absence of relatively high levels of calcium [Citation71].
The observed associations between vitamin D and insulin and glucose metabolism in human have not yet been confirmed by intervention studies. Hence, a causal association has not been established. In a non-randomised study of 10 women with type 2 diabetes, seven of whom were vitamin D deficient at baseline, there was a statistically significant 34% increase from baseline in first-phase insulin secretion during an intravenous glucose load after 1 month of treatment with oral cholecalciferol (D3) at 1332 IU/day [Citation72]. There is also an ongoing randomised placedo-controlled trial in older people testing the effect of the vitamin D supplementation on type 2 diabetes [Citation42].
Vitamin D and cognitive function
Recently, the presence of vitamin D receptor and the vitamin D activating enzyme, 1,-hydroxylase, in the brain has suggested a potential beneficial role of vitamin D in cognitive function. In details, the vitamin D receptor and catalytic enzymes are localised in the areas of the brain involved in complex planning, processing and the formation of new memories. These findings potentially implicate the role of vitamin D in neurocognitive function. Compelling evidence supports a beneficial role for the active form of vitamin D in the developing brain and in adult brain function. Vitamin D exhibits functional attributes that may prove neuroprotective through antioxidative mechanisms, neuronal calcium regulation, immunomodulation, enhanced nerve conduction and detoxification mechanisms [Citation73].
Patients who live at higher latitudes and are at risk of vitamin D deficiency are also more prone to developing schizophrenia [Citation74], and vitamin D deficiency has been associated with depression [Citation29,Citation75] and also with multiple sclerosis [Citation32,Citation76]. Recent studies suggest that vitamin D metabolites may be even important for preserving cognitive function via specific neuroprotective effects [Citation77].
In a recent paper [Citation78] was analysed the relationship between vitamin D and cognitive function. This editorial was focused on three papers, two cross-sectional and one longitudinal. In the latest, Slinin et al. [Citation30] reported results from a longitudinal assessment of community-dwelling men (65 years) participating in the Osteoporotic Fractures in Men (MrOS) Study, enrolled from 2000 to 2002 and followed for 4.6 years. Cognitive function was assessed using the modified Mini-Mental State Examination (3MS), a test of global cognitive function scored on a scale of 0–100 points, 12 and by the Trails B test, a timed test of executive function. Subjects were divided into quartiles based on baseline serum 25OHD concentrations, with the lowest quartile <20 ng/ml. At baseline, the odds ratios for cognitive impairment (defined as 3MS score < 80 or Trails B test time > 225 s) were between 1.6 and 1.8 in the lowest quartile of 25OHD concentrations compared to the highest quartile. However, these odds ratios did not reach statistical significance and were greatly attenuated after controlling for race/ethnicity and education. For incident cognitive impairment, the OR for a significant decline in 3MS score was 1.5 in the lowest quartile of 25OHD concentration compared with the highest quartile and the trend across the quartiles was significant. Control for confounding by race/ethnicity and education, however, slightly attenuated the trend, enough to loose statistical significance. Change in Trails B test time was not different among the 25OHD quartiles. The authors conclude that there is little evidence for an association between vitamin D status and concurrent or incident cognitive impairment. They suggest that additional studies should be carried out including women and tests of other cognitive domains.
Placebo-controlled intervention studies are also needed, to determine if vitamin D supplements will protect against age-related cognitive decline. In the meantime, neurologists and geriatricians should be aware of the high prevalence of vitamin D deficiency in their patient populations and the possibility that supplementation could be beneficial. Adequate intakes of vitamin D for ages 51–70 years and >70 years are currently defined as 10 μg/day (400 IU) and 15 μg/day (600 IU), respectively, or enough to maintain a 25(OH)-vitamin D level of 30 ng/mL or more. These intakes are primarily for maintaining bone health and are evolving standards. The appropriate intake amounts to support brain function in older adults remain to be determined. This conclusion is also in accordance with a recent meta-analysis [Citation31], where a revision of 99 selected studies has been made. Five observational studies met the selection criteria and were included in the final analysis. No prospective cohort study was present. The number of participants ranged from 32 to 9556 community-dwelling older adults (45–65% women). Three studies showed significant positive associations between serum 25OHD concentrations and global cognitive functions, whereas three other studies exploring specific aspects of cognition showed significant associations. The conclusion of this systematic review is that the association between serum 25OHD concentrations and cognitive performance is not yet clearly established.
Vitamin D and non-skeletal actions
The local production of 1,25-dihydroxyvitamin D3 in non-calcium-regulating tissues such as the colon, prostate and breast is thought to regulate up to 200 genes, which help to control cell growth and cellular differentiation and may be responsible for decreasing the risk of cells transforming into a malignant state [Citation79]. 1,25-dihydroxyvitamin D3 has been shown to inhibit cancer cell growth, induce cancer cell maturation, and apoptosis and decrease angiogenesis [Citation79]. Brain, prostate, breast and colon tissues, among others, as well as immune cells have a vitamin D receptor and respond to 1,25-dihydroxyvitamin D3, the active form of vitamin D [Citation29]. In addition, some of these tissues and cells express the enzyme 25-hydroxyvitamin D-1-hydroxylase [Citation79].
1,25-dihydroxyvitamin D3 has a immunomodulatory activity on monocytes and activated T and B lymphocytes [Citation80]. Then, increased production of 1,25-dihydroxyvitamin D3 results in synthesis of cathelicidin, a peptide capable of destroying M. tuberculosis and other infectious agents. When serum levels of 25-hydroxyvitamin D3 fall below 20 ng/ml (50 nmol/l), the monocyte or macrophage is prevented from initiating this innate immune response, which may explain why black Americans, often vitamin D deficient for the sun-protective character of dark skin, are more prone to contracting tuberculosis than white, and tend to have a more aggressive form of the disease. Observational studies that examined the association between low serum vitamin D and risk of active tuberculosis, found that the pooled effect size in random effects meta-analysis was 0.68 with 95% CI 0.43–0.93 [Citation26]. A double-blind randomised controlled trial conducted in 192 healthy adult M. tuberculosis, receiving a single oral dose of 2.5 mg vitamin D or placebo and followed up for 6 weeks, significantly enhanced the ability of participants' whole blood to restrict BCG-lux luminescence in vitro compared with placebo (mean luminescence ratio at follow-up, 0.57 vs. 0.71, respectively; 95% confidence interval for difference, 0.01–0.25; P = 0.03) [Citation46]. A systematic review of randomised controlled clinical trials that studied vitamin D for treatment or prevention of infectious diseases in humans supports further research into adjunctive vitamin D therapy for tuberculosis, influenza and viral upper respiratory tract illnesses [Citation45].
Evidence of diseases associated with vitamin-D deficiency
Cancer and vitamin D
More than 80 years ago, it was reported that living at higher latitudes in the United States is associated with an increased risk of dying of common cancers [Citation81]. In the 1980s and 1990s, several studies confirmed that living at higher latitudes increased the risk of developing and dying of colon, prostate, breast and several other cancers [Citation82]. Because living at higher latitudes diminishes vitamin D production, an association between vitamin D deficiency and cancer mortality was hypothesised. Both men and women exposed to the most sunlight throughout their lives were less likely to die of cancer. Several retrospective and prospective studies with data available on concentrations of vitamin D showed that vitamin D deficiency increases the risk of developing and dying from cancer [Citation83]. It has been reported that adults with vitamin D of <50 nmol/l who were then followed for up to 19 years had a 30–50% have an increased risk of developing colorectal, breast, prostate and many other cancers [Citation83]. A meta-analysis showed that increasing intake of vitamin D to 1000 IU vitamin D3/day would be associated with a decreased risk of colorectal and breast cancer of as much as 50% [Citation84]. Men who ingested >400 IU vitamin D/day had a markedly reduced risk of developing several cancers, including pancreas and oesophagus and non-Hodgkin lymphoma [Citation83]. Lappe et al. [Citation85] reported that postmenopausal women who received 1100 IU vitamin D3 and 1000 mg Ca daily for 4 years reduced their risk of developing cancer by 60%.
However, recent meta-analysis of longitudinal studies and clinical trials showed no association between vitamin D and prostate cancer (PC) [Citation22,Citation44], whereas several studies reporting the association of vitamin D and colon cancer risk showed an inverse relationship between vitamin D and the development of colon cancer [Citation23–25]. This association has not been confirmed in the largest clinical trial realised in postmenopausal women [Citation43].
Autoimmune diseases and vitamin D
The regulatory role of vitamin D in modulating the immune system activity includes inhibitory effects on T cells, B cells and dendritic cells [Citation86]. These suppressive immunologic properties have led to considering its role in autoimmune diseases. Vitamin D has also profound effects on dendritic cells. Dendritic cells have important functions in maintaining both protective immunity and self-tolerance, as immature dendritic cells promote T cell tolerance, whereas mature dendritic cells activate naïve T cells. Mechanisms of action of vitamin D on dendritic cells include actions on the differentiation of monocytes into immature dendritic cells, their maturation and survival. In addition to its functions in maintaining self-tolerance, vitamin D has an important role in protective immunity.
There is a growing body of epidemiologic data linking low levels of serum 25D with autoimmune diseases, such as rheumatoid arthritis (RA) [Citation86] and inflammatory bowel disease [Citation87]. A prospective study on the relationship between vitamin D intake and the risk of RA found that a higher vitamin D intake at baseline provided significant protection from subsequent development of the disease [Citation86]. Women who received >400 IU vitamin D/day were found to have a >40% reduced risk of developing rheumatoid arthritis [Citation27]. However, these findings in RA could not be replicated in a study published this year, which used comparable methods in a different cohort [Citation88].
There is also evidence of an association between vitamin D deficiency and systemic lupus erythematosus (SLE). Initial insights into the prevalence of vitamin D deficiency in SLE come from studies primarily focussed on bone health. The first study measuring vitamin D levels in SLE reported a deficiency of 1,25D in seven of 12 corticosteroid-receiving adolescents [Citation89]. Subsequent studies measuring vitamin D in the context of either bone mineral density (BMD) or fractures or both included a study documenting severe 25 (OH)D deficiency (<25hairsp;nmol/l or 1 ng/ml) in 8% of 107 consecutive patients from the Netherlands [Citation90]. A second study examining BMD in patients with newly diagnosed SLE, established SLE on corticosteroids and age-matched controls reported mean 25(OH)D levels of 27.2 ± 10.05 ng/ml, 19.6 ± 11.9 ng/ml and 40.45 ± 18 ng/ml (respectively) with statistically lower 25(OH)D levels in patients with established SLE compared with controls [Citation91].
A cross-sectional study was specifically evaluated vitamin D levels in SLE come from Copenhagen in young patients. They reported statistically lower levels of 25(OH)D in 21 patients with SLE (mean 13 ng/ml) in comparison with 29 patients with RA (mean 24 ng/ml), patients with osteoarthritis (mean 32 ng/ml) [Citation28]. A second cross-sectional Canadian study of 25 Caucasian patients with SLE reported that more than 50% were vitamin D deficient (using a cutoff of <50 nmol/L or 20 ng/ml) [Citation92].
A recent cross-sectional study recently published has confirmed these results in patients from Shanghai area. Levels of 25D were significantly lower in patients with SLE (11.5 ng/ml) than in patients with RA (54.6 ng/ml) or controls (59.2 ng/ml) [Citation93]. Another recent study from Israel determined 25D levels in a number of autoimmune diseases including MS, myositis, RA, autoimmune thyroid disease and SLE. The mean 25D levels for all diseases were below 20 ng/ml. Patients with SLE (n = 138) had a mean 25D level of 11.9 ± 11.1 ng/ml, which was significantly lower than the mean of 21.6 ng/ml in European controls [Citation94].
Many studies, but not all, have documented an association between higher disease activity and a low level of vitamin D. A significant negative correlation between 25D and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) and European Consensus Lupus Activity Measurement (ECLAM) scores was reported in European patients [Citation86].
Cardiovascular disease, all-cause mortality and vitamin D
It has been recently shown that low levels of 25-hydroxyvitamin D [25(OH)D] are also independently associated with cardiovascular events in patients with and without hypertension suggesting a role of vitamin D for the maintenance of cardiovascular health [Citation15,Citation95,Citation96]. This hypothesis is further supported by the ability of vitamin D to suppress the renin-angiotensin-aldosterone system (RAAS) [Citation16]. Vitamin D deficiency predisposes to up-regulation of the RAAS and hypertrophy of both the left ventricle and vascular smooth muscle cells [Citation17,Citation97]. Furthermore, there is accumulating evidence that vitamin D deficiency may contribute to myocardial dysfunction and arterial hypertension through an increase of the parathyroid hormone that directly produces an increase of blood pressure and an increase of cardiac contractility [Citation98]. Finally, in addition to RAAS activation, the up-regulation of the immune system is often implicated in the pathophysiology of cardiovascular disease [Citation98]. Experimental studies have suggested that vitamin D plays a role in the regulation of several important inflammatory cytokines (such as IL–6 and TNF-alpha) [Citation98]. It has been shown that low levels of 25(OH)D are an independent risk factor of total and cardiovascular mortality in a large cohort of patients referred to coronary angiography [Citation18]. These results are in line with a recent meta-analysis, in which a significant reduction of all-cause mortality was reported for persons receiving vitamin D supplementation [Citation40]. Most of these studies were performed in frail elderly people with vitamin D deficiency [Citation40].
The association between serum 25(OH)D levels and all-cause mortality was also addressed by the Longitudinal Aging Study Amsterdam (LASA), which included 1260 community-dwelling persons aged 65 years and older at baseline. In that study, low vitamin D status was a significant predictor of mortality after adjustments for possible confounders [Citation99].
There is growing evidence that low serum 25(OH)D levels may contribute to heart failure. Vitamin D treatment is associated with improved diastolic function and a regression of myocardial hypertrophy in patients with haemodialysis [Citation100]. Carotid intima-media thickness was also found to be inversely and independently correlated with serum 25(OH)D levels. Recent data from NHANES-III also showed that low serum 25(OH)D concentrations are associated with a higher prevalence of peripheral arterial disease [Citation101]. Furthermore, results from the Framingham Offspring Study showed that patients with 25(OH)D levels below 15 ng/mL (37.5 nmol/l) are at increased risk of incident cardiovascular events, even after adjustments for conventional cardiovascular risk factors [Citation102].
Options for vitamin D therapy
Three options are commonly used to treat vitamin D deficiency including sunlight, artificial UVB light and vitamin D supplements. An exposure of 10–15 min of full-body summer noon-day sun or artificial UVB radiation (such as tanning beds) will input more than 10,000 IU of vitamin D into the systemic circulation of most light-skinned adults. One or two such exposures per week should maintain 25(OH) D levels in an ideal range.
Holick et al. [Citation4] recently reported that for every 100 IU of vitamin D2 or vitamin D3 ingested, there is an increase in circulating 25(OH)D levels of only 1 ng/ml, providing some explanation for why men reporting supplement use have marginally higher concentrations.
The treatment of choice for vitamin D deficiency is vitamin D, cholecalciferol, also known as vitamin D3. Cholecalciferol is available in 400, 1000, 2000, 5000, 10,000, 50,000 and even 300,000 UI capsules. Supplementation with 1000 IU per day will usually result in about a 10 ng/ml elevation of serum 25 (OH) D when given over 3–4 months. Formulation of vitamin D3 of 300,000 IU is also present as intramuscular options, and given 300,000 IU annually corresponds to about 800 IU daily.
The only prescription of vitamin D preparation available in the United States is the vitamin D analogue ergocalciferol (Vitamin D2), available as 50,000 IU capsules.
Specific conditions
Primary and secondary prevention for bone loss: cholecalfiferol 1000 IU/day plus calcium 1 g/day or cholecalfiferol 300,000 IU i.m. biannually.
Inflammatory bowel diseases (IBD): cholecalfiferol 300,000 IU i.m. biannually.
Liver diseases: calcifediol (25 (OH) D3) or 1-alpha (OH) calcidiol.
Kidney diseases: calcitriol 0.5 μg/day plus calcium 1 g/day.
Response to vitamin D treatment
In patients with any stage of chronic kidney disease, 25-hydroxyvitamin D should be measured annually, targeting vitamin D levels of 30 ng/ml or higher, as recommended in the Kidney Disease Outcomes Quality Initiative guidelines from the National Kidney Foundation [Citation103]. It is a misconception to assume that patients taking an active vitamin D analogue have sufficient vitamin D because the response to treatment is not equal in all individuals. Levels of 25-hydroxyvitamin D are inversely associated with parathyroid hormone levels, regardless of the degree of chronic renal failure. Parathyroid glands convert 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, which directly inhibits parathyroid hormone expression. Patients with stage 4 or 5 chronic kidney disease and an estimated glomerular filtration rate of less than 30 ml per minute per 1.73 m² of body-surface area, as well as those requiring dialysis, are unable to make enough 1,25-dihydroxyvitamin D and need to take 1,25-dihydroxyvitamin D3 or one of its less calcaemic analogues to maintain calcium metabolism and to decrease parathyroid hormone levels and the risk of renal bone disease. However, vitamin D compounds do not consistently reduce PTH levels and beneficial effects on patient-level outcomes are unproven [Citation41].
Patients with mild or moderate hepatic failure or intestinal fat-malabsorption syndromes, as well as patients who are taking anticonvulsant medications, glucocorticoids, or other drugs that activate steroid and xenobiotic receptor, require higher doses of vitamin D [Citation104].
In conclusion, vitamin D appears to be an hormone with several actions and is fundamental for many biological systems including bone, skeletal muscle and heart.
Recent studies suggest that assessment of vitamin D status should be recommended not only for prevention and treatment of osteoporosis but also in the global evaluation of cardiovascular disease, sarcopenia, insulin-resistance and cancer in older population.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- HolickMF. Vitamin D deficiency. N Engl J Med 2007;357:266–281.
- MalabananA, VeronikisIE, HolickMF. Redefining vitamin D insufficiency. Lancet 1998;351:805–806.
- HolickMF. MrOs is deficient. J Clin Endocrinol Metab 2009;94:1092–1093.
- HolickMF, ChenTC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080S–1086S.
- OudshoornC, van der CammenTJ, McMurdoME, van LeeuwenJP, ColinEM. Ageing and vitamin D deficiency: effects on calcium homeostasis and considerations for vitamin D supplementation. Br J Nutr 2009;101:1597–1606.
- MaggioD, CherubiniA, LauretaniF, RussoRC, BartaliB, PierandreiM, RuggieroC, MacchiaruloMC, GiorginoR, MinisolaS, et al25(OH)D Serum levels decline with age earlier in women than in men and less efficiently prevent compensatory hyperparathyroidism in older adults. J Gerontol A Biol Sci Med Sci 2005;60:1414–1419.
- OrwollE, NielsonCM, MarshallLM, LambertL, HoltonKF, HoffmanAR, Barrett-ConnorE, ShikanyJM, DamT, CauleyJA; Osteoporotic Fractures in Men (MrOS) Study Group. Vitamin D deficiency in older men. J Clin Endocrinol Metab 2009;94:1214–1222.
- EnsrudKE, TaylorBC, PaudelML, et alSerum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab 2009;94:2773–2780.
- SetonM, JacksonV, LasserKE, DoppeltS, Pierre-JacquesM, ConnellyM. Low 25-hydroxyvitamin D and osteopenia are prevalent in persons > or = 55 yr with fracture at any site: a prospective, observational study of persons fracturing in the community. J Clin Densitom 2005;8:454–460.
- NurmiI, KaukonenJP, LüthjeP, NaboulsiH, TanninenS, KatajaM, KallioML, LeppilampiM. Half of the patients with an acute hip fracture suffer from hypovitaminosis D: a prospective study in southeastern Finland. Osteoporos Int 2005;16:2018–2024.
- BoxerRS, DauserDA, WalshSJ, HagerWD, KennyAM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc 2008;56:454–461.
- WichertsIS, van SchoorNM, BoekeAJ, VisserM, DeegDJ, SmitJ, KnolDL, LipsP. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab 2007;92:2058–2065.
- VisserM, DeegDJ, LipsP; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 2003;88:5766–5772.
- SnijderMB, van SchoorNM, PluijmSM, van DamRM, VisserM, LipsP. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab 2006;91:2980–2985.
- PilzS, DobnigH, NijpelsG, HeineRJ, StehouwerCD, SnijderMB, van DamRM, DekkerJM. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 2009;71:666–672.
- LiYC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem 2003;88:327–331.
- AchingerSG, AyusJC. The role of vitamin D in left ventricular hypertrophy and cardiac function. Kidney Int Suppl 2005;95:S37–S42.
- PilzS, MärzW, WellnitzB, SeelhorstU, Fahrleitner-PammerA, DimaiHP, BoehmBO, DobnigH. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008;93:3927–3935.
- LiuE, MeigsJB, PittasAG, McKeownNM, EconomosCD, BoothSL, JacquesPF. Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr 2009;139:329–334.
- KnektP, LaaksonenM, MattilaC, HärkänenT, MarniemiJ, HeliövaaraM, RissanenH, MontonenJ, ReunanenA. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008;19:666–671.
- SteneLC, JonerG. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr 2003;78:1128–1134.
- YinL, RaumE, HaugU, ArndtV, BrennerH. Meta-analysis of longitudinal studies: Serum vitamin D and prostate cancer risk. Cancer Epidemiol 2009;33:435–445.
- YinL, GrandiN, RaumE, HaugU, ArndtV, BrennerH. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther 2009;30:113–125.
- NgK, WolpinBM, MeyerhardtJA, WuK, ChanAT, HollisBW, GiovannucciEL, StampferMJ, WillettWC, FuchsCS. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer 2009;101:916–923.
- FreedmanDM, LookerAC, ChangSC, GraubardBI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst 2007;99:1594–1602.
- NnoahamKE, ClarkeA. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol 2008;37:113–119.
- MerlinoLA, CurtisJ, MikulsTR, CerhanJR, CriswellLA, SaagKG. Vitamin D intake is inversely associated with rheumatoid arthritis. Arthritis Rheum 2004;50:72–77.
- MüllerK, KriegbaumNJ, BaslundB, SørensenOH, ThymannM, BentzenK. Vitamin D3 metabolism in patients with rheumatic diseases: low serum levels of 25-hydroxyvitamin D3 in patients with systemic lupus erythematosus. Clin Rheumatol 1995;14:397–400.
- HoogendijkWJ, LipsP, DikMG, DeegDJ, BeekmanAT, PenninxBW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry 2008;65:508–512.
- SlininY, PaudelML, TaylorBC, FinkHA, IshaniA, CanalesMT, YaffeK, Barrett-ConnorE, OrwollES, ShikanyJM, et alOsteoporotic Fractures in Men (MrOS) Study Research Group. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology 2010;74:33–41.
- AnnweilerC, AllaliG, AllainP, BridenbaughS, SchottAM, KressigRW, BeauchetO. Vitamin D and cognitive performance in adults: a systematic review. Eur J Neurol 2009;16:1083–1089.
- MungerKL, LevinLI, HollisBW, HowardNS, AscherioA. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838.
- AvenellA, GillespieWJ, GillespieLD, O'ConnellD. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev 2009;2: CD000227.
- Bischoff-FerrariHA, Dawson-HughesB, BaronJA, BurckhardtP, LiR, SpiegelmanD, SpeckerB, OravJE, WongJB, StaehelinHB, et alCalcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr 2007;86:1780–1790.
- Dawson-HughesB, HarrisSS, KrallEA, DallalGE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670–676.
- LipsP, GraafmansWC, OomsME, BezemerPD, BouterLM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med 1996;124:400–406.
- AnnweilerC, SchottAM, BerrutG, FantinoB, BeauchetO. Vitamin d-related changes in physical performance: a systematic review. J Nutr Health Aging 2009;13:893–898.
- Bischoff-FerrariHA, Dawson-HughesB, StaehelinHB, OravJE, StuckAE, TheilerR, WongJB, EgliA, KielDP, HenschkowskiJ. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 2009;339:3692.
- GillespieLD, RobertsonMC, GillespieWJ, LambSE, GatesS, CummingRG, RoweBH. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2009;15: CD007146.
- AutierP, GandiniS. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007;167:1730–1737.
- PalmerSC, McGregorDO, MacaskillP, CraigJC, ElderGJ, StrippoliGF. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med 2007;147:840–853.
- AvenellA, CookJA, MacLennan, G.S., McPhersonGC; RECORD trial group. Vitamin D supplementation and type 2 diabetes: a substudy of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing 2009;38:606–609.
- Wactawski-WendeJ, KotchenJM, AndersonGL, et alCalcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684–696.
- VijayakumarS, MehtaRR, BoernerPS, PackianathanS, MehtaRG. Clinical trials involving vitamin D analogs in prostate cancer. Cancer J 2005;11:362–373.
- YamshchikovAV, DesaiNS, BlumbergHM, ZieglerTR, TangprichaV. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract 2009;15:438–449.
- WejseC, GomesVF, RabnaP, GustafsonP, AabyP, LisseIM, AndersenPL, GlerupH, SodemannM. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2009;179:843–850.
- DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ 2010;340:b5463.
- SimpsonRU, ThomasGA, ArnoldAJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem 1985;260:8882–8891.
- DamTT, von MühlenD, Barrett-ConnorEL. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int 2009;20:751–760.
- HicksGE, ShardellM, MillerRR, BandinelliS, GuralnikJM, CherubiniA, LauretaniF, FerrucciL. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc 2008;56:785–791.
- PlotnikoffGA, QuigleyJM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc 2003;78:1463–1470.
- Bischoff-FerrariHA, DietrichT, OravEJ, HuFB, ZhangY, KarlsonEW, Dawson-HughesB. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or = 60 y. Am J Clin Nutr 2004;80:752–758.
- ShardellM, HicksGE, MillerRR, KritchevskyS, AndersenD, BandinelliS, CherubiniA, FerrucciL. Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci 2009;64:69–75.
- HoustonDK, CesariM, FerrucciL, CherubiniA, MaggioD, BartaliB, JohnsonMA, SchwartzGG, KritchevskySB. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2007;62:440–446.
- ViethR. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol 2004;89:575–579.
- Bischoff-FerrariHA, Dawson-HughesB, WillettWC, StaehelinHB, BazemoreMG, ZeeRY, WongJB. Effect of Vitamin D on falls: a meta-analysis. JAMA 2004;291:1999–2006.
- Bischoff-FerrariHA, WillettWC, WongJB, GiovannucciE, DietrichT, Dawson-HughesB. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005;293:2257–2264.
- PietschmannP, SchernthanerG, WoloszczukW. Serum osteocalcin levels in diabetes mellitus: analysis of the type of diabetes and microvascular complications. Diabetologia 1998;31:892–895.
- IsaiaG, GiorginoR, AdamiS. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care 2001;24:1496.
- BoucherBJ, MannanN, NoonanK, HalesCN, EvansSJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia 1995;38:1239–1245.
- ChiuKC, ChuA, GoVL, SaadMF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004;79:820–825.
- LittorinB, BlomP, SchölinA, ArnqvistHJ, BlohméG, BolinderJ, Ekbom-SchnellA, ErikssonJW, GudbjörnsdottirS, NyströmL, et alLower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006;49:2847–2852.
- The EURODIAB Substudy 2 Study Group. Vitamin D supplement in early childhood and risk for type I (insulin-dependent) diabetes mellitus. Diabetologia 1999;42:51–54.
- HypponenE, LaaraE, ReunanenA, JarvelinMR, VirtanenSM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001;358:1500–1503.
- ScraggR, SowersM, BellC. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–2818.
- FordE S., AjaniUA, McGuireLC, LiuS. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 2005;28:1228–1230.
- BoucherBJ. Inadequate vitamin D status: does it contribute to the disorders comprising syndrome ‘X’. Br J Nutr 1998;79:315–327.
- ZeitzU, WeberK, SoegiartoDW, WolfE, BallingR, ErbenRG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 2003;17:509–511.
- WaltersMR. Newly identified actions of the vitamin D endocrine system. Endocr Rev 1992;13:719–764.
- BeaulieuC, KestekianR, HavrankovaJ, Gascon-BarreM. Calcium is essential in normalizing intolerance to glucose that accompanies vitamin D depletion in vivo. Diabetes 1993;42:35–43.
- IshidaH, SeinoY, SeinoYS, TsudaK, TakemuraJ, NishiS, IshizukaS, ImuraH. Effect of 1,25-dihydroxyvitamin D3 on pancreatic B and D cell function. Life Sci 1983;33:1779–1786.
- BorissovaAM, TankovaT, KirilovG, DakovskaL, KovachevaR. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract 2003;57:258–261.
- BuellJS, Dawson-HughesB. Vitamin D and neurocognitive dysfunction: Preventing “D”ecline?Mol Aspects Med 2008;29:415–422.
- McGrathJ, SeltenJP, ChantD. Long-term trends in sunshine duration and its association with schizophrenia birth rates and age at first registration data from Australia and the Netherlands. Schizophr Res 2002;54:199–212.
- GlothFMIII, AlamW, HollisB. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal effective disorder. J Nutr Health Aging 1999;3:5–7.
- Soilu-HänninenM, LaaksonenM, LaitinenI, ErälinnaJP, LiliusEM, MononenI. A longitudinal study of serum 25-hydroxyvitamin D and intact parathyroid hormone levels indicate the importance of vitamin D and calcium homeostasis regulation in multiple sclerosis. J Neurol Neurosurg Psychiatry 2008;79:152–157.
- LeeDM, TajarA, UlubaevA, PendletonN, O'NeillTW, O'ConnorDB, BartfaiG, BoonenS, BouillonR, CasanuevaFF, et alEMAS study group. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry 2009;80:722–729.
- MillerJW. Vitamin D and cognitive function in older adults: are we concerned about vitamin D-mentia?Neurology 2010;74:13–15.
- NagpalS, NaS, RathnachalamR. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005;26:662–687.
- MathieuC, AdoriniL. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med 2002;8:174–179.
- ApperlyFL. The relation of solar radiation to cancer mortality in North America. Cancer Res 1941;1:191–195.
- GarlandCF, ComstockGW, GarlandFC, HelsingKJ, ShawEK, GorhamED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet 1989;2:1176–1178.
- GiovannucciE, LiuY, RimmEB, HollisBW, FuchsCS, StampferMJ, WillettWC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98:451–459.
- GarlandC, ShekelleRB, Barrett-ConnorE, CriquiMH, RossofAH, OglesbyP. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet 2005;9:307–309.
- LappeJM, Travers-GustafsonD, DaviesKM, ReckerRR, HeaneyRP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007;85:1586–1591.
- KamenD, AranowC. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol 2008;20:532–537.
- JahnsenJ, FalchJA, MowinckelP, AadlandE. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand J Gastroenterol 2002;37:192–199.
- CostenbaderKH, FeskanichD, HolmesM, KarlsonEW, Benito-GarciaE. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis 2008;67:530–535.
- O'ReganS, ChesneyRW, HamstraA, EismanJA, O'GormanAM, DelucaHF. Reduced serum 1,25-(OH)2 vitamin D3 levels in prednisone-treated adolescents with systemic lupus erythematosus. Acta Paediatr Scand 1979;68:109–111.
- BultinkIE, LemsWF, KostensePJ, DijkmansBA, VoskuylAE. Prevalence of and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum 2005;52:2044–2050.
- TeichmannJ, LangeU, StrackeH, FederlinK, BretzelRG. Bone metabolism and bone mineral density of systemic lupus erythematosus at the time of diagnosis. Rheumatol Int 1999;18:137–140.
- HuismanAM, WhiteKP, AlgraA, HarthM, ViethR, JacobsJW, BijlsmaJW, BellDA. Vitamin D levels in women with systemic lupus erythematosus and fibromyalgia. J Rheumatol 2001;28:2535–2539.
- ChenS, SimsGP, ChenXX, GuYY, ChenS, LipskyPE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007;179:1634–1647.
- OrbachH, Zandman-GoddardG, AmitalH, BarakV, SzekaneczZ, SzucsG, DankoK, NagyE, CsepanyT, CarvalhoJF, et alNovel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci 2007;1109:385–400.
- SembaRD, HoustonDK, BandinelliS, SunK, CherubiniA, CappolaAR, GuralnikJM, FerrucciL. Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr 2010;64:203–209.
- GindeAA, ScraggR, Schwartz, RS, CamargoCAJr.Prospective study of serum 25-hydroxyvitamin d level, cardiovascular disease mortality, and all-cause mortality in older U.S. Adults. J Am Geriatr Soc 2009;57:1595–1603.
- LeeJH, O'KeefeJH, BellD, HensrudDD, HolickMF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor?J Am Coll Cardiol 2008;52:1949–1956.
- NemerovskiCW, DorschMP, SimpsonRU, BoneHG, AaronsonKD, BleskeBE. Vitamin D and cardiovascular disease. Pharmacotherapy 2009;29:691–708.
- DobnigH, PilzS, ScharnaglH, RennerW, SeelhorstU, WellnitzB, KinkeldeiJ, BoehmBO, WeihrauchG, MaerzW. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–1349.
- VisserM, DeegDJ, PutsMT, SeidellJC, LipsP. Low serum concentrations of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. Am J Clin Nutr 2006;84:616–622.
- MelamedML, MichosE, PostW, AstorB. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168:1629–1637.
- WangTJ, PencinaMJ, BoothSL, JacquesPF, IngelssonE, LanierK, BenjaminEJ, D'AgostinoRB, WolfM, VasanRS. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–511.
- ArenasMD, Alvarez-UdeF, GilMT, SorianoA, EgeaJJ, MillánI, AmoedoML, MurayS, CarretónMA. Application of NKF-K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease: changes of clinical practices and their effects on outcomes and quality standards in three haemodialysis units. Nephrol Dial Trasplant 2006;21:1663–1668.
- BhuttoA, MorleyJE. The clinical significance of gastrointestinal changes with aging. Curr Opin Clin Nutr Metab Care 2008;11:651–660.