Abstract
Objective. Low total testosterone levels (TT) have been associated with increased morbidity and mortality. However, the prevalence and incidence of testosterone deficiency (TD) in association with its risk has not been assessed systematically to date.
Methods. Data from the prospective population-based Study of Health in Pomerania were used. From the 2117 men aged 20–79 years at baseline, 1490 men with complete TT data were analysed. Crude and age-specific prevalence and incidence rates of TD were estimated by TT levels below the age-specific 10th percentile. Analysis of covariance and Poisson regression models were used to assess the association of socio-demographic characteristics, health-related lifestyle, as well as somatometric, medical and laboratory measures with risk of incident TD.
Results. TD baseline prevalence was 10.4% (N = 155) and incidence 11.7 per 1000 person-years. TT levels showed a significant age-related decline with an unadjusted rate of 0.05 nmol/l per year. Obesity, metabolic syndrome, diabetes and dyslipidaemia were identified as risk factors of incident TD. Subpopulations of men without the revealed risk factors at both examinations maintained constant TT levels over time.
Conclusions. Besides aging alone, lifestyle and different comorbidities were associated with TT level decline, suggesting that the age-related TT decline may be at least partly prevented through the management of potentially modifiable risk factors and health related behaviour.
Introduction
Low levels of total testosterone (TT) have been shown to predict increased morbidity due to metabolic syndrome (MetS) [Citation1,Citation2], stroke [Citation3], atherosclerosis [Citation4,Citation5] and type 2 diabetes [Citation6]. However, the prevalence of low TT levels or testosterone deficiency (TD) in male populations is not known with certainty, mainly due to a lack of consensus about the TT threshold level to define TD. Most investigators use a TT level <10.4 nmol/l (translates to approximately 300 ng/dl using the factor 0.0347) [Citation7], while others advocate cut-off points of 6.94 nmol/l (200 ng/dl) [Citation8], 8.7 nmol/l (251 ng/dl) [Citation9] or 11.7 nmol/l (337 ng/dl) [Citation10]. Although recent guidelines state that there are ‘no generally accepted lower limits of normal’ and recommend cut-offs between 8 and 12 nmol/l (231 and 346 ng/dl, respectively) for treatment decision [Citation11]; serum TT levels between 12 and 40 nmol/l were considered normal in the adult male of all ages [Citation12]. Accordingly, prevalence estimates of TD vary between 5 and 24% [Citation7,Citation8,Citation13–16] with substantial variation according to age, applied definition of TD and study population. Prevalence of TD in primary care in Germany was estimated almost 20% [Citation17]. One of the rare estimates for the incidence of TD (12.3 per 1000 person-years) was reported from the Massachusetts Male Aging Study [Citation8]. To the best of our knowledge, there are no population-based European studies about the prevalence and incidence of TD in healthy males from the community.
Although it has been widely observed that male serum TT levels decline with age [Citation18–21], the data have not been conclusive. While cross-sectional studies have demonstrated very different rates of decline, or even failed to observe a significant decrease in TT with increasing age, longitudinal studies have shown comparably more steep age-related declines [Citation22]. However, recently published findings from longitudinal studies reported an age-independent decline in TT levels [Citation22,Citation23], suggesting different mechanisms involved in the fall of TT levels in the aging men. At this, comorbidity, chronic disease and health-related lifestyle are suggested to accentuate and contribute to these changes [Citation24]. Clarification of the causes of these changes is believed to provide information that preventive action can be taken [Citation25].
However, due to the growing evidence for a secular decline in aging male's TT levels and the persisting uncertainty whether the androgen requirements of elderly men are the same as those of young men, we used age-specific cut-offs for the definition of TD. In comparison with alternative cut-offs for the definition of TD, this study aimed to assess the prevalence and incidence of TD in 1490 men enrolled in the longitudinal population-based Study of Health in Pomerania (SHIP). Furthermore, we aimed to identify risk factors of incident TD from a variety of socio-demographic and health-related lifestyle characteristics, as well as subclinical and clinical conditions; to further investigate the effect of maintained health on TT level trend.
Methods
Study population
SHIP is a population-based cohort study conducted in the north-eastern area of Germany, previously described in more detail [Citation26]. Only individuals with German citizenship and main residency in the study area were included. Selected persons received a maximum of three written invitations. In case of non-response, letters were followed by a phone call or by home visits if contact by phone was not possible. For the baseline examinations, a sample of 3105 eligible men aged 20–79 years was drawn. Finally, 2117 men participated between 1997 and 2001 in the baseline study (response 68.2%). Between 2002 and 2006 all participants were re-invited for follow-up, in which 1589 men (84.4% of eligible men) took part. All participants gave informed written consent. The study was monitored by a review board of independent scientists, and the study protocol is consistent with the principles of the Declaration of Helsinki, as reflected by an a priori approval of the Ethics Committee of the University of Greifswald. Among the 1589 male follow-up participants, complete TT data were available in 1507 men. From that, we excluded men who used sexual hormones (anatomic-therapeutical-chemical [ATC] code G03) (N = 3), testosterone 5α reductase inhibitors (ATC code G04CB) (N = 9) or sexual hormone antagonists (ATC code L02B) (N = 5) at any examination; analysing a final study population of 1490 men.
Measures
Baseline data included a computer-assisted personal interview on socio-demographic characteristics and health-related lifestyle, as well as somatometric, medical and laboratory examinations. Socio-demographic characteristics comprised age, sex, educational level, civil status, occupational level and equalised household income [Citation27]. Information about health-related lifestyle comprised smoking status, high-risk alcohol consumption (>30 g alcohol/day) [Citation28], low physical activity (<1 h/week physical training during summer or winter), dietary intake (measured from a validated food-frequency score (FFS) reflecting the food quality [Citation29] and self-rated health. Healthy lifestyle characteristics included: non-smoking, body mass index (BMI) of 18.5–25.0 kg/m2, consuming five or more fruits and vegetables per day and regular physical activity (≥30 min for ≥5 times per week) and were summed with a score of 1 for each ranging from 0 to 4. Eight healthy behaviours were selected to form a lifestyle score ranging from 0 to 8 including: having never smoked or having stopped smoking, doing a minimum of 3 h of at least moderate physical activity weekly, having less than 6 alcoholic drinks/day and no more than 28/week, eating fish at least three times weekly, eating meat less than six times weekly, never or rarely adding salt to food, possessing a measured BMI of <25.0 kg/m2 and always using reduced fat or skim milk.
Anthropometric measurements were obtained in accordance with World Health Organisation standards using methods developed for use in large-scale epidemiological field work [Citation30]. MetS was defined by any three or more of the five components proposed by the National Cholesterol Education Program Adult Treatment Panel III [Citation31] and was previously described in detail [Citation1]. Also definition of hepatic steatosis by hyperechogenic liver ultrasound pattern and elevated GGT levels was previously published [Citation32].
Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication (ATC code C02). A summative cardiovascular disease (CVD) score was built upon diagnosis of angina pectoris, peripheral artery disease, heart failure stroke or myocardial infarction [Citation33]. Diagnosis of cancer and diabetes based on self-reported physician's diagnosis. Comorbid health status was measured by the Functional Comorbidity Index, which is a summary measure of comorbid diseases selected and weighted according to their association with physical functioning [Citation34]. Accounting of the number of medications used was obtained through manual inventory of medication containers.
Laboratory measurements
Non-fasting blood samples were taken from the cubital vein in the supine position between 07:00 a.m. and 04:00 p.m. Serum aliquots were prepared for immediate analysis or were stored at −80°C for further analysis. Measurements of TT levels were performed during December 2005 and January 2006 from frozen serum aliquots using competitive chemiluminescent enzyme immunoassays on an Immulite 2500 analyser (Siemens Healthcare Medical Diagnostics, Bad Nauheim, Germany) The inter-assay coefficients of variation were 13.2% with a systematic deviation of +2.3% at the 3.2 nmol/l level, and 8.9% with a systematic deviation of +0.24% at the 22.5 nmol/l level.
Total cholesterol (TC) was measured photometrically (Hitachi 704, Roche, Mannheim, Germany), whereas high-density lipoprotein (HDL-C) and low-density lipoprotein (LDL-C) cholesterol was determined after precipitation procedures. Dyslipidaemia was defined as TC > 6.2 mmol/l, or LDL-C > 4.1 mmol/l or HDL-C <1.03 mmol/l, or lipid medication [Citation17]. High-sensitivity C-reactive protein (hs-CRP) was determined immunologically on a Behring Nephelometer II with commercially available reagents from Dade Behring (Dade Behring, Eschborn, Germany) with a test sensitivity of 0.2 mg/l. Leukocyte count was performed within 60 min after blood sampling with a Coulter Max M analyser (Coulter Electronics, Miami, USA). Acute inflammatory reaction was defined by hs-CRP levels >10 mg/l and/or leukocyte count >11.3 Gpt/l [Citation17]. All assays were performed according to the manufacturers' recommendations by skilled technical personal. In addition, the laboratory takes part in official quarterly German external proficiency testing programmes.
Statistical analysis
Descriptive statistics, proportions for categorical variables and means (SD) for continuous variables, were used to describe the study population. Estimates on the crude and age-specific baseline prevalence of TD were computed as number of men with TD divided by the total number of men. Crude and age-specific incidence rates were calculated in men without TD at baseline by dividing the number of follow-up TD cases by the number of person-years at risk. To visually determine the longitudinal trend in TT levels over time, we calculated mean TT levels (baseline + follow-up/2) and plotted them against baseline age. The effect of each baseline covariate on continuous follow-up TT levels was assessed by unstandardised β-coefficients from analysis of covariance (ANCOVA) adjusted for age, blood sampling time and baseline TT levels. To identify potential risk factors of incident TD, the sample was limited to men without prevalent TD at baseline with different threshold levels applied. First, generalised linear models with Poisson distribution, log link function and robust error variances, adjusted for age, blood sampling time and the respective covariate were implemented. Second, to identify relevant covariates in the association of socio- demographic and behavioural as well as clinical and subclinical covariates with incident TD, we conducted stepwise multivariable regression models with forward selection using a P <0.05, forcing age, blood sampling time and respective covariates into the model. Effects were estimated using Results were presented in relative risks (RR) and 95% confidence intervals (95% CI). In a further step, identified covariates with significant impact on TT levels were used to define a subpopulation of healthy men, to perform analysis on TT level trend. Two-sided probability values <0.05 were considered statistically significant. All statistical analyses were performed using Stata 9 (Stata Corporation, College Station, TX).
Results
Cohort characteristics
Selected baseline characteristics of the study sample (complete TT data) were compared to men excluded from analysis (incomplete TT data) (). Included men were significantly younger, higher educated, employed and had generally higher household incomes compared to men excluded from analysis (). While 42.2% of men were physically active, about a quarter (24.3) reported high-risk alcohol consumption and current smoking (27.9%). Included men's mean levels of TT were significantly lower (16.48 nmol/l) compared to excluded men (17.15 nmol/l). Furthermore, included men reported less CVD or comorbid conditions including cancer and diabetes, and fewer medications compared to excluded men (). Although a quarter (25.2% BMI ≥30 kg/m2; 28.2% WC ≥102 cm) of included men was obese, and MetS (40.7%), hypertension (61.6%) and dyslipidaemia (61.8%) were very frequent conditions, we detected any further differences between both groups.
Table I. Descriptive baseline characteristics.
Prevalence and incidence
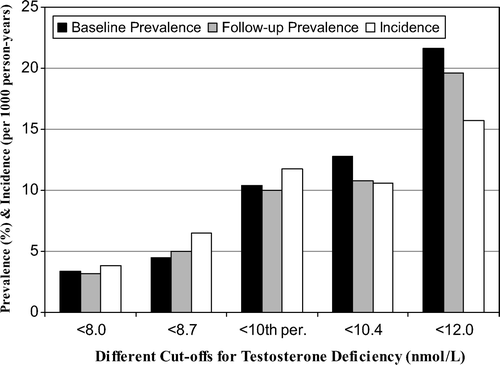
Baseline prevalence estimates of TD below TT levels of 8.0, 8.7, 10.4 and 12.0 nmol/l were 3.4% (N = 50), 4.5% (N = 67), 12.8% (n = 191) and 21.6% (n = 322) (), respectively. Crude incidence of TD defined by TT levels below 10th age-specific percentile from 92 cases during 7838.0 person-years at risk was 11.7 per 1000 person-years, whereas alternative cut-offs yielded incidence rates ranging from 3.8 to 15.7 per 1000 person-years ().
Risk factors of TD
Levels of TT showed a significant dose-response relationship with age (). We estimated a significant prospective age trend in levels of TT with an unadjusted rate of −0.05 nmol/l per year (P < 0.001) and −0.47 nmol/l per decade (P < 0.001). Among the other socio-demographic and behavioural characteristics, only income was inversely associated with TT levels and incident TD, respectively (). Accordingly, the conducted multivariable stepwise forward regression model selected age and income as relevant predictors of incident TD (P < 0.05). We therefore adjusted the models presented in for age, blood sampling time and income. Among the subclinical and clinical covariates presented in , obesity, MetS, dyslipidaemia and diabetes showed significant associations with incident TD. An increase in baseline BMI or WC by one unit was associated with a significant decrease of 0.13 or 0.04 nmol/l in follow-up TT levels (P < 0.001), respectively. Accordingly, men with baseline BMI > 25 kg/m2 had a more than twofold increased risk of incident TD (RR: 2.48; 95% CI: 1.35; 4.56) with comparable risk estimates using alternative cut-offs for the definition of TD (). Men with prevalent MetS (RR: 2.20; 95% CI: 1.37; 3.30), dyslipidaemia (RR: 1.74; 95% CI: 1.11; 2.69) and diabetes (RR: 2.92; 95% CI: 1.04; 3.52) were also identified at increased risk of incident TD. Furthermore, a higher number of comorbid conditions and prescribed medications at baseline was with lower follow-up TT levels and increased risk of incident TD (). We repeatedly performed a stepwise regression model adjusted for age, blood sampling time and income with forward selection of clinical and subclinical covariates, and identified age, income, MetS, dyslipidaemia and BMI as relevant predictors of incident TD (P < 0.05).
Figure 2. Scatterplot for the mean of baseline and follow-up TT levels over baseline age with linear fit line (95% CI). T0, baseline; T1, follow-up.
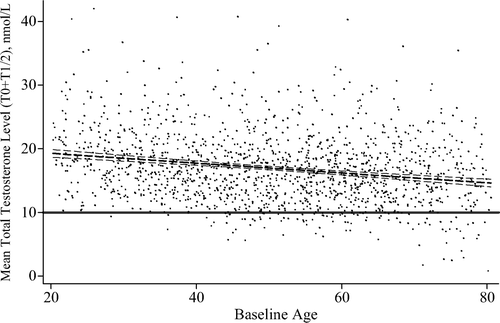
Table II. Estimates for the longitudinal association of socio-demographic and behavioural risk factors with incident testosterone deficiency.
Table III. Estimates for the longitudinal association of subclinical and clinical risk factors with incident testosterone deficiency.
Protective factors of age-associated TT level decline
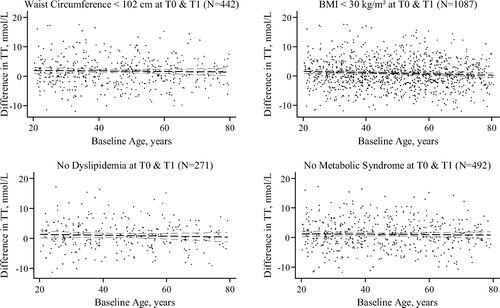
From the previously identified risk factors of TD, we defined subpopulations of men without those conditions at both examinations. Subsequent analysis on longitudinal TT level trend revealed that men who became not obese over the course of the study (WC < 102 cm or BMI < 30 kg/m2) maintained constant TT levels over time (). Furthermore, men without prevalent MetS (N = 503) or dyslipidaemia (N = 227) at baseline and follow-up also maintained constant TT levels over time ().
Discussion
Summary
SHIP provides one of the largest population-based cohorts for the study of the aging male. To the best of our knowledge, this is the first population-based longitudinal study in Europe to present prevalence and incidence estimates of TD in healthy males from the community. Furthermore, the present study confirmed the age-related decline in TT levels with aging and identified obesity, MetS, diabetes and dyslipidaemia as major risk factors of incident TD. But most strikingly, our results suggest that the management of these potentially modifiable risk factors may prevent declining TT levels in the aging male.
Prevalence and incidence
Most population-based studies of TD have arrived at prevalence estimates between 6 and 12% [Citation35], with substantial variation according to age, applied cut-offs, or studied populations. Data from the Boston area indicated an overall prevalence of about 6% among men with a relatively wide age range [Citation8,Citation13]. Wong et al. [Citation16] reported a prevalence of 9.5% among men aged 45–64 years living in Hong Kong. Studies in alternative settings reported higher prevalence estimates of 12% from clinical patients [Citation14] or 19% among German primary care patients [Citation17]. Because diagnostic criteria of TD have not been consistently applied, the present variability in prevalence estimates of TD makes comparisons across studies difficult. However, our prevalence estimates across different cut-offs for the definition of TD appear to correspond well with previous findings. Also incidence rates of TD were comparable with previously published estimates from the Massachusetts Male Aging Study years [Citation8]. Furthermore, the revealed TD prevalence and incidence estimates based on age-specific percentile cut-offs ranged between those originated from fixed cut-offs at 8.7 and 10.4 nmol/l. However, in contrast to fixed cut-offs, age-specific percentile cut-offs may reflect clinical realities, such as the existence of characteristic individual set points for circulating TT levels, below which one, but not another, individual may develop metabolic changes of TD; or the concept of reserve capacity, the possibility that men with TT below the fixed cut-off still may have adequate concentrations to meet their metabolic needs [Citation20]. Therefore, we advocate along with previous recommendations [Citation36], that age-specific percentile cut-offs should be used to estimate the prevalence and incidence of TD.
Risk factors and prevention of TD
Previously published data suggests a plethora of risk factors of TD, including aging [Citation37], unfavourable lifestyle and unhealthy behaviour [Citation10], lower personal income [Citation16], obesity, stroke, cancer [Citation21], polypharmacy [Citation17], diabetes, hypertension [Citation25], comorbidity [Citation38] and MetS [Citation39]. Among the factors inversely related to TD, smoking [Citation38], coffee consumption [Citation21] and employment [Citation25] were discussed. This study confirms the age-related longitudinal decline in TT levels. The magnitude of fall in TT was estimated with an unadjusted rate of 0.05 nmol/l per year and 0.47 nmol/l per decade. Our estimated annual decline is less than that from other longitudinal studies (0.11 nmol/l) [Citation20], but higher that that from previous cross-sectional studies (0.02 nmol/l) [Citation19,Citation38,Citation40].
Our analyses of socio-demographic and behavioural characteristics revealed an inverse association of employment and a negative association of low income concerning risk of incident TD defined by TT levels <8.0 nmol/l. This finding is in line with previous studies reporting that lower personal income is associated with higher risk of TD [Citation16], and employment is associated with increasing TT levels [Citation25]. However, these factors failed to provide a consistent pattern of associations across different definitions of incident TD, suggesting only definition-specific explanatory value. In contrast to previous findings [Citation10,Citation21], we were not able to detect any inverse associations of smoking, coffee consumption or healthy lifestyle with risk of incident TD.
Among the metabolic conditions associated with incident TD, obesity, MetS, dyslipidaemia, and diabetes showed significant effects. Although it has been suggested that WC is better at predicting TT levels than BMI [Citation41], we found no remarkable differences between both measures, with BMI yielding slightly higher effects. However, in accordance with previous studies both measures of central and overall obesity were significantly associated with lower follow-up TT levels [Citation42] and increased risk of incident TD [Citation25]. There was a strong association of MetS and all of its clustered risk factors, including obesity, lipid metabolism and insulin resistance with incident TD. Men with baseline MetS exposed a more than twofold increased risk of incident TD at follow-up. This finding was consistent across alternative cut-offs for the definition of TD, confirming previous reports of an increased risk of incident TD defined by TT levels below 11.0 nmol/l [Citation39] or 10.4 nmol/l [Citation17]. This finding implies reciprocally enhancing effect, as we have previously shown in SHIP that men with low serum TT levels are at increased risk of incident MetS [Citation1] and vice versa. Additionally, diabetes also offers potential bidirectional associations as the presence of lower TT levels in men with diabetes is well known [Citation43,Citation44], and low levels of TT were identified to be predictive of diabetes [Citation45].
Recently reviewed studies of men with prostate cancer who undergo long-term androgen deprivation therapy (ADT) provided evidence of an greater risk of developing dyslipidaemia, insulin resistance, hyperglycaemia and MetS [Citation46]. The observed metabolic and physiological changes may be a direct result of the induced severe TD and might predispose subjects to a greater risk of cardiovascular morbidity and mortality. We found no significant association of CVD considering angina pectoris, peripheral artery disease, heart failure, stroke or myocardial infarction with incident TD. Thus, our data do not support direct effects of these manifest conditions on TT levels. Finding of ultrasonographic liver disease at baseline was associated with significantly lower TT levels at follow-up, whereas hepatic steatosis lacked of predictive ability. However, TD caused by liver disease is well known and multiple pathways, including bidirectional associations, have been discussed [Citation47]. The fact that the intake of six or more medications and comorbidity were associated with lower follow-up TT levels and incident TD reflects previous findings suggesting that multimorbidity and severe illness are associated with suppressed function of the gonadal axis [Citation47]. Comparably, previous findings indicated a TT level decline in men reporting intake of more than six medications [Citation25] and comorbidity [Citation21,Citation38], as well as an increased risk of TD [Citation17]. But even more interestingly was our finding of constant TT levels over time in healthy subpopulations of men without the revealed risk factors of incident TD at both examinations. These results suggest that the age-related decline in TT levels may be prevented through the management of potentially modifiable risk factors including overweight, dyslipidaemia and MetS. This finding is in line with previously discussed implications, suggesting the age-related and potentially preventable and/or reversible decline in TT as a barometer of health [Citation38].
Some limitations of this investigation should be acknowledged. We were not able to define TD syndrome by using both, clinical signs/symptoms plus repeated measurement of morning TT levels, as current guidelines recommend [Citation48]. Because one of the key sources of biological variability in TT levels is time of day, it is reasonable to assume that some men with borderline values may be transiently suppressed by acute conditions or stress, and would not be considered having TD upon repeated testing. However, due to logistical concerns in a large-scale population-based study like SHIP, it was practically impossible to obtain such. However, single TT measurements are believed to be accurate for population studies [Citation49], and we detected any significant effect, when we carefully adjusted for this factor. Furthermore, we sought to limit artifactual changes in TT levels between baseline and follow-up by performing measurements in one central laboratory, following the same collection protocol, and using the same laboratory assays for plasma samples that were stored at −80°C and thawed for the first time [Citation50]. There are several strengths associated with this study. These include a representative population-based sample of men from a defined geographic area, assessment of potentially influential medication in the investigated association and the longitudinal design.
In summary, our findings have several important implications. First, our results provide convincing arguments for altered assessment of male TD by age-specific percentile cut-offs, showing that this definition was able to account for the strong age-dependency in levels of TT. Second, the question arises if the proposed role of TT as independent risk factors of clinical and subclinical conditions is adequate. A risk factor is defined by an aetiologic or causal role in a certain disease [Citation51]. But given the present results, suggesting a bidirectional nature of many of the revealed risk factors of low TT levels and incident TD, reverse causality remains a possibility. Therefore, up to now we propose TT being a risk marker, rather than risk factor, because a risk marker is not assumed to play a direct causal role in the disease process, but it is mainly useful to improve predictive ability. Third, our results provide some support for the concept that ill-health may be a remediable risk factor for reduced circulating TT levels in aging men. Clarification of the underlying causes of these changes in TT levels, whether they are potentially attributable to changes in lifestyle, health status or environmental factors, may provide helpful information which preventive action can be taken.
Acknowledgements
This work is part of the research project Greifswald Approach to Individualised Medicine (GANI_MED). The GANI_MED consortium is funded by the Federal Ministry of Education and Research and the Ministry of Cultural Affairs of the Federal State of Mecklenburg, West Pomerania (03IS2061A). Statistical analysis were further supported by the Community Medicine Research net (CMR) of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research, the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania. The CMR encompasses several research projects that are sharing data of the population-based Study of Health in Pomerania (SHIP; http://www.community-medicine.de). The testosterone reagents used were sponsored by Siemens Healthcare Diagnostics, Eschborn, formerly DPC Biermann GmbH, Bad Nauheim, Germany. Novo Nordisk provided partial grant support for the determination of plasma samples and data analysis.
References
- HaringR, VolzkeH, FelixSB, SchipfS, DorrM, RosskopfD, NauckM, SchoflC, WallaschofskiH. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes. 2009;58:2027–2031.
- KupelianV, PageST, AraujoAB, TravisonTG, BremnerWJ, McKinlayJB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. The Journal of clinical endocrinology and metabolism. 2006;91:843–850.
- YeapBB, HydeZ, AlmeidaOP, NormanPE, ChubbSA, JamrozikK, FlickerL, HankeyGJ. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. The Journal of clinical endocrinology and metabolism. 2009;94:2353–2359.
- SvartbergJ, von MuhlenD, MathiesenE, JoakimsenO, BonaaKH, Stensland-BuggeE. Low testosterone levels are associated with carotid atherosclerosis in men. J Intern Med. 2006;259:576–582.
- VikanT, JohnsenSH, SchirmerH, NjolstadI, SvartbergJ. Endogenous testosterone and the prospective association with carotid atherosclerosis in men: the Tromso study. European journal of epidemiology. 2009;24:289–295.
- FarrellJB, DeshmukhA, BaghaieAA. Low testosterone and the association with type 2 diabetes. The Diabetes educator. 2008;34:799–806.
- HallSA, AraujoAB, EscheGR, WilliamsRE, ClarkRV, TravisonTG, McKinlayJB. Treatment of symptomatic androgen deficiency: results from the Boston Area Community Health Survey. Archives of internal medicine. 2008;168:1070–1076.
- AraujoAB, O'DonnellAB, BrambillaDJ, SimpsonWB, LongcopeC, MatsumotoAM, McKinlayJB. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. The Journal of clinical endocrinology and metabolism. 2004;89:5920–5926.
- ShoresMM, MatsumotoAM, SloanKL, KivlahanDR. Low serum testosterone and mortality in male veterans. Archives of internal medicine. 2006;166:1660–1665.
- YeapBB, AlmeidaOP, HydeZ, NormanPE, ChubbSA, JamrozikK, HankeyGJ, FlickerL. Healthier lifestyle predicts higher circulating testosterone in older men: the Health In Men Study. Clin Endocrinol (Oxf). 2009;70:455–463.
- WangC, NieschlagE, SwerdloffRS, BehreH, HellstromWJ, GoorenLJ, KaufmanJM, LegrosJJ, LunenfeldB, MoralesA, MorleyJE, SchulmanC, ThompsonIM, WeidnerW, WuFC. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2009;12:5–12.
- NieschlagE, BehreH, NieschlagS. Andrology: Male Reproductive Health and Dysfunction. 3rd ed., Berlin: Springer; 2009.
- AraujoAB, EscheGR, KupelianV, O'DonnellAB, TravisonTG, WilliamsRE, ClarkRV, McKinlayJB. Prevalence of symptomatic androgen deficiency in men. The Journal of clinical endocrinology and metabolism. 2007;92:4241–4247.
- LiuCC, WuWJ, LeeYC, WangCJ, KeHL, LiWM, HsiaoHL, YehHC, LiCC, ChouYH, HuangCH, HuangSP. The prevalence of and risk factors for androgen deficiency in aging Taiwanese men. J Sex Med. 2009;6:936–946.
- MorleyJE, KaiserFE, PerryHM, 3rd, PatrickP, MorleyPM, StauberPM, VellasB, BaumgartnerRN, GarryPJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism: clinical and experimental. 1997;46:410–413.
- WongSY, ChanDC, HongA, WooJ. Prevalence of and risk factors for androgen deficiency in middle-aged men in Hong Kong. Metabolism: clinical and experimental. 2006;55:1488–1494.
- SchneiderHJ, SieversC, KlotscheJ, BohlerS, PittrowD, LehnertH, WittchenHU, StallaGK. Prevalence of low male testosterone levels in primary care in Germany: cross-sectional results from the DETECT study. Clin Endocrinol (Oxf). 2009;70:446–454.
- FeldmanHA, LongcopeC, DerbyCA, JohannesCB, AraujoAB, CovielloAD, BremnerWJ, McKinlayJB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of clinical endocrinology and metabolism. 2002;87:589–598.
- GrayA, FeldmanHA, McKinlayJB, LongcopeC. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. The Journal of clinical endocrinology and metabolism. 1991;73:1016–1025.
- HarmanSM, MetterEJ, TobinJD, PearsonJ, BlackmanMR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of clinical endocrinology and metabolism. 2001;86:724–731.
- SvartbergJ, MidtbyM, BonaaKH, SundsfjordJ, JoakimsenRM, JordeR. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. European journal of endocrinology / European Federation of Endocrine Societies. 2003;149:145–152.
- AnderssonAM, JensenTK, JuulA, PetersenJH, JorgensenT, SkakkebaekNE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. The Journal of clinical endocrinology and metabolism. 2007;92:4696–4705.
- TravisonTG, AraujoAB, O'DonnellAB, KupelianV, McKinlayJB. A population-level decline in serum testosterone levels in American men. The Journal of clinical endocrinology and metabolism. 2007;92:196–202.
- SnyderPJ. Decreasing testosterone with increasing age: more factors, more questions. The Journal of clinical endocrinology and metabolism. 2008;93:2477–2478.
- TravisonTG, AraujoAB, KupelianV, O'DonnellAB, McKinlayJB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. The Journal of clinical endocrinology and metabolism. 2007;92:549–555.
- VolzkeH, AlteD, SchmidtCO, RadkeD, LorbeerR, FriedrichN, AumannN, LauK, PiontekM, BornG, et alCohort Profile: The Study of Health in Pomerania. International journal of epidemiology.
- KawachiI, KennedyBP. The relationship of income inequality to mortality: does the choice of indicator matter?Social science & medicine (1982). 1997;45:1121–1127.
- AlteD, LuedemannJ, RoseHJ, JohnU. Laboratory markers carbohydrate-deficient transferrin, gamma-glutamyltransferase, and mean corpuscular volume are not useful as screening tools for high-risk drinking in the general population: results from the Study of Health in Pomerania (SHIP). Alcoholism, clinical and experimental research. 2004;28:931–940.
- WinklerG, DoringA. Validation of a short qualitative food frequency list used in several German large scale surveys. Zeitschrift fur Ernahrungswissenschaft. 1998;37:234–241.
- WHO. Measuring obesity - classification and description of anthropometric data. Report on a WHO consultation of the epidemiology of obesity. Warsaw, 21–23 October1987.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421.
- HaringR, WallaschofskiH, NauckM, DorrM, BaumeisterSE, VolzkeH. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology. 2009;50:1403–1411.
- PocockSJ, McCormackV, GueyffierF, BoutitieF, FagardRH, BoisselJP. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trials. BMJ. 2001;323:75–81.
- GrollDL, ToT, BombardierC, WrightJG. The development of a comorbidity index with physical function as the outcome. Journal of clinical epidemiology. 2005;58:595–602.
- TravisonTG, AraujoAB, HallSA, McKinlayJB. Temporal trends in testosterone levels and treatment in older men. Current opinion in endocrinology, diabetes, and obesity. 2009;16:211–217.
- SchatzlG, MadersbacherS, TemmlC, Krenn-SchinkelK, NaderA, SregiG, LapinA, HermannM, BergerP, MarbergerM. Serum androgen levels in men: impact of health status and age. Urology. 2003;61:629–633.
- LapauwB, GoemaereS, ZmierczakH, Van PottelberghI, MahmoudA, TaesY, De BacquerD, VansteelandtS, KaufmanJM. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. European journal of endocrinology / European Federation of Endocrine Societies. 2008;159:459–468.
- WuFC, TajarA, PyeSR, SilmanAJ, FinnJD, O'NeillTW, BartfaiG, CasanuevaF, FortiG, GiwercmanA, et alHypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. The Journal of clinical endocrinology and metabolism. 2008;93:2737–2745.
- LaaksonenDE, NiskanenL, PunnonenK, NyyssonenK, TuomainenTP, ValkonenVP, SalonenJT. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. The Journal of clinical endocrinology and metabolism. 2005;90:712–719.
- MullerM, den TonkelaarI, ThijssenJH, GrobbeeDE, van der SchouwYT. Endogenous sex hormones in men aged 40–80 years. European journal of endocrinology / European Federation of Endocrine Societies. 2003;149:583–589.
- SvartbergJ, von MuhlenD, SundsfjordJ, JordeR. Waist circumference and testosterone levels in community dwelling men. The Tromso study. European journal of epidemiology. 2004;19:657–663.
- MohrBA, BhasinS, LinkCL, O'DonnellAB, McKinlayJB. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. European journal of endocrinology / European Federation of Endocrine Societies. 2006;155:443–452.
- Barrett-ConnorE, KhawKT, YenSS. Endogenous sex hormone levels in older adult men with diabetes mellitus. American journal of epidemiology. 1990;132:895–901.
- SparkRF. Testosterone, diabetes mellitus, and the metabolic syndrome. Current urology reports. 2007;8:467–471.
- LaaksonenDE, NiskanenL, PunnonenK, NyyssonenK, TuomainenTP, ValkonenVP, SalonenR, SalonenJT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041.
- HakimianP, BluteM, Jr., KashanianJ, ChanS, SilverD, ShabsighR. Metabolic and cardiovascular effects of androgen deprivation therapy. BJU international. 2008;102:1509–1514.
- KaragiannisA, HarsoulisF. Gonadal dysfunction in systemic diseases. European journal of endocrinology / European Federation of Endocrine Societies. 2005;152:501–513.
- WangC, NieschlagE, SwerdloffR, BehreHM, HellstromWJ, GoorenLJ, KaufmanJM, LegrosJJ, LunenfeldB, MoralesA, et alInvestigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30:1–9.
- LiuPY, DeathAK, HandelsmanDJ. Androgens and cardiovascular disease. Endocrine reviews. 2003;24:313–340.
- FriedrichN, VolzkeH, RosskopfD, StevelingA, KrebsA, NauckM, WallaschofskiH. Reference ranges for serum dehydroepiandrosterone sulfate and testosterone in adult men. J Androl. 2008;29:610–617.
- WangTJ. New cardiovascular risk factors exist, but are they clinically useful?European heart journal. 2008;29:441–444.