Abstract
Osteoporosis in elderly men is becoming an important health issue with the aging society. Elderly men with androgen deficiency are exposed to osteoporosis and can be treated with testosterone replacement. In this study, Eurycoma longifolia (EL), a plant with androgenic effects, was supplemented to an androgen-deficient osteoporotic aged rat as alternative to testosterone. Aged 12 months old Sprague-Dawley rats were divided into groups of normal control (NC), sham-operated (SO), orchidectomised-control (OrxC), orchidectomised and supplemented with EL (Orx + El) and orchidectomised and given testosterone (Orx + T). After 6 weeks of treatment, serum osteocalcin, serum terminal C-telopeptide Type 1 collagen (CTX) and the fourth lumbar bone calcium were measured. There were no significant differences in the osteocalcin levels before and after treatment in all the groups. The CTX levels were also similar for all the groups before treatment. However, after treatment, orchidectomy had caused significant elevation of CTX compared to normal control rats. Testosterone replacements in orchidectomised rats were able to prevent the rise of CTX. Orchidectomy had also reduced the bone calcium level compared to normal control rats. Both testosterone replacement and EL supplementation to orchidectomised rats were able to maintain the bone calcium level, with the former showing better effects. As a conclusion, EL prevented bone calcium loss in orchidectomised rats and therefore has the potential to be used as an alternative treatment for androgen deficient osteoporosis.
Introduction
Osteoporosis is characterised by low bone mineral density resulting in weak bones with increased risk of fracture. It is a major public health problem due to high fracture rates, decrease in the quality of life of osteoporotic subjects and high healthcare costs [Citation1]. According to the World Health Organisation (WHO), osteoporosis occurs when the bone mineral density falls to more than 2.5 SD below the standard reference of the maximum bone mineral density of young adult female. If this criterion is referred to the maximal bone mineral density of the young adult male, one to two million men in United States would have osteoporosis [Citation2].
Most of the attention has been given to osteoporosis in women because of the dramatic bone loss after menopause compared to the gradual bone loss in men with aging. Recently, more attention has been given to male osteoporosis due to the increase in the number of men having osteoporosis. It is estimated that 20% of osteoporotic cases are men and the number is increasing due to the aging society. The risk of osteoporotic fractures in men is 1 in 5 after the age of 50 [Citation3].
The main cause of osteoporosis in men is androgen deficiency [Citation4]. This may occur in the elderly due to normal aging or in young men with undescended testes, tumour of the testis or chromosomal abnormality. These men are exposed to osteoporosis due to deficiency of testosterone which is required to maintain bone density [Citation4]. The rate of bone loss in androgen deficient men was found to be similar to postmenopausal women [Citation5]. Naturally, serum testosterone level declines at 0.4% per year from 39- to 70-years old [Citation6]. This is almost the same as the percentage bone loss per year at the femoral neck (0.3%) in healthy men after the age of 50 [Citation7]. The main treatment to prevent diseases related to androgen deficiency is androgen replacement therapy where testosterone is injected intramuscularly [Citation8]. Some patients may refuse treatment because of the painful administration of testosterone and its associated adverse effects especially prostate cancer.
Therefore, an alternative treatment which is safe and effective with convenient oral intake is required to treat and prevent this type of osteoporosis in men. Eurycoma longifolia (EL) is a traditional medical plant, known as tongkat Ali in Malaysia, tung saw in Thailand, pasak bumi in Indonesia and cay ba bihn in Vietnam [Citation9]. This plant is in under the family of Simaroubaceae. The diameter of the trunk is around 10 cm and it can grow up to 10 m high. The leaf is in pinnate shape and the length of the leaf is about 20–40 cm. The flower of E.longifolia is dioecious while the fruit is ovoid in shape and will turn to dark brown colour when it is ripe [Citation10]. The root is part of the plant believed to have the medicinal values to enhance male sexuality and fertility and as anti-aging agent [Citation11–13]. The water-soluble extract of EL contains mainly phenolic components, tannins, high-molecular-weight polysaccharide, glycoprotein and mucopolysacharides. It is believed that these water-soluble components are the active ingredients which are biologically active in rendering the observed properties in animal studies.
EL has been used to improve male sexuality due to its aphrodisiac effects but nowadays, it is not only available commercially in various preparations as health supplements but is also added to drinks such as coffee and tea.
The androgen-like properties of EL were evident in studies which have shown that male rats supplemented with EL mated more frequently and have faster penile erection compared to control rats [Citation14,Citation15]. The aim of the study is to explore the potential of the androgen-like EL as an alternative to testosterone in the treatment of androgen-deficient osteoporosis in males.
Material and methods
Male Sprague-Dawley rats (aged 12 months) were obtained from the UKM Animal House. The rats were housed in a plastic cage at 29 ± 3°C under natural day/night cycle. They were fed commercial food pellets and tap water ad libitum. They were allowed to adjust to the new environment for a week before the study was started. The study was approved by the UKM Animal Ethics Committee (PP/FAR/2008/NAZRUN/13-FEB/217-FEB-2008-FEB-2010).
Forty male Sprague-Dawley rats, aged 12 months were divided into groups of normal control (NC), sham-operated (Sham), orchidectomised-control (OrxC), orchidectomised and supplemented with EL (Orx + El), orchidectomised and given testosterone replacement (Orx + T). Body weights were measured before the start of treatment and then weekly until the end of the study.
Aged orchidectomised rats were used as the model for androgen-deficient osteoporosis. Before performing orchidectomy, the rats were anesthetised with Ketapex:Xylazil (1:1). A 2-cm ventral midline incision was made in the scrotum and the skin was retracted to expose the tunica. The tunica was pierced and the testes were pushed out and raised to expose the underlying blood vessels and tubules. The spermatic chord was clamped and tied with absorbable catgut suture at the confluence of the blood vessels and epididymis. The testes were removed and all deferential vessels and ducts were replaced back into the tunica.
Eurycoma longifolia aqueous extract was supplied by Phytes Biotek Sdn Bhd. (Malaysia). It was extracted from the root of the plant using a patented high pressure water extraction (US Patent No: US7,132,117 B2). The extract was in brownish powder form. It contained bioactive 22.0% eurypeptide, 41.1% glycosaponin and 1.6% eurycomanone. EL aqueous extract powder was dissolved in normal saline and given via oral gavages at the doses of 15 mg/kg rat weight daily at 9 am for 6 weeks [Citation16]. Testosterone was purchased from TCI UK Ltd (UK). It was diluted in olive oil (Bertolli, Italy) and 8 mg/kg was injected intramuscularly once daily at 9 am for 6 weeks [Citation17]. Blood samples were collected before the start of treatment and after 6 weeks of treatment. Blood samples were obtained from the retro-orbital vein after anesthetising the rats with ether. After 3 h, the blood was centrifuged at 3000 rpm for 10 min and the serum stored at temperature of −70°C.
At the end of treatment, the rats were sacrificed humanely and the fourth lumbar vertebrae were dissected out and cleansed of all soft tissues. The bones were dried in an oven at 100°C for 24 h, then ashed in a furnace at 800°C for 12 h. The ash was weighed and dissolved in 3 ml nitric acid and then diluted in lanthanum chloride. Calcium chloride was measured with an Atomic Absorption Spectrophotometer (Shimadzu AA-680) at 422.7 nm.
Bone biochemical markers of serum Osteocalcin and C-terminal telopeptide of type 1 collagen (CTx) were measured before and after treatment using ELISA technique with ELISA reader (VERSAmax, Sunnyvale, USA). Kits used were Rat Osteocalcin ELISA (Biomedical Technologies, Herlev, Denmark) and RatlapsTM ELISA CTX-1 (Nordic Biosciences, IDS UK).
The results are expressed as mean ± SEM. The statistical significance of the data has been determined using one-way analysis of variance (ANOVA) and post hoc Tukey test. The level of significance was taken as p < 0.05.
Results
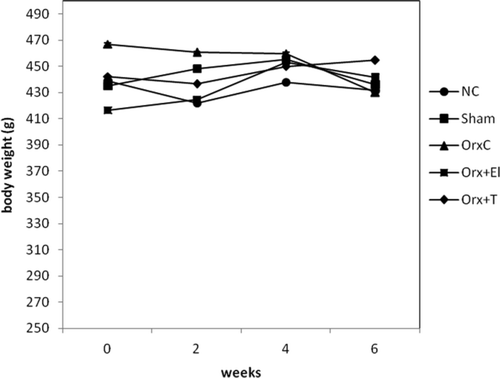
The mean body weights of the different groups of rats were not significantly different before the study and at the end of the study. Their weights remain steady throughout the study ().
Figure 1. Mean body weight throughout the study. There was no significant difference in body weight between the groups (p < 0.01). NC: normal control group; Sham: sham-operated; OrxC: orchidectomised-control; Orx + El: orchidectomised and supplemented with EL; Orx + T: orchidectomised and given testosterone replacement.
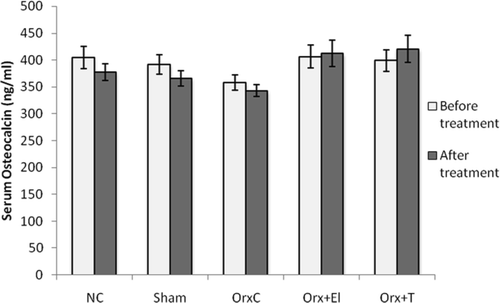
Before treatment, there was no significant difference in the serum osteocalcin levels for all the groups. After 6 weeks of treatment, there was also no difference in the serum osteocalcin levels for all the groups. These results indicated no difference in the bone formation rate before and after treatment for all the groups ().
Figure 2. Mean serum osteocalcin levels of all the group before and after treatment. Data presented as mean ± SD (p < 0.01). NC: normal control group; Sham: sham-operated; OrxC: orchidectomised-control; Orx + El: orchidectomised and supplemented with EL; Orx + T: orchidectomised and given testosterone replacement.
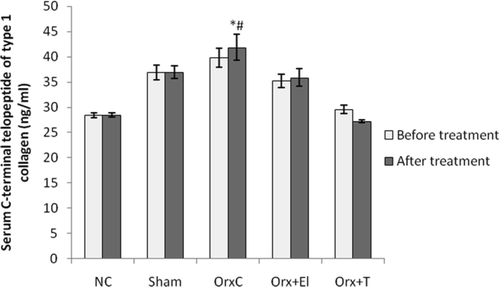
As for the serum CTx levels, before treatment there were no significant difference for all the groups. After 6 weeks of treatment, the CTx level of OrxC group was significantly elevated compared to NC group. Testosterone replacement has managed to prevent the rise of CTx level induced by orchidectomy. The CTx level appeared to be lowered in the EL group; however, the change was not significant ().
Figure 3. Mean serum C-terminal telopeptide of type 1 collagen of all the group before and after treatment. Data presented as mean ± SD (p < 0.01). *Significant difference compared to NC group. #Significant difference compared to Orx + T group. NC: normal control group; Sham: sham-operated; OrxC: orchidectomised-control; Orx + El: orchidectomised and supplemented with EL; Orx + T: orchidectomised and given testosterone replacement.
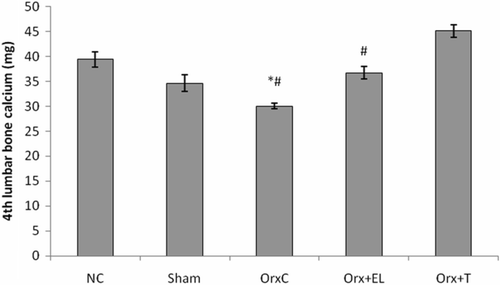
In the next parameter, the amount of calcium in the fourth lumbar vertebrae was measured. Orchidectomy was found to cause significant bone calcium losses which were consistent with osteoporosis due to testosterone deficiency. When the orchidectomised rats were given testosterone replacement, the bone calcium was preserved as observed in the OrxC + T group. EL supplemented to orchidectomised rats was also able to preserve the bone calcium but with lower efficacy compared to testosterone ().
Figure 4. Mean 4th lumbar bone calcium for all the groups. Data presented as mean ± SD (p < 0.01). *Significant difference compared to NC group. #Significant difference compared to Orx + T group. NC: normal control group; Sham: sham-operated; OrxC: orchidectomised-control; Orx + El: orchidectomised and supplemented with EL; Orx + T: orchidectomised and given testosterone replacement.
Discussion
Osteoporotic fracture in men is associated with higher morbidity and mortality than in women [Citation18]. This would have a huge impact on the life of the sufferer and their family. Therefore, androgen deficient men who are exposed to osteoporosis require testosterone replacement therapy. Intramuscular injections produce a stable testosterone level but this route of administration is painful and would have to be given by a trained medical personnel. Side-effects of testosterone replacement therapy include prostate cancer, liver damage, nausea, vomiting, limb swellings and painful erections.
There is a need to find an alternative agent to testosterone which could offer protection against osteoporosis but at the same time can be taken orally with minimal side-effects. To the best of our knowledge, there are no studies that have focused on the effects of EL on osteoporosis. Therefore, we have conducted a study to determine the effects of EL in an orchidectomised aged rat model, the accepted animal model for androgen-deficient osteoporosis in men [Citation19]. The rat's bone have a similar response to treatment as human bone [Citation20] and changes in their bone mass can be adequately extrapolated to humans [Citation21]. Testosterone deficiency due to orchidectomy was shown to cause reduction in bone mineral density in rat models which may lead to osteoporosis [Citation22–24].
Based on the results, the osteocalcin levels remain unchanged in orchidectomised rats but the CTx levels were found to be elevated. These indicate that the state of androgen deficiency may have influence on bone resorption only, leading towards bone loss. Testosterone replacement totally prevented the CTx elevation induced by orchidectomy. Several human studies have reported similar reductions in bone resorptive markers with testosterone therapy [Citation25–27]. EL appeared to exhibit similar actions but the changes did not reach statistical significance. Orchidectomy had also led to bone calcium loss which was prevented with testosterone replacement. EL was also able to significantly prevent bone calcium loss due to orchidectomy. However, it was less effective compared to testosterone replacement. Perhaps higher doses of EL are required to match the effects of testosterone.
EL supplementations were shown to increase muscle density of male rats [Citation15] and increase body mass and arm circumference of men [Citation28]. The mechanism of EL in producing these androgenic effects is still unclear. There were reports that it may increase free testosterone by promoting dissociation of testosterone from sex-hormone binding globulin [Citation29] or by directly raising the testosterone level in men [Citation30]. Recent human trials have proven that it has anti-stress and anti-aging effects. This may be accounted by its ability to normalise growth hormone while its high content level of superoxide dismutase enables it to scavenge superoxide-free radicals and inhibit lipid peroxidation [Citation31–34]. Other suggested actions of EL include raising HDL levels and lowering blood glucose in diabetic.
The dose of EL used in the present study appeared to have the potential to treat androgen-deficient osteoporosis but was not enough to match the effects of testosterone. This dose was about twice the dose of EL that was shown to be sufficient to increase sperm counts in normal rats [Citation16]. Perhaps, higher doses should be used as the current dose was considered to be a very safe dose. According to Satayavivad et al. [Citation35], the oral lethal dose 50 (LD50) of the aqueous extract of EL is more than 3000 mg/kg. In a human study, subjects taking up to of 600 mg per day of EL did not experience any adverse effects and they had normal full blood count, renal and liver function tests [Citation36].
Conclusion of our study is that EL has great potential as an alternative agent to testosterone replacement in treating androgen-deficient osteoporosis in men. It has good safety profile and convenient oral route of administration. In future, similar studies may be repeated with higher doses of EL. It may also be combined together with testosterone to treat osteoporosis. This combination may lower the dose of testosterone needed and therefore reduce its side effects.
Acknowledgements
We thank the Faculty of Medicine UKM for providing the grant FF-0104-2008 for this study.
References
- Sambrook P, Cooper C. Osteoporosis. Lancet 2006;367:2010–2018.
- Bilezikian JP. Osteoporosis in men. J Clin Endocrinol Metab 1999;84:3431–3434.
- Disease statistics. [Internet]. Washington, D.C.: National Osteoporosis Foundation. 2004. http://www.nof.org/osteoporosis/stats.htm. Last accessed 5 February 2010.
- Looker AC, Orwoll ES, Johnston CC Jr, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Heyse SP. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 1997;12:1761–1768.
- Blunt BA, Klauber MR, Barrett-Connor EL, Edelstein SL. Sex differences in bone mineral density in 1653 men and women in the sixth through ten decades of life: the Rancho Bernardo study. J Bone Miner Res 1994;9:1333–1338.
- Stepan JJ, Lachman M, Zverina J, Pacovsky V, Baylink DJ. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J Clin Endocrinol Metab 1989;69:523–527.
- Gray A, Feldman HA, Mc Kinlay JB, Longcpe C. Age, disease and changing sex hormone levels in middle-ages men: results of the Massachusetts male aging study. J Clin Endocrinol Metab 1991;73:1016–1025.
- Aminorroaya A., Kelleher S, Conway AJ, Ly LP, Handelsman DJ. Adequacy of androgen replacement influences bone density response to testosterone in androgen-deficient men.Eur J Endocrinol 2005;152:881–886.
- Goreja WG. Tongkat Ali: tree that cures a hundred diseases. New York: Amazing Herbs Press; 2004. pp 1–42.
- Goh SH, Chuah CH, Mok J.S.L, Soepadmo E. Malaysian medicinal plants for the treatment of cardiovascular disease. Kuala Lumpur: Pelanduk Publication Sdn Bhd.; 1995.
- Burkill IH, Hanif M. Malay village medicine, The Garden Bulletin, Strait Settlements. 1930;167–332.
- Ali JM, Saad JM. Biochemical effect of Eurycoma longifolia jack on the sexual behavior, fertility, sex hormone and glycolysis. Dissertation Paper For Bachelor Of Science, Department Of Biochemistry, University Of Malaya; 1993.
- Ang HH, Sim MK; Effect of Eurycoma Longifolia Jack on sexual behavior of male rats. Arch Pharmacol Res 1997;20:656–658.
- Ang HH, Cheang HS. Effects of Eurycoma longifolia jack on laevator ani muscle in both uncastrated and testosterone-stimulated castrated intact male rats. Arch Pharm Res 2001;24:437–440.
- Ang HH, Ngai TH, Tan TH. Effects of Eurycoma longifolia Jack on sexual qualities in middle aged male rats. Phytomedicine 2003;10:590–593.
- Norhazlina AW, Norfilza MM, Halim WNHA, Dass S. The effect of Eurycoma longifolia jack on spermatogenesis in estrogen treated rats. Clinic 2010;65:93–98.
- Nwe KHH, Morat PB, Khalid BAK. Opposite effects of sex steroids on 11β-hydroxysteroid dehydrogenase activity in the normal and adrenalectomized rat testis. Gen Pharmacol 1997;28:661–664.
- Johnell O, Kanis J, Gullberg G. Mortality, morbidity, and assessment of fracture risk in male osteoporosis. Calcif Tissue Int 2001;69:182–184.
- Vanderschueren D, Jans I, Van Herck E, Moermans K, Verhaeghe J, Bouillon R. Time-related increase of biochemical markers of bone turnover in androgen-deficient male rats. Bone Miner 1994;26:123–131.
- Abe T, Chow JWM, Lean JM, Chambers TJ. Estrogen does not restore bone lost after ovariectomy in the rat. J Bone Miner Res 1993;8:831–838.
- Jee WSS. Animal models in the prevention and treatment of osteopenia: forward. Bone 1995;17:113S–114S.
- Gunness M, Orwoll E. Early induction of alteration in cancellous and cortical bone histology after orchidectomy in mature rats. J Bone Miner Res 1996;11:1531–1538.
- Ima-Nirwana S, Norazlina M, Khalid BAK. Pattern of bone mineral density in growing male and female rats after gonadectomy. J ASEAN Fed Endoc Soc 1995;16:21–35.
- Rosen HN, Tollin S, Balena R, Middlebrook VL, Moses AC, Yamamoto M, Zein AJ, Greenspan SL. Bone density is normal in male rats treated with finasteride. Endocrinol 1995;136:1391–1387.
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, Dlewati A, Strom BL. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Med Endocrinol 1999;84:1966–1972.
- Kenny AM, Prestwood KM, Marcello KM, Raisz LG. Determinants of bone density in healthy older men with low testosterone levels. J Gerontol Med Sci A 2000;55:1–6.
- Kenny AM, Prestwood KM, Gruman CA. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A 2001;56:266–272.
- Hamzah S, Yusof A. The ergogenic effects of eurycoma longifolia jack: a pilot study. Br J Sports Med 2003;37:464–470.
- Shawn T, Kraemer W. The cortisol connection. California: Hunter House Inc.; 2007. pp 102–113.
- Tambi MI. Nutrients and Botanicals for optimizing Men's Health. Examining the evidence for Eurycoma longifolia jack, the Malaysian Ginseng in men's health. Third Asia Pacific Forum on Andrology; 2009 Oct 10–13; Nanjing. Shanghai: Nature Publishing Group; 2009. 180 p.
- Tambi MIM. Water soluble extract of Eurycoma Longifolia in enhancing testosterone in males. International Trade Show and Conference, Supply Side West, The Venetian, Las Vegas, Nevada, USA – Supplement Book; 2003.
- Tambi MIM. Standardised water soluble extract of Eurycoma Longifolia (LJ100) on men's health. IJA 2005;28(Suppl. 1) 25–54.
- Tambi MIM. Standardised water soluble extract of Eurycoma Longifolia LJ100 maintains healthy aging in man. AMJ 2006;9.
- Tambi MIM. Eurycoma Longifolia jack: a potent adaptogen in the form of water-soluble extract with the effect of maintaining men' health. AJA 2006;8.
- Satayavivad J, Noppamas S, Aimon S, Yodhathai T. Toxicological and antimalaria activity of Eurycoma longifolia Jack extracts in mice. Thai J Phytopharmacy 1998;5:14–27.
- Tambi MI. Standardized water soluble extract of Eurycoma Longifolia LJ100 maintains healthy aging in man. In: Lunenfeld B, editor. Abstracts of the 5th World Congress on the Aging Male. February 9–12, 2006, Salzburg, Austria. Aging Male 2006;9. [Abstract]
