Abstract
Benign prostatic hyperplasia (BPH) is a medical condition affecting a wide range of the aging male population resulting in various degrees of lower urinary tract symptoms (LUTS). Today, a variety of medical therapies and minimally invasive BPH treatment modalities are available. Medical therapy includes α1 blockers, 5α reductase inhibitors and combination therapy. When these options fail, surgery is indicated. Transurethral resection of the prostate (TURP) is still considered the gold standard surgical treatment for BPH. Nevertheless, numerous minimally invasive treatment alternatives are available that are comparable in effectiveness to TURP, with significantly less morbidity. In this article, current treatment options for BPH are reviewed with respect to their indications, long-term safety and efficacy in relieving BPH related LUTS. The selection of the type of BPH treatment should be based on the physician's experience, patient's co-morbidities as well as the prostate size and clinical disease progression.
Introduction
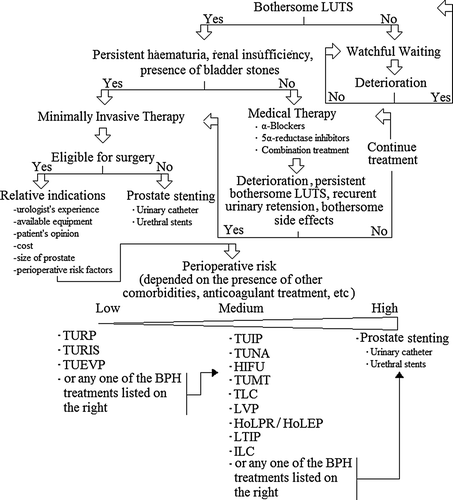
Benign prostatic hyperplasia (BPH) is a normal condition of the prostate appearing in the majority of aging males. Nevertheless, the condition is associated with an increased risk for lower urinary tract symptoms (LUTS) that can significantly affect a patient's quality of life. Despite the fact that the true incidence of clinical BPH has yet to be determined, the prevalence is high with the proportion of men with moderate to severe symptoms doubling with each decade of life [Citation1,Citation2]. Nearly 30% of men older than 60 years of age experience troublesome LUTS due to BPH [Citation3]. A watchful waiting (non-medical, non-surgical) policy in addition to lifestyle modifications (fluid management, avoidance of caffeine and use of alcohol) and specific changes in behaviour (bladder retraining, double voiding and urethral milking) are suitable for all men demonstrating BPH related LUTS, that do not complain of high levels of bother [Citation1,Citation4]. However, at 1 and 5 years, respectively, 15% and 35% of patients following watchful waiting will deteriorate and seek further management [Citation5,Citation6]. The management of BPH is a very complex process given that available treatment alternatives demonstrate different clinical outcomes, side effects and economic costs (). Moreover, promising novel minimally invasive treatment options are emerging, adding complexity to the decision making process of today's urologist. In this article, the medical and minimally invasive treatment modalities for the management of BPH are reviewed with the aim of updating physicians and assisting them in making informed treatment decisions.
Medical therapy options
Medical therapy is the first line of treatment for most patients experiencing clinical BPH. The goal of such treatment is to decrease LUTS, prevent BPH-associated complications and improve a patient's quality of life [Citation7]. The main disadvantage of pharmacologic management of BPH is the need for a patient's commitment to a long-term treatment, which in turn is associated with high long-term cost. Three main medical therapy approaches are used, α1-adrenergic receptors block, inhibition of 5α reductase inhibitors and combination therapy between these two types of therapy.
α1 Blockers
Prostate adrenoreceptor blockade is thought to reduce smooth muscle tone, decreasing the dynamic element of prostatic obstruction. Initially evaluated non-selective α-blockers were soon abandoned due to the systemic effects of general α-receptor blockage (e.g. hypotension). Today, a wide variety of α-blockers selective for the α1 adrenoreceptor subtype are available. These can be divided into α1 blockers (doxazosin, indoramin, prazosin and terazosin) and selective α1 blockers (tamsulosin and alfuzosin). The latter are relatively selective for the ‘A’ subtype of α1 adrenoreceptors that are mainly located in the prostate. Consequently, selective α1 blockers tend to affect less the α1B adrenoreceptors of systemic blood vessels, reducing the incidence of orthostatic and vasodilatory side effects. Recently, silodosin, a highly selective inhibitor of the α1A adrenoreceptor has been introduced causing practically no orthostatic hypotension and no clinically important effects on the heart (no effect on heart rate, PR segment, QRS complex or morphologic ECG data) [Citation8]. In contrast to promising clinical effectiveness, significant ejaculation disorders have been reported with silodosin [Citation9]. Mid or long-term comparative studies examining the effectiveness of silodocin versus the other commercial available α1 blockers is lacking.
Currently, no significant difference between different α1 blockers in terms of efficacy has been proven. After the initiation of therapy, α1 blockers exhibit an early onset of efficacy (within <1 week) with regard to both symptoms and flow rate improvement [Citation10]. Long-term data derived from large scale controlled trials demonstrate that α blocker treatment maintains its efficacy for up to 5 years [Citation11–13]. Overall symptoms tend to improve by 30–40% and flow rates by 16–25%, compared with placebo [Citation14]. Nevertheless, about one-third of men will not experience significant symptom reduction. In the latter case, that the drug is proven ineffective over an 8-week trial, treatment should be discontinued [Citation1]. Several well designed studies have proved that in case of acute urinary retention α1 blockers administration increases the possibility of a successful trial to remove the indwelling catheter [Citation15,Citation16]. Consequently, α1 blocker administration in the latter case is considered common practice.
5α-Reductase inhibitors
5α-reductase is the enzyme converting testosterone to dihydrotestosterone (DHT). Human prostate is a DHT-dependent organ. DHT deprivation results in prostate shrinkage leading to a significant LUTS reduction in case of BPH. Currently, available 5α-reductase inhibitors include finasteride and dutasteride. Finasteride inhibits type 2 5α-reducatse isoenzyme, leading to a suppression of DHT by 70% in the serum and 90% in the prostate. Dutasteride inhibits the action of both type 1 and 2 5α-reductase isoenzymes leading to a reduction of up to 90% in the circulating DHT. Clinical benefits of both inhibitors are considered equal [Citation17].
Numerous randomised, placebo-controlled studies have verified the efficacy of 5α-reductase inhibitors in the treatment of BPH. Significant long-term reduction of prostate size by 20–30% and improvement in urinary flow rates and symptom scores have been well documented in large series of patients [Citation18–21 ]. Actual effectiveness should be awaited as late as 6 months after treatment initiation and has been proved to be substantially maintained even after 6–10 years of treatment [Citation22,Citation23]. Nevertheless, not all patients are benefited by 5α reductase inhibitors. Prostate size and basic prostate specific antigen (PSA) levels are the main predictors of outcome. Men with small prostate size (<40 ml) and low PSA levels are less likely to be benefited by treatment with a 5α-reducatse inhibitor [Citation24,Citation25]. 5α-Reductase inhibitors are considered very safe and their adverse effects are considered minimal, mainly related with sexual function (decreased libido, impotence and decreased ejaculate).
It is not the purpose of this article to discuss prostate cancer. Nevertheless, questions have been raised regarding the potential of 5α-reductase inhibitor treatment to mask PSA increase in case of prostate cancer. It is well known that 5α reductase inhibitors lower serum PSA levels. Proscar Long-term Efficacy and Safety Study (PLESS) group concluded that treatment with finasteride preserves usefulness of PSA in the detection of prostate cancer, since, by doubling PSA serum levels, an accurate estimation can be expected [Citation26]. In addition, the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial revealed that treatment with dutasteride improved significantly the diagnostic utility of PSA for all prostatic tumours (p < 0.0001), even for high grade cancer (p < 0.02) [Citation27]. Moreover, long-term finasteride therapy does not affect the histologic features of benign prostatic tissue and prostate cancer on needle biopsy [Citation28]. Consequently, 5α-reductase inhibitors do not mask early detection of localised prostate adenocarcinomas. On the contrary, Prostate Cancer Prevention Trial (PCPT) and REDUCE trial demonstrated that 5α-reductase inhibitors actually reduce the risk for prostate cancer in average and high risk (elevated PSA levels) population accordingly [Citation27,Citation29]. Nevertheless, the comments on the PCPT and REDUCE trial are controversial, with many authors still being concerned that despite the fact that 5α-reductase inhibitors do reduce the total risk for prostate cancer, they increase the risk of high grade prostate cancer.
Combination therapy
Combination therapy with α1 blockers and 5α-reductase inhibitors could potentially be beneficial due to synergistic action. Current literature on the subject reveals controversies. McConnell et al. evaluated 3047 patients with BPH in a long-term double blind trial. The effect of placebo, doxazosin, finasteride and combination therapy on the risk of clinical progression of BPH was studied. Doxazosin and finasteride reduced the risk of clinical progression of BPH 39% and 34%, respectively, compared to placebo. Combination treatment with doxazosin and finasteride resulted in a 66% risk reduction, which was significantly greater than the risk reduction with each agent alone [Citation13].
In contrast, the Prospective Europen Doxazosin and Combination Therapy trial (PREDICT) showed no significant difference between doxazosin and combination treatment of doxazosin and finasteride within a 52 week follow-up [Citation30]. Moreover, several studies have shown that α-blocker therapy can be withdrawn in the majority of men following initial combination therapy with 5α reductase inhibitors, since patients initially receiving combination therapy with finasteride or dutasteride and an α-blocker were found to experience no significant symptom deterioration after discontinuing the α-blocker [Citation31,Citation32].
Recently, Naslund et al. evaluated the impact of delaying 5α-reductase inhibitor therapy in men on α1 blocker therapy to treat BPH. In total, 6896 patients with BPH treated by combination therapy with α1 blockers and 5α-reductase inhibitors were included in the cohort. Εach 30 day delay in starting 5α-reductase inhibitor therapy after initiating α1 blockers was shown to result in increasing the likelihood for clinical progression, acute urinary retention and prostate related surgery up to 21.1%, 18.6% and 26.7%, respectively [Citation33]. Nevertheless, it was not elucidated whether the protection against clinical progression was due to combination treatment or an effect produced by 5α-reductase inhibitor treatment alone.
Minimal invasive treatment
Whenever medical therapy of BPH has failed and bothersome LUTS persist surgical intervention is indicated. Apart from clinical BPH refractory to medical therapy, other indications for surgery include refractory or recurrent urinary retention, persistent haematuria, renal insufficiency (rare) and the presence of bladder stones [Citation1]. A number of different surgical treatment modalities are available. Transurethral resection of the prostate (TURP) is considered the gold standard approach. Modifications of the conventional technique and other minimal invasive alternatives have emerged aiming to provide symptomatic relief while avoiding the morbidity associated with the conventional approach. TURP, transurethral and transrectal non-laser treatments as well as laser-based treatment options will be discussed in this review.
Trasurethral resection of the prostate
TURP is considered the gold standard approach for BPH since it provides definitive relief in most patients and its effectiveness is supported by extensive study data. TURP uses an electrical loop to remove prostate's inner portion via a transurethral approach. It is considered the most common surgical treatment for BPH worldwide comprising 95% of all BPH-related surgical approaches. It is the treatment of choice for prostates sized 30–80 ml. A major meta-analysis including 29 randomised controlled studies demonstrated that TURP resulted in a percent improvement of LUTS exceeding 70% (mean range 66–76%) and increases in Qmax of 115% (range 80–150%) [Citation35]. Prolonged hospitalisation and reasonably high rates of complications are considered the main disadvantages of the technique. Intra- and post-operative complications are correlated with size of the prostate and the duration of the procedure. In a large scale prospective evaluation of 10,654 patients who underwent TURP, the most common short-term complications were urinary retention (5.8%), need for surgical revision (5.6%), significant urinary tract infection (3.6%), haematuria requiring transfusion (2.9%) and dilutional hyponatremia (1.4%) [Citation36].
Transurethral and transrectal no-laser alternatives
Transurethral electrovaporisation of the prostate (TUEVP) uses a roller ball electrode that is rolled multiple times over prostate tissue inducing electrovaporisation to the desired depth. In a formal meta-analysis including 20 randomised controlled trials, TUEVP provided equivalent improvement to that of TURP in LUTS reduction and Qmax improvement for up to a year of follow-up. TUEVP demonstrated a more favourable profile for transfusion rates, hospital stay and catheterization time. Nevertheless, it demonstrated higher rates of urinary retention and need for re-intervention [Citation37].
Transurethral resection of the prostate in saline (TURIS) is a technique very similar to conventional TURP. The only difference is that it uses a bipolar current with both electrodes within the cystoscope and therefore can be used with saline as the irrigant. The latter decreases the risk of dilutional hyponatremia and allows procedures of longer duration [Citation38]. Ho et al. conducted a prospective randomised study in 100 patients comparing TURIS with TURP. No TURP syndrome was found in the TURIS arm of the study in contrast to two cases in the TURP group. Both treatments were found clinically equivalent at 1-year follow-up [Citation39]. In a single-centre randomised trial of 202 patients TURIS was compared to TURP. Both approaches were similar in operative duration, resection weight and radicality of resection. Nevertheless, TURIS caused 34% less bleeding than TURP, with the difference being greatest (81%) for the largest blood losses [Citation40].
Transurethral incision of the prostate (TUIP) is a minimal invasive transurethral alternative treatment option indicated for patients with small volume prostate glands (<30 ml). It involves a deep internal incision from the urethra to the external capsule in each prostate lobe. Its main advantage is that it limits the rates of retrograde ejaculation and consequently is particularly indicated for younger men interested in maintaining fertility. Moreover, it is associated with a lower incidence of complications, fewer blood transfusion rates and shorter operative time and hospital stay than TURP [Citation41]. Recently, Lourenco et al. conducted a meta-analysis of short- and long-term data from randomised controlled trials comparing TUIP with TURP. Both techniques appeared to offer equivalent symptomatic improvement for men with mild to moderate BPH. Nevertheless, a higher re-operation rate was revealed for TUIP. As the authors concluded, choosing TUIP should be based on the balance between the lower risk of perioperative morbidity and the higher risk of subsequent re-operation [Citation42].
Transrectal high-intensity focused urtrasound (HIFU) is a minimally invasive treatment option that uses a focused high intensity ultrasound beam to cause deep prostate tissue lesions without damaging superficial structures. Its use has recently been expanded in the treatment of prostate cancer. In the case of the treatment of BPH, transrectal HIFU is well-tolerated, but requires general or spinal anaesthesia or heavy intravenous sedation. It is practically a bloodless treatment option with no major intraoperative blood loss. The most prominent side-effect is prolonged urinary retention, lasting from 3 to 19 days. Hematospermia and urinary tract infections are also common complications. Lu et al. evaluated the midterm effectiveness of the approach in 150 consecutive cases of BPH. HIFU was proved safe and effective for up to 1 year postoperatively [Citation43]. Nevertheless, longer follow-up results reveal a high rate of symptom recurrence. In a study examining the long-term outcome of 80 patients with a follow-up of up to 4 years, it was demonstrated that 43.8% of patients underwent TURP due to insufficient therapeutic response [Citation44].
Transurethral needle ablation (TUNA) delivers low level radiofrequency energy to the prostate using needles inserted into the gland trasurethraly. It is considered a very safe technique with no blood loss, very low rates of complications and few anaesthetic requirements. Moreover, it does not require long hospital stay. Early postoperative morbidity includes irritative voiding symptoms lasting up to 4–6 weeks [Citation45]. Numerous studies have demonstrated that TUNA significantly improves subjective and objective BPH parameters with respect to baseline. Nevertheless, its efficacy is considered inferior to that of TURP and it is not as durable, declining in the long-term, resulting in the need for a relative high rate of secondary treatment [Citation46,Citation47]. Moreover, it is considered an expensive treatment option for BPH compared to other alternatives [Citation48].
Transurethral microwave thermotherapy (TUMT) is a safe and office-based alternative treatment option with the advantage that it has no requirements for anaesthesia and no blood loss. TUMT transmits, through an antenna, electromagnetic radiation to the prostate that is converted to heat. Resultant intraprostatic elevation of temperature leads to ablative coagulative necrosis. Initial results with TUMT were controversial due to the fact that there are many different machines with different antenna designs, cooling capabilities and patterns of necrosis. Kaye et al. conducted a meta-analysis of published randomised controlled studies comparing high energy TUMT with conventional TURP. TUMT was considered highly effective with minimum rates of morbidity at 1 year follow-up. Nevertheless, its efficacy was inferior to that of TURP. Newly developed TUMT devices are more effective than previously used lower-energy devices, especially in aims of improving objective end points [Citation49]. Long-term investigation of these new devices is needed. Finally, it is considered an expensive treatment option for BPH compared to other alternatives [Citation48].
Prostate stenting (PS) using permanent urethral stents is an alternative treatment option indicated for BPH patients with medium to severe LUTS who are poor candidates for surgical approaches (usually due to concomitant comorbidities). Current experience with such stents indicates that in well selected patients, PS can offer long-term efficacy (up to 12 years) with patient satisfaction [Citation50,Citation51]. Incontinence, persisting detrusor dysfunction and stent migration are commonly reported complications [Citation51]. More than half of stented cases are expected to fail during the initial months post-insertion. This problem has prompted the suggestion that an initial placement of a temporary stent be used instead of a permanent stent. When treatment is considered successful, the temporary stent should be exchanged with a permanent one [Citation52].
Laser-based treatment
Transurethral laser coagulation (TLC) is a laser ablation method where a side-fire laser fiber, held a small distance away from the target, is used to deliver laser energy from a transurethral orientation to the prostate. Delivered energy coagulates but does not vaporise tissue. The coagulated tissue eventually necroses and sloughs, relieving the obstruction. TLC is considered an effective surgical treatment for BPH demonstrating improvements in symptom scores, quality of life and flow rate equivalent to those attained after TURP. Moreover it is characterised by lower transfusion rates than TURP [Citation53]. Nevertheless, the major disadvantage of the technique is that it is characterised by high rates of prolonged, postoperative urinary catheterisation and a higher incidence of post-procedure irritative voiding symptoms. Moreover, long-term studies (up to 4-year follow-up) demonstrated inferior results for TLC in terms of efficacy and durability, compared with conventional electrovaporisation. Residual obstructive adenoma appears the main cause of long-term failure for TLC [Citation54].
Laser vaporisation of the prostate (LVP) is a technique similar to conventional electrovaporisation of the prostate. LVP uses high density laser energy to induce vaporisation of tissue. An immediate urethral patency is achieved, shortening catheterisation duration in the initial postoperative period. Due to the excellent coagulative properties of laser vaporisation, the technique has been proven safe and feasible even in patients undergoing anti-coagulation treatment, demonstrating minimum blood loss [Citation55]. In a recent randomised controlled study involving 1 year of follow-up, LVP was compared with conventional TURP. LVP produced equivalent improvements in flow rates and LUTS reduction to that seen with TURP. Moreover, compared with TURP, LVP markedly reduced the length of hospital stay, duration of postoperative catheterization and adverse effect rate [Citation56]. Durability of improvement in outcomes with LVP has been documented for up to 5 years with an overall re-treatment rate of 8.9% [Citation57]. Finally, compared to all other alternative treatment modalities for BPH, LVP is considered the most cost effective option [Citation48].
Holmium laser resection of the prostate (HoLRP) is a technique that directs laser energy in such a way that the prostate is resected by incising sections of tissue. Similarly to TURP, multiple small prostate chips are dissected from the obstructing prostate lobes, initially fall into the bladder, and then are retrieved through the ureteroscope. Alternatively, in Holmium laser enucleation of the prostate (HoLEP), the prostate is initially divided into its three anatomical lobes. Each lobe is then enucleated in an antegrade fashion creating the same result that is achieved via open prostatectomy [Citation58]. Several well designed studies have demonstrated that both HoLRP and HoLEP produce equivalent results as those with TURP and open prostatectomy, with significantly less perioperative morbidity, catheterisation time and hospital stay [Citation59–62 ]. Holmium laser provides excellent hemostasis leading to minimum blood loss in comparison to alternative treatment options and is safely applicable even in patients on anticoagulation therapy [Citation63,Citation64].The technique, although considered time consuming with prolonged operative times, is well suited to large prostates even greater than 100 g [Citation62]. Outcome improvement has been reported to be durable for up to 6 years [Citation65].
Laser incision of the prostate (LTIP) is a technique similar to conventional electrocautery TUIP. It is indicated for the treatment of small prostate glands (<30 g). Its main advantage is the very low incidence of postoperative catheterisation. Cornford et al. reported only a 3% rate of postoperative catheterisation in 100 consecutive patients subjected to holmium YAG LTIP. This rate was low enough to consider the approach as catheterless [Citation66].
Interstitial laser coagulation (ILC) is a transurethral thermal technique similar to ultrasound (HIFU) and microwave (TUMT) prostate thermotherapies. Using laser energy, it induces intraprostatic temperature elevation and tissue coagulation without destroying the prostatic urethra. Blood loss in ILC is considered negligible. ILC results improvements in symptom reduction and quality of life measures comparable to that seen with TURP [Citation67]. Nevertheless, the approach has been related to a high incidence of postoperative infections and prolonged (up to 24 days) catheterisation [Citation68].
Conclusions
In conclusion, a watchful waiting policy is suitable for all men demonstrating BPH-related LUTS that do not complain of high levels of bother. Medical therapy with α-blockers is characterised by a rapid effectiveness in symptom reduction in contrast with 5α-reductase inhibitors that require several months for the initiation of their clinical effectiveness. 5α-reductase inhibitors are particularly indicated for patients with enlarged prostate volumes, and their long-term effectiveness against clinical progression has been well documented. When conservative management has failed, surgery is indicated. TURP is still considered the gold standard surgical procedure for BPH. Nevertheless it is associated with significant morbidity. Several minimally invasive techniques have been added to the armamentarium of today's urologist demonstrating promising results that are generally comparable to those achieved with TURP. Laser-based treatments are safe and effective and are associated with minimum morbidity. Thus, they are gaining more and more popularity relative to other treatment modalities. Above all, selection of type of treatment should be based on the physician's experience and the patient's co-morbidities as well as prostate size and clinical progression ().
Figure 1. Treatment decision making flowchart. TURP: transurethral prostatectomy, TUEVP: transurethral electovaporisation, TURIS: trasurethral resection in saline, TUIP: transurethral incision of the prostate, HIFU: transrectal high intensity focused ultrasound, TUNA: transurethral needle ablation, TUMT: transurethral microwave thermotherapy, PS: prostatic stenting, TLC: transurethral laser coagulation, LVP: laser vaporization of the prostate, HoLPR/HoLEP: Holmium laser resection of the prostate/Holmium laser enucleation of the prostate, LTIP: laser transurethral incision of the prostate, ILC: interstitial laser coagulation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Guidelines on benign prostatic hyperplasia. European Association of Urology. Internet. 2004. Electronic Citation. www.uroweb.org. Last accessed 2004.
- Sagnier PP, McFarlane G, Teillac P, Botto H, Richard F, Boyle P. Impact of symptoms of prostatism on level of bother and quality of life of men in the French community. J Urol 1995;153:669–673.
- Chapple CR. BPH disease management. Eur Urol 1999;36 (Suppl 3):1–6.
- Brown C, Yap T, Cromwell D, Rixon L, Steed L, Mulligan K, Mundy A, Newman SP, van der Meulen J, Emberton M. Self management for men with lower urinary tract symptoms: randomized controlled trial. BMJ 2007;334:25–28.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. New Engl J Med 1995;332:75–79.
- Netto NR Jr, de Lima ML, Netto MR, D'Ancona CA. Evaluation of patients with bladder outlet obstruction and mild international prostate symptom score followed up by watchful waiting. Urol 1999;53:314–316.
- Nickel JC. The economics of medical therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia. Curr Urol Rep 2006;7:282–287.
- Morganroth J, Lepor H, Hill LA, Volinn W, Hoel G. Effects of the selective α 1a-adrenoceptor antagonist silodosin on ECGs of healthy men in a randomized, double-blind, placebo- and moxifloxacin-controlled study. Clin Pharmacol Ther 2010;87: 609–613.
- Homma Y, Kawabe K, Takeda M, Yoshida M. Ejaculation disorder is associated with increased efficacy of silodosin for benign prostatic hyperplasia. Urology 2010;76: 1446–1450.
- Roehrborn CG. Efficacy of α-adrenergic receptor blockers in the treatment of male lower urinary tract symptoms. Rev Urol 2009;11 (Suppl 1):1–8.
- Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, Morrill BB, Gagnier RP, Montorsi F; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010;57:123–131.
- Roehrborn CG. BPH progression: concept and key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int 2008;101 (Suppl 3):17–21.
- McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, Lepor H, McVary KT, Nyberg LM Jr, Clarke HS, The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003;349:2387–2398.
- Djavan B, Marberger M. Meta-analysis on the efficacy and tolerability of α1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol 1999;36:1–13.
- McNeil SA, Daruwala PD, Mitchel ID, Shearer MG, Hargreave TB. Sustained-release alfuzosin and trial without catheter after acute urinary retention: a prospective placebo-controlled. BJU Int 1999;84:622–627.
- Chan PSF, Wong WS, Chan LW, Cheng CW. Can terazosin (α blocker) relieve acute urinary retention and obviate the need for an indwelling urethral catheter? Br J Urol 1996;77 (Suppl 1):27.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol 2004;6:31–39.
- Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, Andriole GL, Geller J, Bracken BR, Tenover JS, The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992;327:1185–1191.
- Andersen JT, Ekman P, Wolf H, Beisland HO, Johansson JE, Kontturi M, Lehtonen T, Tveter K. Can finasteride reverse the progress of benign prostatic hyperplasia? A two year placebo-controlled study. The Scandinavian BPH study group. Urology 1995;46:631–637.
- Nickel JC, Fradet Y, Boake RC, Pommerville PJ, Perreault JP, Afridi SK, Elhilali MM. Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: results of a 2-year randomised controlled trial (the PROSPECT Study). CMAJ 1996;155:1251–1259.
- Vaughan D, Imperato-McGinley J, McConnell J, Matsumoto AM, Bracken B, Roy J, Sullivan M, Pappas F, Cook T, Daurio C, Long-term (7 to 8-year) experience with finasteride in men with benign prostatic hyperplasia. Urology 2002;60:1040–1044.
- Ekman P. Maximum efficacy of finasteride is obtained within 6 months and maintained over 6 years. Follow-up of the Scandinavian Open-extension Study. The Scandinavian Finasteride Study Group. Eur Urol 1998;33:312–317.
- Lam JS, Romas NA, Lowe FC. Long-term treatment with finasteride in men with symptomatic benign prostatic hyperplasia: 10-year follow-up. Urology 2003;61:354–358.
- Bruskewitz R, Girman CJ, Fowler J, Rigby OF, Sullivan M, Bracken RB, Fusilier HA, Kozlowski D, Kantor SD, Johnson EL, Effect of finasteride on bother and other healthrelated quality of life aspects associated with benign prostatic hyperplasia. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology 1999;54:670–678.
- Marberger MJ, Andersen JT, Nickel JC, Malice MP, Gabriel M, Pappas F, Meehan A, Stoner E, Waldstreicher J. Prostate volume and serum prostate-specific antigen as predictors of acute urinary retention. Combined experience from three large multinational placebo-controlled trials. Eur Urol 2000;38:563–568.
- Andriole GL, Guess HA, Epstein JL, Wise H, Kadmon D, Crawford ED, Hudson P, Jackson CL, Romas NA, Patterson L, Treatment with finasteride preserves usefulness of prostate specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology 1998;52:195–201.
- Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010;362:1192–1202.
- Yang XJ, Lecksell K, Short K, Gottesman J, Peterson L, Bannow J, Schellhammer PF, Fitch WP, Hodge GB, Parra R, Does long-term finasteride therapy affect the histologic features of benign prostatic tissue and prostate cancer on needle biopsy? PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology 1999;53:696–700.
- Kaplan SA, Roehrborn CG, Meehan AG, Liu KS, Carides AD, Binkowitz BS, Heyden NL, Vaughan ED Jr. PCPT: Evidence that finasteride reduces risk of most frequently detected intermediate- and high-grade (Gleason score 6 and 7) cancer. Urology 2009;73:935–939.
- Kirby RS, RoehrbornC, Boyle P, Bartsch G, Jardin A, Cary MM, Sweeney M, Grossman EB; Prospective European Doxazosin and Combination Therapy Study Investigators. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazocin and Combination Therapy (PREDICT) trial. Urology 2003;61:119–126.
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of α-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001;58:203–209.
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. α-Blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5-α reductase inhibitor dutasteride. Eur Urol 2003;44:461–466.
- Naslund M, Eaddy MT, Hogue SL, Kruep EJ, Shah MB. Impact of delaying 5-α reductase inhibitor therapy in men on α-blocker therapy to treat BPH: assessment of acute urinary retention and prostate-related surgery. Curr Med Res Opin 2009;25:2663–2669.
- Lepor H. The role of gonadotropin-releasing hormone antagonists for the treatment of benign prostatic hyperplasia. Rev Urol 2006;8:183–189.
- Madersbacher S, Marberger M. Is transurethral resection of the prostate still justified? Br J Urol 1999;83:227–237.
- Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, Lack N, Stief CG; Urology Section of the Bavarian Working Group for Quality Assurance. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. J Urol 2008;180:246–249.
- Poulakis V, Dahm P, Witzsch U, Sutton AJ, Becht E. Transurethral electrovaporization vs transurethral resection for symptomatic prostatic obstruction: a meta-analysis. BJU Int 2004;94:89–95.
- Botto H, Lebret T, Barrι P, Orsoni JL, Hervi JM, Lugagne PM. Electrovaporization of the prostate with the Gyrus device. J Endourol 2001;15:313–316.
- Ho HS, Yip SK, Lim KB, Fook S, Foo KT, Cheng CW. A prospective randomized study comparing monopolar and bipolar transurethral resection of prostate using transurethral resection in saline (TURIS) system. Eur Urol 2007;52:517–522.
- Fagerstrom T, Nyman CR, Hahn RG. Bipolar transurethral resection of the prostate causes less bleeding than the monopolar technique: a single-centre randomized trial of 202 patients. BJU Int 2010;105:1560–1564.
- Yang Q, Peters TJ, Donovan JL, Wilt TJ, Abrams P. Transurethral incision compared with transurethral resection of the prostate for bladder outlet obstruction: a systematic review and meta-analysis of randomized controlled trials. J Urol 2001;165: 1526–1532.
- Lourenco T, Shaw M, Fraser C, MacLennan G, N'Dow J, Pickard R. The clinical effectiveness of transurethral incision of the prostate: a systematic review of randomised controlled trials. World J Urol 2010;28:23–32.
- Lü J, Hu W, Wang W. Sonablate-500 transrectal high-intensity focused ultrasound (HIFU) for benign prostatic hyperplasia patients. J Huazhong Univ Sci Technol Med Sci 2007;27:671–674.
- Madersbacher S, Schatzl G, Djavan B, Stulnig T, Marberger M. Long-term outcome of transrectal high-intensity focused ultrasound therapy for benign prostatic hyperplasia. Eur Urol 2000;37:687–694.
- Schatzl G, Madersbacher S, Lang T, Marberger M. The early postoperative morbidity of transurethral resection of the prostate and of four minimally invasive treatment alternatives. J Urol 1997;158:105–110.
- Bouza C, López T, Magro A, Navalpotro L, Amate JM. Systematic review and meta-analysis of transurethral needle ablation in symptomatic benign prostatic hyperplasia. BMC Urol 2006;6:14–30.
- Zlotta AR, Giannakopoulos X, Maehlum O, Ostrem T, Schulman CC. Long-term evaluation of transurethral needle ablation of the prostate (TUNA) for treatment of symptomatic benign prostatic hyperplasia: clinical outcome up to five years from three centers. Eur Urol 2003;44:89–93.
- Stovsky M, Griffiths R, Duff S. A clinical outcomes and cost analysis comparing photoselective vaporization of the prostate to alternative minimally invasive therapies and transurethral prostate resection for the treatment of benign prostatic hyperplasia. J Urol 2006;176:1500–1506.
- Kaye JD, Smith AD, Badlani GH, Lee BR, Ost MC. High-energy transurethral thermotherapy with CoreTherm approaches transurethral prostate resection in outcome efficacy: a meta-analysis. J Endourol 2008;22:713–718.
- Masood S, Djaladat H, Kouriefs C, Keen M, Palmer JH. The 12-year outcome analysis of an endourethral wallstent for treating benign prostatic hyperplasia. BJU Int 2004;94:1271–1274.
- Goepel M, Senge A, Otto T, Rübben H. Long-term results of wall stent implantation in benign prostatic hyperplasia and high risk status. Urologe A 1997;36:151–156.
- Grimsley SJ, Khan MH, Lennox E, Paterson PH. Experience with the spanner prostatic stent in patients unfit for surgery: an observational study. J Endourol 2007;21:1093–1096.
- Cowles RS, Kabalin JN, Childs S, Lepor H, Dixon C, Stein B, Zabbo A. A prospective randomized comparison of transurethral resection to visual laser ablation of the prostate for the treatment of benign prostatic hyperplasia. Urology 1995;46:155–160.
- Abdel-Khalek M, El-Hammady S, Ibrahiem el-H. A 4-year follow-up of a randomized prospective study comparing transurethral electrovaporization of the prostate with neodymium: YAG laser therapy for treating benign prostatic hyperplasia. BJU Int 2003;91:801–805.
- Karatas OF, Alkan E, Horasanli K, Luleci H, Sarica K. Photoselective vaporization of the prostate in men with a history of chronic oral anti-coagulation. Int Braz J Urol 2010;36:190–197.
- Bouchier-Hayes DM, Van Appledorn S, Bugeja P, Crowe H, Challacombe B, Costello AJ. A randomized trial of photoselective vaporization of the prostate using the 80-W potassium-titanyl-phosphate laser vs transurethral prostatectomy, with a 1-year follow-up. BJU Int 2010;105:964–969.
- Hai MA. Photoselective vaporization of prostate: five-year outcomes of entire clinic patient population. Urology 2009;73: 807–810.
- Suardi N, Gallina A, Salonia A, Briganti A, Dehò F, Zanni G, Abdollah F, Naspro R, Cestari A, Guazzoni G, Holmium laser enucleation of the prostate and holmium laser ablation of the prostate: indications and outcome. Curr Opin Urol 2009;19:38–43.
- Montorsi F, Naspro R, Salonia A, Suardi N, Briganti A, Zanoni M, Valenti S, Vavassori I, Rigatti P. Holmium laser enucleation versus transurethral resection of the prostate: results from a 2-center, prospective, randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol 2004;172:1926–1929.
- Westenberg A, Gilling P, Kennett K, Frampton C, Fraundorfer M. Holmium laser resection of the prostate versus transurethral resection of the prostate: results of a randomized trial with 4-year minimum long-term follow-up. J Urol 2004;172:616–619.
- Naspro R, Suardi N, Salonia A, Scattoni V, Guazzoni G, Colombo R, Cestari A, Briganti A, Mazzoccoli B, Rigatti P, Holmium laser enucleation of the prostate versus open prostatectomy for prostates >70 g: 24-month follow-up. Eur Urol 2006;50:563–568.
- Kuntz RM, Lehrich K. Transurethral holmium laser enucleation versus transvesical open enucleation for prostate adenoma greater than 100 gm: a randomized prospective trial of 120 patients. J Urol 2002;168:1465–1469.
- Gupta N, Sivaramakrishna, Kumar R, Dogra P, Seth A. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of >40 g. BJU Int 2006;97:85–89.
- Elzayat E, Habib E, Elhilali M. Holmium laser enucleation of the prostate in patients on anticoagulant therapy or with bleeding disorders. J Urol 2006;175:1428–1432.
- Gilling PJ, Aho TF, Frampton CM, King CJ, Fraundorfer MR. Holmium laser enucleation of the prostate: results at 6 years. Eur Urol 2008;53:744–749.
- Cornford PA, Biyani CS, Powell CS. Transurethral incision of the prostate using the holmium:YAG laser: a catheterless procedure. J Urol 1998;159:1229–1231.
- Kursh ED, Concepcion R, Chan S, Hudson P, Ratner M, Eyre R. Interstitial laser coagulation versus transurethral prostate resection for treating benign prostatic obstruction: a randomized trial with 2-year follow-up. Urology 2003;61:573–578.
- Liedberg F, Adell L, Hagberg G, Palmqvist IB. Interstitial laser coagulation versus transurethral resection of the prostate for benign prostatic enlargement – a prospective randomized study. Scand J Urol Nephrol 2003;37:494–497.
