Abstract
Objective: To determine the effect of long-term testosterone replacement therapy (TRT) on depression symptoms in hypogonadal men. Methods: Data were from TRiUS, a multicenter, 12-month observational registry (N = 849) of hypogonadal men prescribed 1% testosterone gel. Measures including total testosterone (TT) were assessed at baseline and months 3, 6, and 12. Depression symptoms were measured with Patient Health Questionnaire-9 (PHQ-9), a validated self-report questionnaire. A PHQ-9 score decrease of ≥5 represents clinical improvement. Results PHQ-9 scores were available for 762/849 TRiUS participants at baseline. Overall, 92.4% (704/762) demonstrated some level of depressive symptoms, with 17.3% (132/762) having moderately severe (score 15–19) to severe (score 20–27) symptoms. Subcohorts with significantly (p ≤ 0.03) more moderately severe to severe symptoms were: <60 years old, TT levels <250 ng/dl (<8.68 nmol/l), HIV/AIDS-positive, or used antidepressants or opioids. TT levels and PHQ-9 scores improved significantly (p < 0.01) by 3 months of TRT. At 12 months PHQ-9 scores showed a clinically meaningful mean improvement of 5.62 points, patients with moderately severe to severe symptoms decreased from 17.3% to 2.1% (5/233), and subcohorts, including those defined by age (<60 years) and antidepressant use, had improved PHQ-9 scores ≥5. Conclusion: TRT may reduce depression symptoms in hypogonadal men, including middle-aged men and those using antidepressants.
Introduction
Over a lifetime, depression is the most likely mental health disorder suffered by individuals [Citation1]. While the rate of depression is generally higher in women, this gender difference disappears after the age of 65, at which point men are just as likely to suffer from depression [Citation2]. Symptoms of depression are commonly found in middle-aged to elderly men, and are among several symptoms (low libido, ED, hot flushes, fatigue, irritability, poor concentration, reduced physical performance, and sleep disturbance) associated with hypogonadism, or testosterone deficiency (TD) [Citation3].
Testosterone levels in men drop approximately 3.2 ng/dl (0.11 nmol/l) each year after the age of 30 [Citation4]. Diagnosis of TD includes a history of symptoms and biochemical evidence of low testosterone, usually defined as total testosterone (TT) levels <300 ng/dl (<10.4 nmol/l) and free testosterone (FT) levels <50 pg/ml (<173.5 pmol/l) [Citation3,Citation5–8]. Presenting symptoms of TD include sexual, cognitive, and emotional parameters, such as decreased sexual function and drive, poor concentration/memory, decreased energy, and depressive mood [Citation3,Citation8–10].
Some studies have found that testosterone replacement therapy (TRT) can relieve depression symptoms associated with hypogonadism [Citation11–13]. A meta-analysis of 7 clinical studies showed that TRT significantly improved symptoms of depression in hypogonadal men when compared with placebo [Citation12]. However, it is unclear if TRT can help across a range of symptom severity, and if specific subcohorts of hypogonadal men stand to benefit more or less based upon other characteristics.
For our study, we used data collected through the Testim® Registry in the US (TRiUS), the first prospective observational cohort registry of hypogonadal men on TRT. Depression symptoms were evaluated with the Patient Health Questionnaire-9 (PHQ-9), a short, 9-question, validated instrument for evaluating depression levels [Citation14–16]. We sought to determine if low testosterone was associated with the severity of depression symptoms in a large, hypogonadal male population and if TRT over a 12-month period significantly improved these symptoms.
Methods
Patients and design
Data were taken from the TRiUS registry (N = 849), which has been described in detail previously [Citation17]. TRiUS was a prospective, 12-month observational cohort registry of hypogonadal men on Testim 1% (testosterone gel). Inclusion criteria were: hypogonadal males naïve to Testim therapy who had completed an institutional review board-approved informed consent form. Exclusion criteria were: any hypersensitivity to ingredients in testosterone gel or any known or suspected carcinoma of the breast or prostate cancer. Non-responders to previous TRT were eligible for enrollment.
Hypogonadal diagnosis, dosing, follow-up visit scheduling, and laboratory testing and blood draws were performed by physicians according to their customary practice. For data analysis, follow-up visits were grouped into 3-, 6-, and 12-month visit time periods if they fell within 30 days prior to, or 60 days after, the visit period. If the patient had multiple visits during a time period, the visit closest to month 3, 6, or 12 was used for analysis.
Outcomes
TT, FT, bioavailable testosterone (BT), and sex hormone–binding globulin (SHBG) levels were measured at baseline and months 3, 6, and 12. To measure depressive symptoms, the PHQ-9 questionnaire was administered at baseline (day 0) and at follow-up visits. Nine questions are included in the questionnaire, and each item is scored from 0 (not at all) to 3 (nearly every day). The total PHQ-9 score can range from 0–27, with higher numbers denoting greater severity of depression. The level of depression severity was scored according to Kroenke et al. [Citation16]:
0 = No depression symptoms
1–4 = Minimal depression symptoms
5–9 = Mild depression symptoms
10–14 = Moderate depression symptoms
15–19 = Moderately severe depression symptoms
20–27 = Severe depression symptoms
Clinical improvement of depression symptoms are measured by a reduction of ≥5 on the PHQ-9 score [Citation18].
Statistical analysis
Descriptive statistics were used to characterize the demographics of the patient population and effect of TRT. Percentages are presented for all categorical data and mean ± standard deviation (SD) are presented for all numerical data, unless otherwise stated. Changes in testosterone levels and depression scores from baseline were tested using paired t-tests. Changes in percentages of patients defined by depression level were tested using the test of symmetry for correlated proportions. Testosterone levels were correlated (Spearman rho) with total PHQ-9 scores and each PHQ-9 question. p-values <0.05 were considered statistically significant. All analyses were performed using SAS (version 9.1, SAS Institute, Inc., Cary, NC).
Results
Baseline characteristics
At baseline, PHQ-9 results were available for 90% (762/849) of patients. Of patients with PHQ-9 results, TT levels were available for 87% (660/762) and FT levels were available for 54% (409/762). In this population, the mean ± SD PHQ-9 score was 8.63 ± 6.18 (mild depression) and the TT and FT levels were 284 ± 145 ng/dl (9.85 ± 5.03 nmol/l) and 43 ± 65 pg/dl (149.2 ± 225.6 nmol/l), respectively ().
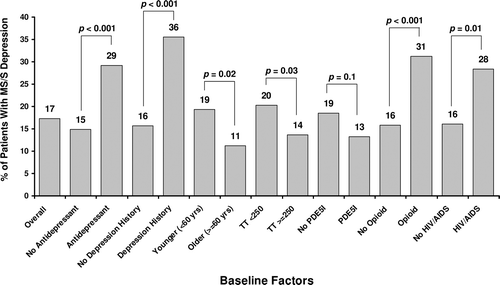
In general, hypogonadal patients showed a Gaussian distribution along the depression severity scale and patients with the most severe depression symptoms had the lowest TT levels (). However, total PHQ-9 scores did not statistically correlate with any testosterone laboratory measures (TT, FT, or SHBG). At baseline, 17.3% (132/762) of the population had moderately severe to severe depression symptoms (score ≥15), 52% (396/762) had mild to moderate symptoms (score 5–14), and 31% (234/762) had minimal to no symptoms (score <5). The percentages of patients with moderately severe to severe depression symptoms was significantly higher in subcohorts defined by antidepressant or opioid use, self-reported depression history, age <60 years, TT levels <250 ng/dl (<8.68 nmol/l), and HIV/AIDS (), but not obesity (data not shown).
Table I. Values for TT, FT, BT, SHBG, and total PHQ-9 scores at baseline and 3, 6, and 12 months of TRT.
Figure 1. Distribution of patients at baseline with depression symptoms ranging from none to severe. Symptom severity was determined using the total PHQ-9 score for each patient. The mean total testosterone level for each degree of symptom severity is listed below the graph. PHQ-9 = Patient Health Questionnaire-9.
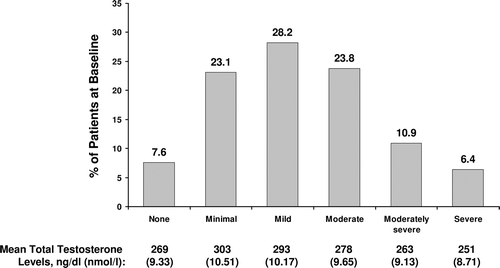
Figure 2. Percentage of patients with moderately severe to severe depression at baseline in the overall population and by subgroup. Subgroups with significantly more patients with moderately severe to severe depression were characterized by antidepressant use, self-reported depression history, age, baseline total testosterone level, opioid use, and HIV/AIDS. HIV/AIDS = human immunodeficiency virus/acquired immunodeficiency syndrome; PDE5I = phosphodiesterase type 5 inhibitors; TT = total testosterone.
Effect of testosterone replacement therapy
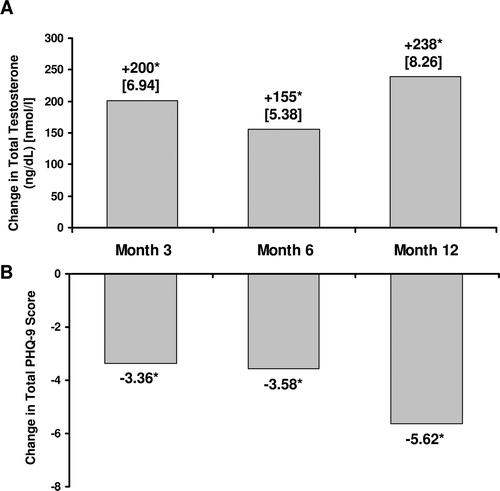
After TRT was initiated, TT and FT levels significantly increased in patients available for assessment at months 3, 6, and 12 () and corresponded with significantly (p < 0.01) decreasing total PHQ-9 scores (). Maximum benefit was seen at 12 months following TRT with a mean PHQ-9 score decrease of 5.62 ± 6.24 points, representing a clinical improvement (≥5 points [Citation18]) in depression symptoms.
Figure 3. Change in total testosterone levels (A) and total PHQ-9 scores (B) after initiation of TRT. *p < 0.01 versus baseline. PHQ-9 = Patient Health Questionnaire-9; TRT = testosterone replacement therapy; TT = total testosterone.
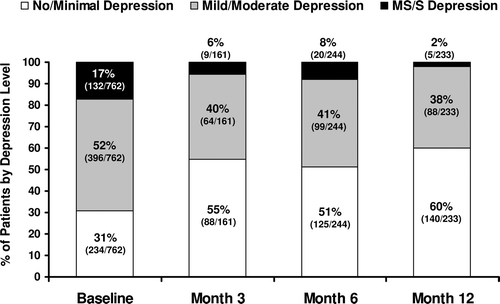
After initiation of TRT, the percentage of patients with no or minimal depression symptoms (score <5) increased whereas the percentages of patients with mild to moderate (score 5–14) and moderately severe to severe (score 15–27) depression symptoms decreased (). Maximum benefit was seen at 12 months, when the percentage of patients with no or minimal symptoms increased significantly (p < 0.0001) from 31% (234/762) at baseline to 60% (140/233), and the percentage of patients with moderately severe to severe depression decreased significantly (p < 0.0001) from 17% (132/762) at baseline to 2% (5/233).
Figure 4. Reduction in the percentages of patients with PHQ-9 scores denoting mild/moderate and moderately severe/severe depression symptoms, and increase in the percentage of patients with no/minimal depression symptoms, after initiation of TRT. The denominator within each bar is the number of patients available for PHQ-9 testing at that time point. Significant improvements were seen for all patient groups at all time points (p < 0.0001), though maximal improvement was not seen until 12 months of TRT. PHQ-9 scores: No or minimal depression symptoms, <5; mild to moderate depression symptoms, 5–14; moderately severe to severe depression symptoms, 15–27. PHQ-9 = Patient Health Questionnaire-9; TRT = testosterone replacement therapy; MS/S = moderately severe to severe depression.
For individual questions on the PHQ-9 assessment, improvements in PHQ-9 scores were significant (p < 0.01) at all time points for eight out of nine questions, though maximum improvement was not seen until 12 months TRT (). The three questions with the greatest improvements were #4 (Feeling tired or having little energy), #5 (Trouble falling or staying asleep/sleeping too much), and #3 (Little interest or pleasure in doing things). At 12-months post TRT, there were no significant correlations between TT and individual PHQ-9 scores (data not shown).
Table II. Baseline values and change in PHQ-9 scores from baseline, total and by question.
We examined the correlation of testosterone measures and total PHQ-9 scores over all time points and found statistically significant correlations only at 6 months of TRT. At 6 months, total PHQ-9 scores significantly and negatively correlated with TT (Spearman r = −0.38, p < 0.0001), FT (Spearman r = −0.22, p = 0.04), and SHBG (Spearman r = −0.43, p = 0.03).
Effect of TRT in subcohorts
After 12 months of TRT, total PHQ-9 scores were reduced to the level of mild depression in antidepressant users (total score 5–9) and minimal depression (total score <5) in all other subcohorts.
Clinical improvement (decrease in score ≥5 [Citation18]) was seen in patients who at baseline were <60 years, were taking antidepressants, had low-normal to normal TT levels (≥250 ng/dl [≥8.68 nmol/l]), were not taking phosphodiesterase type 5 (PDE5) inhibitors, and did not have HIV/AIDS (). Both opioid users and non-users also showed clinical improvement. Clinical improvement was not seen in older (≥60 years) patients, those with low TT levels (<250 ng/dl [<8.68 nmol/l]), those on PDE5 inhibitors, those not taking antidepressants, and those with HIV/AIDS.
Figure 5. Change in total PHQ-9 scores (mean ± SE) after 12 months TRT in the overall population and in subgroups. A decrease of ≥5 points (dotted line) represents a meaningful clinical response to treatment. Subgroups with significantly different responses were characterized by age, baseline total testosterone level, and PDE5 inhibitor use (bars to the left of the dashed line). PHQ-9 = Patient Health Questionnaire-9; SE = standard error; TRT = testosterone replacement therapy; TT = total testosterone.
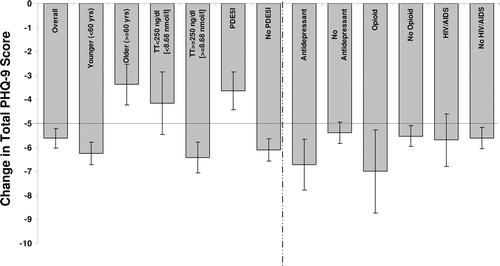
Positive prognostic factors were defined as characteristics of subcohorts that showed both clinical improvement and statically significant improvement over patients not in the subcohort; positive prognostic factors included baseline age <60 years, TT ≥250 ng/dl (≥8.68 nmol/l), and no PDE5 inhibitor use (, left of dashed line). Surprisingly, HIV/AIDS status was not a prognostic factor even though those positive for HIV/AIDS had higher PHQ-9 scores at baseline.
Discussion
Our results show that in a large population of hypogonadal men, 92.4% of patients demonstrated depression symptoms as measured by the PHQ-9, ranging from minimal to severe. The percentage of patients with moderately severe to severe depression symptoms was 17.3% in the overall population, and when analyzed by subcohort, the percentage of patients with moderately severe to severe depression symptoms was significantly greater in subcohorts defined by medication usage, age (<60 years), and TT levels. After 12 months of TRT, the percentage of patients with moderately severe to severe depression symptoms significantly decreased from 17.3% to 2.1% and PHQ-9 scores significantly improved. Overall, the mean drop in PHQ-9 score after 12 months of TRT was 5.62 ± 6.24, which represents a clinically meaningful improvement [Citation18] in depression symptoms. Subcohorts defined by age (<60 years) and antidepressant use also had PHQ-9 scores decrease ≥5 points, indicating a clinically meaningful benefit with 12 months of TRT use.
Although depressed mood is generally considered a symptom of hypogonadism [Citation3], few large epidemiological studies of the general population have found an association between depressed mood and low testosterone. Studies have found that depression symptoms were significantly associated with BT [Citation19] or could be selected for with low FT thresholds [Citation20]. Still, two other studies found no association between depression symptoms and testosterone levels in the general population [Citation21,Citation22].
However, in smaller studies, there is evidence that depression symptoms and testosterone levels may be linked. Patients with depression symptoms were significantly more likely to have lower testosterone levels [Citation2,Citation23–25] and hypogonadal men were significantly more likely to have or develop depression symptoms [Citation26–28]. Similarly, we found that while testosterone levels did not correlate with PHQ-9 scores at baseline, patients with the most severe depression symptoms also had the lowest total testosterone levels, and that the subcohort defined by total testosterone levels <250 ng/dl (<8.68 nmol/l) had a significantly higher prevalence of patients with moderately severe to severe depression symptoms than patients with higher testosterone levels.
In general, in hypogonadal populations, much like the population studied here, TRT has been shown to have a positive effect on mood. Some studies have shown significant improvements after 4–6 weeks [Citation29–31], whereas others have suggested that maximal improvement may not be reached until after 6 months of TRT or later [Citation32,Citation33]. In accordance with these studies, we saw statistical improvements within 3 months of TRT, but clinically meaningful improvements only at 12 months of TRT. Variability in the timing of improvement for individual PHQ-9 items may reflect the time necessary to reach different testosterone level thresholds; the lack of consistent correlations between symptoms and testosterone levels [Citation21,Citation22] suggests that these thresholds may be different for each patient. Improvement was observed on 8 of 9 individual items on the PHQ-9, although the degree of improvement varied somewhat, with the greatest improvement observed in the items that evaluate energy, sleep, and interest/pleasure. This is not entirely surprising, considering that these symptoms are somewhat nonspecific for depression; that is, these are symptoms associated with testosterone deficiency in general [Citation3] but that also overlap with symptoms of depression assessed by the PHQ-9, and therefore are likely to be improved with TRT.
In our subcohort analysis, we found that hypogonadal patients on antidepressants saw a clinically meaningful benefit on TRT after 12 months. This outcome supports an initial finding by Pope et al. [Citation13] that described improved depression symptoms over placebo after TRT in hypogonadal men with major depressive disorder on antidepressant therapy. However, Pope and colleagues found that these results were not replicated in a larger population [Citation34]. It could be that major depressive disorders are not amenable to improvement with TRT. In depressed populations, studies have shown that low testosterone is more often associated with the incidence of milder, long-term depressive episodes (dysthymic disorder) [Citation24,Citation25], than with intense, short-term depressive episodes (major depressive disorder) [Citation35–40]. A study by Seidman et al. [Citation41] focused on hypogonadal men with dysthymic disorder and the effect of augmenting antidepressant therapy with TRT. In this population, 6 weeks of TRT showed significant improvement over placebo, and a 69% antidepressant response versus 10% in the placebo group.
In our subcohort analysis, we identified characteristics of patients who did not show a clinically meaningful improvement in depression scores, i.e., older patients (>60 years old), those on PDE5 inhibitors, and those with TT levels <250 ng/dl (<8.68 nmol/l). It should be noted that in this univariate analysis, age, PDE5 inhibitor use, and low TT characteristics could be overlapping characteristics and describe the same group of men in our hypogonadal population.
Some would argue that the PHQ-9 cannot effectively screen for and diagnose depressive disorders as defined by the DSM-IV [Citation15], although scores ≥15 have been found to identify major depressive disorder with 95% specificity [Citation16]. We found that long-term TRT reduced the number of patients with PHQ-9 scores ≥15 from 17.3% to 2.1%, though it should be remembered that we did not have a placebo for comparison. In addition, we found that men on antidepressants saw a mean improvement of −6.72 points, which surpasses the threshold of−5 points [Citation18] for a clinically adequate treatment response. Thus, our results agree with the initial results from Pope et al. [Citation13], suggesting that testosterone may be useful as an adjunctive therapy for depressed hypogonadal men refractory to treatment with selective serotonin reuptake inhibitors (SSRIs). However, it should be noted that final PHQ-9 scores for hypogonadal men on antidepressants was >5, suggesting that this population still had mild depressive symptoms after 12 months of TRT; thus, despite antidepressant and TRT use, there was residual symptomatology which may require additional forms of therapy. It may be that patients on antidepressants with residual symptoms had more severe disease at baseline.
Because testosterone levels naturally decline with age [Citation42], one would expect that older men may be particularly vulnerable to depression and hypogonadism [Citation23,Citation28]. In elderly male populations (>70 years), men with depression were more likely to demonstrate low FT [Citation2,Citation27] and TT [Citation2]. Low FT was even found to increase the risk of future depression [Citation27]. However, we found that patients younger than 60 had a higher prevalence of moderately severe to severe depression at baseline and showed a significantly greater response to TRT than those older than age 60.
Other studies support our finding that middle-aged hypogonadal men may be particularly prone to depression. An observational study of men in the Massachusetts area found that hypogonadism was highest among men 40–60 years of age, presumably due to high levels of stress, anxiety, and depression stemming from the work and life factors of middle age [Citation43]. And in a study of middle-aged men, clinically significant depression was three times as frequent in hypogonadal men than in their eugonadal counterparts [Citation26].
Middle-aged and men suffering from depressive symptoms may not realize that they could be hypogonadal, although “midlife-onset male dysthymic disorder” is becoming recognized as a distinct disorder treatable with testosterone [Citation41]. Effective treatment of this population is of particular importance because, among other consequences such as decreased ability to respond to adverse health events, depression can reduce income-generating years due to disability or early retirement [Citation44]. Guidelines are still being developed on how to treat hypogonadal men with depression [Citation11].
The limitations of our study include those typical of a registry study, including the major limitation of having no placebo control. In addition, testosterone dosing was non-randomized and variable, not all patients were available for all follow-up visits, and, because physician behavior was not directed, there were no standardized criteria for hypogonadal diagnosis and treatment and no standardized time for blood draw for laboratory measures. Lastly, we did not have the mental health status of patients professionally evaluated before or after treatment for hypogonadism. Given these limitations, we cannot make any conclusions on whether TRT treats depression as an illness; rather, we can only draw conclusions on the effect of TRT on the depressive symptoms associated with a typical hypogonadal patient population.
Our study of the effect of long-term TRT on depressive symptoms in hypogonadal men is the first to examine subcohorts defined by age, TT levels, and concomitant drug use. We found that patients within these parameters had a significantly greater prevalence of moderately severe to severe depression, and that those who were younger, who had relatively higher testosterone levels, and who were not on PDE5 inhibitors at baseline realized the most benefit. Our data support observations that TRT may improve depression symptoms in hypogonadal men. Because depression symptoms and hypogonadal symptoms can overlap, this study reinforces the need to test for low testosterone levels in men presenting with depression, especially in middle-aged populations.
Declaration of interest: Funding for the TRiUS registry, and to support the preparation of this manuscript, was provided by Auxilium Pharmaceuticals, Inc. The authors wish to thank Tara Gupta, PhD of MedVal Scientific Information Services, LLC, for providing medical writing and editorial assistance. Dr. Khera: Allergan: research funding; Auxilium: speaker; Slate: speaker. Dr. Bhattacharya: Amgen: speaker; Auxilium: consultant, steering committee; Bristol-Myers Squibb: speaker; Novartis: speaker; Solvay: speaker. Dr. Blick: Auxilium: investigator, speaker; Tibotec: advisor/consultant, speaker; ViiV: advisor/consultant, speaker; Virxsys: research funding, advisor/consultant. Dr. Kushner: Auxilium: employee. Dr. Nguyen: Auxilium: employee. Dr. Miner: Auxilium: research funding; advisor; Endo: advisor; GlaxoSmithKline: research funding. The data from this analysis were presented as a platform at the American Urological Association 105th Annual Meeting, May 29–June 3, 2010 in San Francisco, California (Khera M et al. J Urol 2010;183(4 suppl):e578. Abstract 1499) and at the International Society of Men’s Health 7th World Congress, 28–30 October 2010 in Nice, France (Miner M et al. J Mens Health 2010;7(3):348. Abstract 213). A paper describing the methodology and baseline demographics and comorbidities of the TRiUS population has been published (Miner MM, Khera M, Bhattacharya R et al. Baseline data from the TRiUS registry: symptoms and comorbidities of testosterone deficiency. Postgrad Med. 2011;123:17–27).
References
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593–602.
- Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry 2008;65:283–289.
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–2559.
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86:724–731.
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, et al.; International Society of Andrology; International Society for the Study of Aging Male; European Association of Urology; European Academy of Andrology; American Society of Andrology. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol 2009;55:121–130.
- AACE Hypogonadism Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr Pract 2002;8:439–456.
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009;37:5–20.
- Carnegie C. Diagnosis of hypogonadism: clinical assessments and laboratory tests. Rev Urol 2004;6 Suppl 6:S3–S8.
- O’leary MP. Development of an index to evaluate symptoms in men with androgen deficiency. Rev Urol 2003;5 Suppl 1:S11–S15.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004;350:482–492.
- Kanayama G, Amiaz R, Seidman S, Pope HG Jr. Testosterone supplementation for depressed men: current research and suggested treatment guidelines. Exp Clin Psychopharmacol 2007;15:529–538.
- Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract 2009;15:289–305.
- Pope HG Jr, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105–111.
- Löwe B, Schenkel I, Carney-Doebbeling C, Göbel C. Responsiveness of the PHQ-9 to Psychopharmacological Depression Treatment. Psychosomatics 2006;47:62–67.
- Wittkampf K, van Ravesteijn H, Baas K, van de Hoogen H, Schene A, Bindels P, Lucassen P, et al. The accuracy of Patient Health Questionnaire-9 in detecting depression and measuring depression severity in high-risk groups in primary care. Gen Hosp Psychiatry 2009;31:451–459.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613.
- Miner MM, Khera M, Bhattacharya RK, Blick G, Kushner H. Baseline data from the TRiUS registry: symptoms and comorbidities of testosterone deficiency. Postgrad Med 2011;123:17–27.
- Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care 2004;42:1194–1201.
- Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab 1999;84:573–577.
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, et al.; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–135.
- Araujo AB, Durante R, Feldman HA, Goldstein I, McKinlay JB. The relationship between depressive symptoms and male erectile dysfunction: cross-sectional results from the Massachusetts Male Aging Study. Psychosom Med 1998;60:458–465.
- Berglund LH, Prytz HS, Perski A, Svartberg J. Testosterone levels and psychological health status in men from a general population: The Tromsø study. Aging Male 2011;14:37–41.
- Makhlouf AA, Mohamed MA, Seftel AD, Niederberger C, Neiderberger C. Hypogonadism is associated with overt depression symptoms in men with erectile dysfunction. Int J Impot Res 2008;20:157–161.
- Seidman SN, Araujo AB, Roose SP, Devanand DP, Xie S, Cooper TB, McKinlay JB. Low testosterone levels in elderly men with dysthymic disorder. Am J Psychiatry 2002;159:456–459.
- Markianos M, Tripodianakis J, Sarantidis D, Hatzimanolis J. Plasma testosterone and dehydroepiandrosterone sulfate in male and female patients with dysthymic disorder. J Affect Disord 2007;101:255–258.
- Hintikka J, Niskanen L, Koivumaa-Honkanen H, Tolmunen T, Honkalampi K, Lehto SM, Viinamäki H. Hypogonadism, decreased sexual desire, and long-term depression in middle-aged men. J Sex Med 2009;6:2049–2057.
- Joshi D, van Schoor NM, de Ronde W, Schaap LA, Comijs HC, Beekman AT, Lips P. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010;72:232–240.
- Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162–167.
- Jockenhövel F, Minnemann T, Schubert M, Freude S, Hübler D, Schumann C, Christoph A, et al. Timetable of effects of testosterone administration to hypogonadal men on variables of sex and mood. Aging Male 2009;12:113–118.
- Dean JD, Carnegie C, Rodzvilla J, Smith T. Long-term effects of testim® 1% testosterone gel in hypogonadal men. Rev Urol 2004;6 Suppl 6:S22–S29.
- McNicholas TA, Dean JD, Mulder H, Carnegie C, Jones NA. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU Int 2003;91:69–74.
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 2004;89:2085–2098.
- Giltay EJ, Tishova YA, Mskhalaya GJ, Gooren LJ, Saad F, Kalinchenko SY. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J Sex Med 2010;7:2572–2582.
- Pope HG Jr, Amiaz R, Brennan BP, Orr G, Weiser M, Kelly JF, Kanayama G, et al. Parallel-group placebo-controlled trial of testosterone gel in men with major depressive disorder displaying an incomplete response to standard antidepressant treatment. J Clin Psychopharmacol 2010;30:126–134.
- Undén F, Ljunggren JG, Beck-Friis J, Kjellman BF, Wetterberg L. Hypothalamic-pituitary-gonadal axis in major depressive disorders. Acta Psychiatr Scand 1988;78:138–146.
- Levitt AJ, Joffe RT. Total and free testosterone in depressed men. Acta Psychiatr Scand 1988;77:346–348.
- Rubin RT, Poland RE, Lesser IM. Neuroendocrine aspects of primary endogenous depression VIII. Pituitary-gonadal axis activity in male patients and matched control subjects. Psychoneuroendocrinology 1989;14:217–229.
- Doering CH, Brodie HK, Kraemer HC, Moos RH, Becker HB, Hamburg DA. Negative affect and plasma testosterone: A longitudinal human study. Psychosom Med 1975;37:484–491.
- Sachar EJ, Halpern F, Rosenfeld RS, Galligher TF, Hellman L. Plasma and urinary testosterone levels in depressed men. Arch Gen Psychiatry 1973;28:15–18.
- Rizvi SJ, Kennedy SH, Ravindran LN, Giacobbe P, Eisfeld BS, Mancini D, McIntyre RS. The relationship between testosterone and sexual function in depressed and healthy men. J Sex Med 2010;7:816–825.
- Seidman SN, Orr G, Raviv G, Levi R, Roose SP, Kravitz E, Amiaz R, Weiser M. Effects of testosterone replacement in middle-aged men with dysthymia: A randomized, placebo-controlled clinical trial. J Clin Psychopharmacol 2009;29:216–221.
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 2005;26:833–876.
- Guay A, Seftel AD, Traish A. Hypogonadism in men with erectile dysfunction may be related to a host of chronic illnesses. Int J Impot Res 2010;22:9–19.
- Conti RM, Berndt ER, Frank RG. Early retirement and public disability insurance applications: Exploring the impact of depression. Cambridge, MA: National Bureau of Economic Research; May, 2006.