Abstract
Introduction: The clinical significance of low to low-normal testosterone (T) levels in men remains debated. Aim: To analyze the effects of raising serum T on lean body mass (LBM), fat mass (FM), total body mass, and health-related quality-of-life (HRQoL). Methods: Randomized, double-blind, placebo-controlled study. Men, aged 50–80 years, with serum total T<15 nmol/L and bioavailable T < 6.68 nmol/L, and a Aging Males’ Symptoms (AMS) total score >36, received 6 months treatment with transdermal 1% T gel (5–7.5 mg/day; n =183) or placebo gel (n =179), followed by 12 months open-label with T in all. Results: After 6 months, LBM increased in T- treated patients by 1.28 ± 0.15 kg (mean ± SE) and FM decreased by 1.16 ± 0.16 kg, with minor changes with placebo (LBM +0.02 ± 0.10 kg and FM −0.14 ± 0.12 kg; all p < 0.001, T group vs. placebo). Changes were largely similar across subgroups of age, baseline total testosterone, and baseline BMI. Total HRQoL improved compared with placebo (p < 0.05, T group vs. placebo). Conclusions: Six months 1% T gel improved body composition and HRQoL in symptomatic men with low to low-normal T, with further improvements over the following 12 months.
Introduction
Late-onset hypogonadism (LOH) is defined as a “clinical and biochemical syndrome associated with advancing age and characterized by symptoms and a deficiency in serum testosterone” [Citation1–3]. The exact prevalence of LOH is unclear, and obviously depends on the criteria of defining LOH [Citation4,Citation5] and whether epidemiological or clinical studies of men presenting with clinical symptoms are analyzed. For instance, one widely cited clinical study indicated that androgen deficiency affected approximately 39% of men aged ≥45 years reporting to primary care centers in the USA [Citation6], while large epidemiological studies find much lower prevalence rates, with a prevalence rate of only 2.1% in men aged 40–79 years [Citation5,Citation7].
Clinical signs and symptoms of LOH include diminished sexual desire, reduced erection frequency and quality, alterations in cognitive function and mood, sleep disturbances, and osteoporosis. Changes in body composition, such as decreased LBM and an increased visceral fat mass, are also characteristic of LOH [Citation2,Citation3]. Not surprisingly, the symptomatology associated with LOH may significantly reduce a patient’s quality-of-life.
Several small studies have demonstrated the efficacy of various testosterone formulations – including intramuscular injections, oral agents, and transdermal patches and gels – in restoring serum testosterone levels and improving the clinical signs and symptoms of hypogonadism in younger men [Citation8]. The number of large, rigorously-designed, placebo-controlled studies on efficacy and safety of testosterone therapy for LOH in aging males is, however, very limited [Citation9–11].
Aims
The aim of this randomized, placebo-controlled study was to investigate the efficacy and safety of treatment with a 1% testosterone gel in men aged 50–80 years with hypogonadal to low-normal total serum testosterone (<15.0 nmol/L) and bioavailable serum testosterone lower than 6.68 nmol/L along with putative symptoms of testosterone deficiency.
Patients and methods
Primary and secondary study objectives
The primary objective of the study was the assessment of changes in LBM after 6 months of treatment with testosterone gel compared with placebo.
A secondary study objective was assessment of changes in LBM during open label treatment with testosterone gel from study month 6 to 18, and assessment of changes in LBM in subgroups of patients. Other secondary study objectives were assessment of changes in testosterone serum levels, fat mass, total body mass, bone density, AMS rating scale, and monitoring of safety parameters.
Study design and patients
The first part of the study over 6 months had a randomized, double-blind, placebo-controlled design, to assess the efficacy and safety of a transdermal hydroalcoholic 1% testosterone gel (Testogel®; Bayer Pharma AG, Berlin, Germany). The study was conducted in eight countries (Austria, Finland, Germany, Ireland, Italy, Spain, Sweden, and UK). Men aged 50–80 years with symptoms of testosterone deficiency were eligible for inclusion. Diagnosis of symptomatic testosterone deficiency required all of the following: (1) serum total testosterone <15 nmol/L (<4.3 ng/mL), (2) bioavailable testosterone <6.68 nmol/L (< 1.93 ng/mL), and (3) an Aging Males’ Symptoms (AMS) rating scale total score >36 [Citation12].
The methods used to identify potential patients for screening varied between study centres. Some centres used newspaper and radio adverts and leaflets in primary care settings to identify patients with AMS scores suitable for inclusion. Other methods of patient identification included approaching primary care physicians and/or specialist clinics (e.g. erectile dysfunction clinics) for suitable referrals.
Two separate samples of baseline testosterone were taken before 10 a.m. and the mean value was used to determine inclusion of the patient, and a baseline value of serum total testosterone level of <15 nmol/L as well as bioavailable serum testosterone < 6.68 nmol/L was required for inclusion in the study. A recent study indicates that total testosterone is not sensitive enough to detect hypogonadism unless the serum level of total testosterone is 400 ng/dL or higher [Citation13].
These values are higher than in most other studies investigating the effects of testosterone administration to elderly men [Citation14–17]. However, Snyder et al. in their landmark study using testosterone in men over 65 years of age to study effects on body composition had chosen a threshold of 475 ng/dL (16.5 nmol/L [Citation14]). There is no generally accepted lower-limit-of-normal for serum testosterone and the effects of testosterone therapy in relation to baseline testosterone levels are largely unknown. Recent evidence suggests that symptoms of testosterone deficiency accumulate as total testosterone levels fall below 15 nmol/L [Citation18]. The European Male Aging study [Citation5] showed that though sexual symptoms have a low threshold, there is a wide range of serum testosterone levels at which these symptoms occur, for instance, the onset of a decreased level of vigorous activity was observed when serum testosterone was below 13 nmol/L (3.7 ng/mL), slightly above the widely cited 12 nmol/L cut-off value for hypogonadism.
Levels of serum total testosterone and bioavailable testosterone were assessed at a central laboratory. The testosterone levels were measured by electrochemiluminescence immunoassay (ECLIA) technique on a Roche Elecsys or Modular E170 analyzer. The intra-assay coefficient of variation (CV) was 2.1% and inter-assay CV was 2.8% for the range of testosterone values measured. The bioavailable testosterone was calculated by using the formula of Vermeulen et al. by using the total testosterone, the levels of sex hormone binding globulin and of albumin [Citation19].
Eligible patients were willing to avoid significant changes in physical activity and lifestyle during the study. The main exclusion criteria were: use of androgen therapy or anabolic steroids within the previous 12 months; contraindication or hypersensitivity to 1% testosterone gel; body mass index (BMI) >35 kg/m2; prostate specific antigen (PSA) level ≥4 ng/mL or severe symptomatic benign prostatic hyperplasia (International Prostate Symptom Score [IPSS] ≥20); suspected or past history of prostate or breast cancer; hematocrit >50%; prolactin >25 ng/mL; metallic implants (except in the head); concurrent use of cytochrome P450-inducing medications (e.g. quinidine, ketoconazole, macrolides), corticotrophins, or oxyphenbutazone; presence of sleep apnoea, polycythemia, hypothalamic-pituitary disorders, psychiatric disorders, uncontrolled diabetes mellitus or diabetes mellitus with vascular changes, uncontrolled thyroid disorders, hypertension or epilepsy; and severe cardiac, and hepatic or renal insufficiency.
The study was approved by the respective independent ethics committees and institutional review boards, and was conducted in accordance with ICH Good Clinical Practice Guidelines and the Declaration of Helsinki. Patients provided informed written consent.
Treatment assignment and study medications
Of the 1316 men screened, 362 were eligible for inclusion and they were randomized in parallel to receive testosterone gel (5 g, equivalent to 50 mg testosterone) or placebo gel (5 g) using a computer-based randomization system. Randomization was performed by the Central Randomization Group of Bayer Pharma AG (Berlin, Germany).
Patients received testosterone gel or identical placebo gel in a foil sachet (2.5 or 5 g gel) for daily topical self-administration. Gel was applied to clean, dry, healthy skin over both shoulders, both arms or the abdomen at the same time of day (preferably in the morning). Double-blind treatment continued for 6 months. At month 3 (visit 4) there was an option to increase the dose based on the clinical response of the patient. Depending on the clinical response of the patient, and if total testosterone was not above 28 nmol/L (>8.1 ng/mL), the dose of testosterone or placebo gel could be increased after 3 months to 7.5 g (75 mg testosterone), and if the effects were still not satisfactory, a further increase to 10 g (100 mg testosterone) followed during the open-label phase.
To maintain blinding, total testosterone measurements were performed at the central laboratory. The investigator was warned if values of total testosterone were >28 nmol/L (>8.1 ng/mL) and in these men the dosage was decreased until laboratory measurement values were indicating values <28 nmol/L. At the end of the 6 month randomized, double- blind treatment phase (month 6, visit 5), patients who had their dose increased at month 3 (at visit 4) or at month 6, had their treatment unblinded. During the double-blind phase 39 men discontinued. At the end of the 6-month, double-blind study period 323 (89.2%) of the original 362 patients opted to continue testosterone treatment in an open-label extension phase for an additional 12 months, that was completed by 244 (67.4% of the original study group) men.
Baseline visits were performed ≤ 21 days after screening. During the baseline visit, patients were randomized to treatment with testosterone or placebo, and DEXA scans were performed. Treatment started the day after the baseline visit, and selective assessments were performed at 6 weeks and 3, 9 and 12 months. Full assessments were made at 6 and 18 months.
Hormone assays
Blood samples were collected at baseline, at the 3-month and all subsequent follow-up visits. Unexpectedly high levels of serum total testosterone (>52 nmol/L) were detected in some patients at 3 and 6 months, probably due to contamination caused by the presence of a high concentration of the study drug in the skin at the site of blood sampling. To identify contaminated testosterone measurements, all available blood samples were re-assessed and levels of 5α-dihydrotestosterone (DHT) and estradiol (E2) were measured. If the TT/E2 or TT/DHT ratio was found to be greater than the mean normal TT/E2 or TT/DHT ratio plus 2 × SD, the sample was considered to be contaminated and the patient’s testosterone value was excluded from the final analysis of serum total testosterone levels. In total, samples for 1/172 and 1/169 testosterone-treated patients at 3 and 6 months, respectively, met the criteria for exclusion. No samples from placebo-treated patients met the exclusion criteria.
Efficacy assessments
Body composition
LBM, fat mass and total body mass were measured using whole-body dual-energy X-ray absorptiometry (DEXA), which was conducted at each study centre using apparatus from GE Healthcare (Chalfont St Giles, Buckinghamshire, UK) or Hologic Inc (Bedford, MA, USA). DEXA scans were performed with patients in the supine position and lasted 7–15 min. To ensure the accuracy and consistency of DEXA measurements during the study, apparatus quality control was managed by Bio-Imaging Technologies, BV (Leiden, the Netherlands). Instrument calibration was performed at the start of the study and at the end of the open label extension phase, using a body composition phantom (Bio-Imaging Variable Composition Phantom VCP).
Health-related quality-of-life
Health-related quality-of-life (HRQoL) was assessed using the AMS rating scale. This self-administered scale includes 17 symptom-related items evaluated on a five-point scale (1 = no symptoms, 5 = very severe symptoms [Citation12]). Translations of the AMS were performed in accordance with international recommendations for linguistic and cultural adaptation of HRQoL measures. Total AMS scores and scores for the psychological, somatic and sexual subscales of the scale were calculated. For the computation of the total scores 3 of 17 missing items were allowed, subsequently imputed with the mean of the remaining item values. For the total scale, a score ≤ 26 indicates no impairment and ≥ 50 severe impairment. For the psychological subscale, a score of ≤ 5 indicates no impairment and ≥ 12 severe impairment. For the somatic subscale, a score of ≤ 8 indicates no impairment and a score of ≥ 19 severe impairment. For the sexual subscale, a score of ≤ 5 indicates no impairment and a score of ≥11 severe impairment.
Safety assessments
A complete medical and surgical history was obtained during screening. Physical examinations and vital sign monitoring were performed during screening, at 6 months, and during the 12 months open-label study. Clinical laboratory assessments of hepatic and hematological function, serum lipids and serum hormone levels were performed at a central laboratory. Treatment was stopped if hematocrit levels were > 54%.
Prostate safety was monitored by digital rectal examination and measurement of IPSS and PSA levels. Several safety guidelines for testosterone administration to elderly men regard this as adequate [Citation2,Citation8]. Treatment was stopped if PSA levels increased by >1 ng/mL and >50% above screening values, or if prostate cancer or breast cancer was suspected. Adverse events were monitored throughout the study and are presented descriptively.
Statistical analysis
The primary objective of the study was to analyze the difference in the change in LBM (i.e. primary endpoint) at 6 months following treatment with testosterone vs. placebo. It was estimated that 286 patients were needed to detect a clinically significant difference of 1 kg at a significance level of 5% with 80% power. To allow for patient dropout, target enrolment was 360.
Body composition data are presented for all patients in the intention-to-treat (ITT) population who underwent DEXA-scanning at the baseline visit as planned. For patients who underwent DEXA scans at the baseline visit but not at month 6, baseline body composition data were used to replace the missing 6-month data; this method of analysis ensured a conservative approach to establishing the efficacy of active treatment (testosterone therapy). HRQoL data are presented for all ITT patients who completed AMS questionnaires as planned. All other data, unless otherwise indicated, are presented for the ITT population.
Analysis of variance was used to test for possible treatment effects, and to compare the effects following treatment with testosterone vs. placebo. The primary endpoint was also analyzed in subgroups by patient age (<65 and ≥65 years), baseline serum total testosterone (<10, 10 to <12, and 12 to <15 nmol/L), and BMI (below or ≥ 30 kg/m2). Changes in fat mass, total body mass and AMS (sub)scales were also analyzed using the methodology described for the primary endpoint. All tests were two-tailed with p < 0.05 denoting statistical significance. The software used was SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
Patients and study medication
A total of 1315 patients were screened for eligibility. The ITT population included 362 patients (testosterone, n = 183; placebo, n = 179). In total, 168/183 (91.8%) patients in the testosterone group and 155/179 (86.6%) in the placebo group completed the first 6 months of the study thus exceeding the target number of 286 patients. Of the testosterone/testosterone treatment group 118 men and of the placebo/testosterone treatment group 126 completed the 12 months unblinded study phase.
There were no relevant differences in baseline demographics or clinical characteristics between groups receiving testosterone and placebo (). Overall, 68/183 (37.2%) of testosterone-treated and 57/179 (31.8%) of the placebo-treated patients were aged ≥65 years.
Table I. Baseline demographic and clinical characteristics in the ITT population, according to the study group.
Compliance to study medication – assessed as the proportion of patients who applied ≥70% of planned total doses – was comparable across treatment groups (testosterone, 175/183 [95.6%]; placebo, 169/179 [94.4%]). At month 3, the dose of study medication (testosterone or placebo gel) was increased to 7.5 g in 113/174 (64.9%) patients in the testosterone group and 124/165 (75.2%) patients in the placebo group.
Serum total testosterone
Serum total testosterone levels increased following treatment with testosterone. Mean (SD) total testosterone levels at 3 and 6 months in the testosterone group were 20.23 (20.45) and 20.12 (15.38) nmol/L, respectively, vs. 10.82 (3.27) and 10.77 (3.43), respectively, in the placebo group. Total testosterone levels in the placebo group at 3 and 6 months were comparable to baseline levels. Thereafter, serum testosterone levels remained stable in the testosterone group while serum testosterone rose to levels, similar to the T treatment group (21.8 (10.17) nmol/L) in the former placebo group. Age <65 or ≥ 65 was no factor in serum testosterone achieved after testosterone administration (data not shown).
Efficacy
Body composition
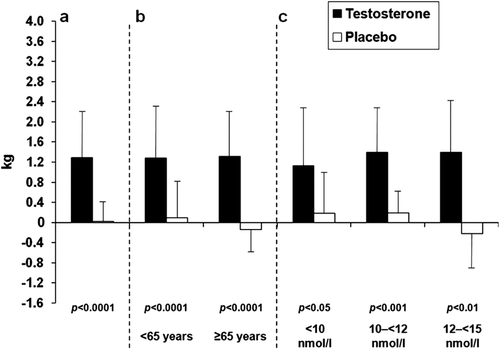
The primary endpoint of the study – the change in LBM after 6 months – was statistically significant in favour of testosterone. The mean (± SE) increase in LBM was +1.28 ± 0.15 and +0.02 ± 0.10 kg in testosterone and placebo groups, respectively (p < 0.001 for group difference; ; ). Over the next 12 months of the study there was no further increase in LBM in the group treated with testosterone but in the group previously on placebo and later treated with testosterone LBM increased significantly, and reached similar values at the end of the study as encountered in the group receiving testosterone during the double-blind phase of the study. Increases in LBM following testosterone therapy were also significant when patients were grouped according to age or baseline serum total testosterone (). Interestingly, there were also gains in the group of men whose baseline serum testosterone levels were between 12 and <15 nmol/L ().
Table II. Outcomes at 6 months during the randomized phase, according to treatment group.
Figure 1. Changes in outcomes during the trial according to treatment status. Error bars represent standard error (SE). *: p < 0.05 for difference between the two groups. **: p < 0.001 for difference between the two groups. is based on data from individual patients without applying imputation methods.
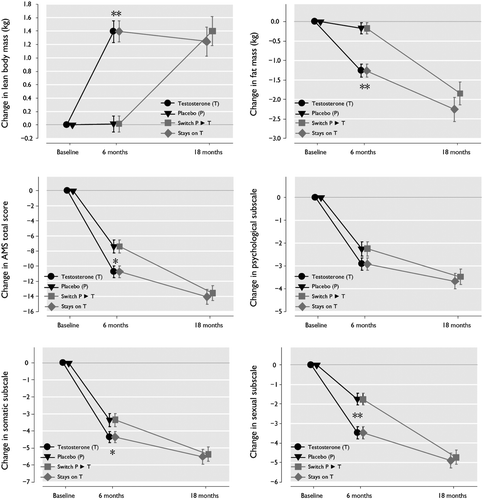
Figure 2. Mean (SD) changes in lean body mass at 6 months with testosterone versus placebo in all patients (a), and in patients grouped by age (b) and by baseline serum total testosterone levels (c).
Significant decreases in fat mass were associated with testosterone therapy; after 6 months, the mean (SD) decrease in fat mass following testosterone and placebo treatment was – 1.16 ± 0.16 and – 0.14 ± 0.12 kg, respectively (p < 0.001 for group difference; ; ). This decrease in fat mass was largely similar across subgroups of age, baseline total testosterone, and baseline BMI, although testosterone-associated changes in fat mass were not statistically significant vs. placebo in all subgroups (). The effect on body fat mass was somewhat more pronounced in the obese population (i.e. BMI ≥30 kg/m2; ). Over the next 12 months of the study there was a statistically significant progressive decrease of fat mass. In the testosterone treated group at month 18, fat mass had decreased further (by an additional – 1.11 ± 0.24 kg to a total of 2.45 ± 0.48 kg. In the placebo group that switched to testosterone after 6 months an almost similar decrease in fat mass was seen at the end of the 18 month study ().
Table III. Effect of Testosterone on the main outcomes at 6 months during the randomized phase, in subgroups.
Changes in bone mineral density, bone mass, and total body mass were not significantly different between the randomized groups (p = 0.41; p = 0.96, and p = 0.29, respectively) ().
Health-related quality-of-life
HRQoL assessed with the AMS rating scale improved in both treatment groups. Testosterone therapy was associated with a significantly greater decrease in the mean (± SD) AMS total score at 6 months (testosterone, – 10.54 ± 0.75 vs. placebo – 7.19 ± 0.78; p = 0.002) (; ). Scores on the AMS rating scale remained above or slightly below 36 which value had been taken as a cut-off value to diagnose symptomatic LOH. Analysis of the AMS subscales revealed statistically significant decreases in the psychological (testosterone, – 2.89 ± 0.26 vs. placebo – 2.12 ± 0.29; p = 0.049) () and sexual (testosterone, – 3.37 ± 0.31 vs. placebo – 1.69 ± 0.27; p < 0.001) () subscale scores following testosterone therapy, and a similar difference that approached statistical significance for the somatic subscale scores (p = 0.053). Over the next 12 months of the study there was a progressive decrease of scores on the AMS and its subscales (). At month 18, the mean total AMS score for all patients was 33.4 ± 9.82.
Safety
Adverse events
During the double-blind treatment phase, 10/183 (5.5%) and 12/179 (6.7%) patients in the testosterone and placebo groups, respectively, discontinued the study due to adverse events. Adverse event profiles were similar in the groups receiving testosterone and placebo. The number of patients experiencing one or more serious adverse events was 7/183 (3.8%) and 6/179 (3.4%) in testosterone and placebo groups, respectively. Five patients experienced serious adverse events considered to be potentially related to the study medication (testosterone group). One patient was diagnosed with prostate cancer and subsequently treated with radical prostatectomy. Another underwent a prostate biopsy in which intraepithelial neoplasia was diagnosed, and the third experienced a worsening of prostate-related symptoms which resolved following transurethral resection of the prostate (TURP). A fourth patient had a tendon rupture of the biceps, and a fifth a deep vein thrombosis.
One patient in the testosterone group died of coronary artery disease; this cause of death was not considered related to the study medication.
Hematocrit and prostate safety
The number of patients in the testosterone group that discontinued the study due to increased hematocrit (>54%) during the first 6 months of treatment was 2/183 (1.1%). No patients in the placebo group discontinued due to an increased hematocrit. In the 12 months extension period 21/249 patients left the study because of elevations of the hematocrit.
Mean (range) PSA levels in the testosterone group at baseline, 3 and 6 months were 1.25 (0.1–3.7, n = 183), 1.32 (0.2–4.9, n = 173) and 1.44 (0.3–5.5, n = 169) ng/mL, respectively. Corresponding values in the placebo group were 1.31 (0.2–3.7, n = 179), 1.27 (0.2–8.1, n = 165) and 1.23 (0.2–4.9, n = 155) ng/mL. A total of 2/183 (1.1%) patients in the testosterone group and 4/179 (1.1%) in the placebo group during the double-blind phase discontinued due to increased PSA (Table IV); of these, one patient in the testosterone group also had increased hematocrit. For the group that received testosterone gel during the first 6 months and during the open-label treatment phase PSA mean values (in ng/mL) were as follows: 1.25 (SD 0.79) at screening and 1.58 (SD 1.08) at month 18. Mean change from screening for these patients was +0.18 ng/mL (SD 0.59) at month 6 and +0.29 ng/mL (SD 0.68) at month 18. Twenty-nine patients prematurely discontinued their study participation over the open label phase due to increases of PSA ().
Table IV. Discontinuations due to increases of serum PSA >1 ng/mL and/or >50% above value at screening.
Mean (SD) IPSS scores at baseline and 6 months, respectively, were 7.1 (5.1, n = 183) and 6.4 (5.6, n = 166) in the testosterone group and 8.1 (5.8, n = 179) and 8.7 (7.2, n = 155) in the placebo group. Subsequent treatment with testosterone gel until month 18 resulted in mean values of 6.1 (4.97). Abnormal digital rectal examinations at baseline and 6 months were reported in 26/181 (14.4%) and 16/164 (9.8%) of testosterone-treated patients, and 24/176 (13.6%) and 13/153 (8.5%) of patients in the placebo group. The percentage of abnormal findings in all patients who received testosterone gel throughout the study was 11.7 % at baseline and 11.3 % after month 18.
Discussion
The stringent, placebo-controlled design of this study over the first 6 months, the recruitment of a large number of patients (n = 362), dose titration of testosterone and scores on the AMS as an inclusion criterion, enabled the robust analysis of the efficacy and safety of testosterone therapy in men with total serum testosterone < 15.0 nmol/L and bioavailable serum testosterone < 6.68 nmol/L. The study met its primary aim to demonstrate significant increases of LBM during testosterone therapy compared to placebo (mean increase with testosterone, 1.28 kg) in men with low or low-normal testosterone levels and symptoms of androgen deficiency. These favorable increases in LBM were accompanied by significant decreases in fat mass (mean decrease, 1.16 kg). There was little effect of testosterone therapy on total body mass, probably because testosterone-associated changes in lean body mass and fat mass were of similar magnitude. Hormone assays revealed that serum total testosterone levels were restored to the normal range in patients receiving testosterone therapy (mean value at 6 months, 20.1 nmol/L).
The beneficial effects of testosterone therapy on body composition appeared to be largely independent of baseline serum total testosterone levels in this patient group, provided they were <15.0 nmol/L; indeed, changes in LBM and fat mass with testosterone versus placebo were also significant in patients within the highest baseline total testosterone subgroup (12 to <15 nmol/L). Most guidelines for treatment of LOH define the cut-off point for eligibility for testosterone administration at ≤12 nmol/L, while our study included subjects with testosterone levels <15.0 nmol/L and found benefits in the group with testosterone levels between 12 and <15 nmol/L, similar to earlier studies [Boyanov et al. (T < 15 nmol/L [Citation20]), Snyder et al. <16.5 nmol/L [Citation15], Marin et al. (T < 20 nmol/L [Citation21])), Allan et al (T < 15 nmol/L [Citation22]) providing evidence that changes in body composition indeed occur in borderline eugonadal men.
Testosterone-associated changes in fat mass were not statistically significant in the ≥80 years subgroup or the 10 to <12 nmol/L baseline total testosterone subgroup. However, the study was not sufficiently powered to detect differences among all subgroups. Other possible reasons include potential differences in the time course of muscle accretion and adipose-tissue mobilization between patient subgroups. Our blinded placebo-controlled phase was of rather short duration (6 months). A longer duration might have found more positive effects as in the study of Snyder et al. [Citation23] using transdermal testosterone and Page et al. [Citation14], or in open-label studies [Citation24,Citation25] using parenteral testosterone. Two recent publications indicate that there is a time course of the effects of testosterone administration and that a range of improvements of metabolic parameters follow improvements of body composition. The present study was relatively short and did not allow to observe the potential benefits of the intervention on variables other than body composition [Citation26,Citation27].
Body composition data from this study provide valuable information regarding which patients might be suitable for testosterone therapy. For example, the relationships between therapeutic outcomes and baseline testosterone levels are largely unknown. Current recommendations state that symptomatic LOH should be associated with serum total testosterone levels <8–12 nmol/L to qualify for testosterone treatment [Citation28]. However, there is no generally accepted lower-limit-of-normal for serum testosterone for testosterone deficiency in older men, and a cross-sectional cohort study in men aged 50–86 years has shown that symptoms and metabolic risk factors associated with LOH accumulate as total testosterone levels fall below 15 nmol/L [Citation18]. In the current large prospective placebo-controlled study, testosterone therapy significantly increased LBM and decreased fat mass in men with baseline total testosterone levels between 12 and <15 nmol/L and bioavailable serum testosterone lower than 6.68 nmol/L [Citation18,Citation29].
Overall, the effects of testosterone on body composition in men with symptoms of androgen deficiency and serum total testosterone <15 nmol/L in this study are comparable with previously reported studies with testosterone gel involving men with total testosterone ≤10.4 nmol/L [Citation10,Citation17,Citation30]. Further subgroup analyses in this study revealed significant testosterone-associated improvements in LBM in men aged ≥65 years. The mean age of men in previous studies of this formulation was approximately 50 years (range 19–68 years [Citation17,Citation30]). In the study of Snyder and colleagues [Citation15,Citation31], body composition was measured only in men aged >65 years with baseline serum total testosterone <16.5 nmol/L [Citation15]. After 36 months, increases in lean body mass and decreases in fat mass were significantly greater following treatment with a scrotal testosterone patch compared with placebo [Citation31]. In contrast to the present study, however, the magnitude of body composition changes was not independent of baseline serum testosterone; in fact, a significant and inverse relationship between the effects of testosterone therapy on body composition and baseline serum testosterone was reported. The latter was not confirmed by Di Sante et al. [Citation32] who demonstrated biological effects of testosterone administration irrespective of baseline total testosterone levels. Saad et al. reported that increments in testosterone predicted treatment effects [Citation33]. Whether these findings reflect differences in the pharmacokinetic properties of the testosterone formulations used in the present study and that of Snyder et al. [Citation15] is unclear.
In androgen-deficient men, alterations in body composition coexist with metabolic complications, and epidemiologic studies have shown that low testosterone levels are strongly associated with metabolic syndrome [Citation34–37]. Testosterone therapy has been reported so far in studies – of short duration and limited sample size – to have a protective role in metabolic syndrome and to improve glucose control, dyslipidaemia and visceral adiposity in men with or without overt type 2 diabetes [Citation38–42], but these variables of the metabolic syndrome were not tested in our study. However, the primary endpoint of this study, LBM, is probably a highly relevant variable modifying the long-term risk of insulin resistance and metabolic syndrome [Citation43,Citation44]. In addition, lower LBM or muscle mass in contrast to other signs and symptoms of hypogonadism has been shown to be typical not only for patients with severe, but also moderate LOH [Citation45]. Future studies should investigate the prospective relevance of testosterone-induced changes in LBM, along with changes in fat mass, on the incidence of newly diagnosed metabolic syndrome or diabetes mellitus in testosterone-treated hypogonadal patients.
To our knowledge, this is the first reported use of the AMS rating scale in a large, randomised, placebo-controlled, clinical study. This scale was developed to assess the severity of symptoms of aging in men and to measure the effects of treatment on HRQoL [Citation46,Citation47]. A modest but significant reduction in AMS total scores was associated with testosterone treatment in this study, which was progressive over time. The clinical relevance is not certain since scores were still in the upper range of normal or above the normal range.
Only a trend towards significance was found for the differences in the somatic subscale scores of the AMS among the two groups, but the analysis of the AMS psychological and sexual subscales revealed statistically significant improvements that were also documented in other studies [Citation16,Citation17]. The improvement in the subscale of sexual functioning was the strongest compared to the placebo group; also pronounced improvements were found in subscales which measure subjective symptoms. A possible explanation might be that androgen-dependent prevalence of loss of libido is already present in men whose total testosterone concentrations are < 15 nmol/L [Citation3]. This testosterone level was the cut-off point for inclusion in this study and other androgen-dependent complaints presenting only at lower serum testosterone thresholds might have been overall less prevalent in our study population. The findings of this study are not only in support of previously reported beneficial effects of testosterone therapy on quality-of-life [Citation48], but also support the use of the AMS rating scale in this patient population.
Testosterone therapy was generally well tolerated during the study. The number of patients who discontinued the study (21/249) due to raised hematocrit levels in the 12 months extension phase, however, reiterates the need for hematological monitoring in men receiving testosterone therapy. In this study, increases in serum PSA were only slightly greater in the testosterone group compared with placebo. Thirty-one testosterone-treated men left he study because of increases of serum PSA >1 ng/mL and / or >50% above screening values. One man was diagnosed with prostate cancer and one with prostatic intraepithelial neoplasia, and a third one experienced a worsening of prostate-related symptoms, which resolved following transurethral prostate resection. Digital rectal examination and PSA measurements remain mandatory in men with LOH prior to, and at regular intervals during, testosterone treatment [Citation8,Citation28].
Conclusions
This large, randomized, double-blind, placebo-controlled study demonstrated that 6 months’ treatment with testosterone improves LBM and HRQoL and decreases fat mass in men with serum total T < 15.0 nmol/L, bioavailable T < 6.68 nmol/L and symptoms of androgen deficiency. Over the following 12 months there were progressive decreases in fat mass and AMS scores, indicating progressive improvement of HRQoL. Testosterone therapy was well tolerated in this patient population. Careful monitoring of potential side effects is mandatory.
Acknowledgements
The European Testogel® Study Team: Austria: R Herwig, Innsbruck; A Jungwirth, Salzburg; M Lamche, Vienna; G Nell, Vienna; G Pinggera, Innsbruck; N Schmeller, Salzburg; P Schramek, Vienna; H Trummer, Graz; Finland: O Lukkarinen, Oulu; V Nurmenniemi, Helsinki; T Tammela, Tampere. Germany: H M Behre, Halle; G Haidl, Bonn; B Lochmann, Dresden; G Meissner, Magdeburg; H Stahl, Leipzig; Italy: V Bonifacio, Rome; F Colombo, Milan; C Imbimbo, Napoli; C Moretti, Rome; A Fabbri, Rome; Ireland: M Byrne, Dublin; J Nolan, Dublin; Spain: J M Cuadrado, Barcelona; I Moncada, Madrid; E Ruiz Castañé, Barcelona; J R Tolrá, Barcelona; Sweden: S Arver, Stockholm; A Giwercman, Malmö; G Johannsson, Göteborg; UK: P-M Bouloux, London; J Fraser, Wigan; A Grossman, London; R Pawa, Wigan; R Ross, Sheffield; H Shaw, Reading; H Thomas, Cardiff; study management: J Kelly, Berlin, Germany; statistical analyses: F Hiemeyer, Berlin, Germany.
Declaration of Interest: Josep R. Tolrá, Vincenzo Bonifacio and Michael Lamche have nothing to declare. Teuvo L. J. Tammela has received grant support from Bayer Pharma. Stefan Arver, Hermann M. Behre and Erik J. Giltay have received grant support and/or lecture fees from Bayer Pharma. Judy Kelly and Florian Hiemeyer are employed by Bayer Pharma. This study was sponsored by Bayer Pharma AG, Berlin, Germany.
References
- Mäkinen JI, Huhtaniemi I. Androgen replacement therapy in late-onset hypogonadism: current concepts and controversies – a mini-review. Gerontology 2011;57:193–202.
- Wang C, Nieschlag E, Swerdloff RS, Behre H, Hellstrom WJ, Gooren LJ, Kaufman JM, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2009;12:5–12.
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol 2009;5:673–681.
- Page ST. Reproductive endocrinology: Late-onset hypogonadism: evidence for diagnostic criteria. Nat Rev Endocrinol 2010;6:602–603.
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, et al.; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–135.
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762–769.
- Liu PY, Beilin J, Meier C, Nguyen TV, Center JR, Leedman PJ, Seibel MJ, et al. Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab 2007;92:3599–3603.
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–2559.
- Cunningham GR. Testosterone replacement therapy for late-onset hypogonadism. Nat Clin Pract Urol 2006;3:260–267.
- Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602–612.
- Gooren LJ. Androgens and male aging: Current evidence of safety and efficacy. Asian J Androl 2010;12:136–151.
- Heinemann LA. Aging Males’ Symptoms scale: a standardized instrument for the practice. J Endocrinol Invest 2005;28:34–38.
- Anawalt BD, Hotaling JM, Walsh TJ, Matsumoto AM. Performance of total testosterone measurement to predict free testosterone for the biochemical evaluation of male hypogonadism. J Urol 2012;187:1369–1373.
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 2005;90:1502–1510.
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 1999;84:2647–2653.
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R; North American AA2500 T Gel Study Group. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab 2003;88:2673–2681.
- Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, et al.; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839–2853.
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 2006;91:4335–4343.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672.
- Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 2003;6:1–7.
- Mårin P, Holmäng S, Jönsson L, Sjöström L, Kvist H, Holm G, Lindstedt G, Björntorp P. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 1992;16:991–997.
- Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab 2008;93:139–146.
- Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab 2000;85:2670–2677.
- Haider A, Gooren LJ, Padungtod P, Saad F. Improvement of the metabolic syndrome and of non-alcoholic liver steatosis upon treatment of hypogonadal elderly men with parenteral testosterone undecanoate. Exp Clin Endocrinol Diabetes 2010;118:167–171.
- Haider A, Gooren LJ, Padungtod P, Saad F. Concurrent improvement of the metabolic syndrome and lower urinary tract symptoms upon normalisation of plasma testosterone levels in hypogonadal elderly men. Andrologia 2009;41:7–13.
- Saad F, Aversa A, Isidori AM, Zafalon L, Zitzmann M, Gooren L. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol 2011;165:675–685.
- Saad F, Haider A, Giltay EJ, Gooren LJ. Age, obesity and inflammation at baseline predict the effects of testosterone administration on the metabolic syndrome. Horm Mol Biol Clin Invest 2011;6:193–199.
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, et al.; International Society of Andrology; International Society for the Study of Aging Male; European Association of Urology; European Academy of Andrology; American Society of Andrology. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol 2009;55:121–130.
- Black AM, Day AG, Morales A. The reliability of clinical and biochemical assessment in symptomatic late-onset hypogonadism: can a case be made for a 3-month therapeutic trial? BJU Int 2004;94:1066–1070.
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 2004;89:2085–2098.
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, Dlewati A, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 1999;84:1966–1972.
- Di Sante S, Conners WP, Morgentaler A. Influence of baseline serum testosterone on changes in body composition in response to testosterone therapy. J Sex Med 2012;9:585–593.
- Saad F, Gooren L, Haider A, Yassin A. Effects of testosterone gel followed by parenteral testosterone undecanoate on sexual dysfunction and on features of the metabolic syndrome. Andrologia 2008;40:44–48.
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, Ble A, Egan J, et al. Association between hormones and metabolic syndrome in older Italian men. J Am Geriatr Soc 2006;54:1832–1838.
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Salonen R, Rauramaa R, Salonen JT. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol 2003;149:601–608.
- Svartberg J. Epidemiology: testosterone and the metabolic syndrome. Int J Impot Res 2007;19:124–128.
- Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol 2005;174:827–834.
- Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 2009;30:726–733.
- Mårin P, Arver S. Androgens and abdominal obesity. Baillieres Clin Endocrinol Metab 1998;12:441–451.
- Mårin P, Holmäng S, Gustafsson C, Jönsson L, Kvist H, Elander A, Eldh J, et al. Androgen treatment of abdominally obese men. Obes Res 1993;1:245–251.
- Kapoor D, Malkin CJ, Channer KS, Jones TH. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol (Oxf) 2005;63:239–250.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006;154:899–906.
- Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 2011;96:2898–2903.
- Kim TN, Park MS, Lim KI, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Res Clin Pract 2011;93:285–291.
- Tajar A, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, Bartfai G, et al.; EMAS Group. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab 2012;97:1508–1516.
- Heinemann LA, Zimmermann T, Vermeulen A, Thiel C, Hummel W. A new ‘aging males’ symptoms’ rating scale. Aging Male 1999;2:105–114.
- Heinemann LA, Saad F, Zimmermann T, Novak A, Myon E, Badia X, Potthoff P, et al. The Aging Males’ Symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes 2003;1:15.
- Seftel A. Testosterone replacement therapy for male hypogonadism: part III. Pharmacologic and clinical profiles, monitoring, safety issues, and potential future agents. Int J Impot Res 2007;19:2–24.
