Abstract
Objective: We investigated the effects of oral testosterone undecanoate (TU) on bone mineral density (BMD), lean body mass (LBM) and body fat mass (BFM) in aging men with symptomatic testosterone deficiency (TD).
Methods: Three hundred twenty-two men ≥50 years with TD symptoms and calculated free testosterone <0.26 nmol/L participated in a multicenter, double-blind, placebo-controlled trial. Patients were randomized to placebo, oral TU 80 mg/d, oral TU 160 mg/d, or oral TU 240 mg/d, administered as divided doses with normal meals. BMD of the hip and lumbar spine were evaluated by dual energy X-ray absorptiometry (DEXA), and body composition (LBM and BFM) by whole body DEXA.
Results: Oral TU significantly increased BMD at Month 12 at the lumbar spine (240 mg/d), total hip (240 mg/d), and trochanter and intertrochanter (160 and 240 mg/d) compared with placebo. Oral TU significantly increased LBM at Months 6 and 12 for all oral TU groups compared with placebo. BFM significantly decreased at Month 6 (all oral TU groups) and Month 12 (160 mg/d) compared with placebo. The effects on BMD and body composition showed a clear dose response.
Conclusions: Treatment with oral TU led to improvement in BMD, LBM and BFM in aging men with symptomatic TD.
Introduction
In addition to sexual dysfunction, depressed mood, and fatigue, testosterone deficiency (TD) in men can also result in a reduction in bone mineral density (BMD) (resulting in osteopenia, osteoporosis and increased risk of fractures), a reduction in lean body mass (LBM) (associated with diminution in muscle volume and strength), and an increase in body fat mass (BFM) [Citation1]. A number of randomized controlled trials have demonstrated the beneficial effects of testosterone therapy on BMD and body composition in men with TD [Citation2–13]. However, the results of these studies have often been inconsistent, which might in part be related to variability in the duration of treatment, various routes of administration and/or small sample size, and lack of uniform inclusion criteria. Moreover, in most trials of testosterone therapy in TD patients, dose-response relationships have not been investigated.
Andriol® Testocaps® is an oral testosterone undecanoate (TU) formulation that is taken with normal meals. Following its intestinal absorption with ingested lipids, TU is incorporated into post-meal chylomicrons and transported to the systemic circulation by the lymphatic system, thereby circumventing the portal circulation and liver and avoiding both rapid metabolism and the potential hepatotoxicity that has been reported with other oral testosterone therapies [Citation14,Citation15]. The TU is rapidly hydrolyzed by esterases, liberating testosterone [Citation14]. When taken with normal meals, oral TU produces a dose-dependent increase in serum testosterone to levels within the physiological range [Citation16,Citation17].
We report the results of a 1 year, randomized, placebo-controlled study investigating the effects of three daily doses of oral TU (80, 160 and 240 mg/d; taken as divided doses with normal meals) in 322 men at least 50 years of age with symptomatic TD (based on a positive score on the Androgen Deficiency in Aging Males [ADAM questionnaire] [Citation18] and a morning calculated free testosterone measurement of <0.26 nmol/L). We previously reported the safety and TD ratings scale data from this study [Citation19]. Treatment with oral TU resulted in an improved total Aging Males’ Symptoms (AMS) rating scale score at Month 6 at all doses tested, but differences relative to placebo were not statistically significant [Citation19]. There was greater improvement in the AMS rating scale score in patients >60 years when compared with patients ≤60 years (p = 0.001) [Citation19]. The AMS sexual symptoms domain improved with oral TU 160 mg/d at Months 6 (p = 0.008) and 12 (p = 0.012) compared with placebo [Citation19]. Treatment was well tolerated and there were no between-group differences in adverse events or discontinuation rates [Citation19]. The present report presents the findings from this study for the effects of oral TU therapy on the prespecified hard efficacy endpoints of BMD, biochemical bone markers (serum osteocalcin and serum type I collagen C-telopeptide), and body composition (LBM and BFM).
Methods and materials
This study was a multicenter, randomized, double-blind, placebo-controlled trial, involving 14 centers in seven European countries. The trial was conducted between November 2001 (first subject entered) and July 2004 (last assessment of the last subject). This trial was registered at www.clinicaltrials.gov under number NCT00434824.
Study population
Men were eligible for the study if they were at least 50 years of age, with a body mass index (BMI) between 18 and 34 kg/m2, and symptomatic TD (as identified by a positive ADAM questionnaire score [Citation18] and a morning calculated free testosterone level of <0.26 nmol/L) [Citation20]. The calculated free testosterone cut-off level of 0.26 nmol/L was chosen on the basis of it being two standard deviations below the normal range in young men, as assessed in a sample of 150 men [Citation21]. Detailed exclusion criteria for the present study have been published previously [Citation19]. Patients whose cause of androgen deficiency was other than aging were excluded from the study. Patients were not to have been treated with androgens, anabolic steroids, bisphosphonates, calcitonin, or fluoride (sodium fluoride tablets for caries prevention were allowed) within the last two years prior to screening, and these drugs could not be taken as concomitant medications during the study. Calcium/Vitamin D supplementation prior to and/or during the study was allowed. The study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and local regulatory agencies. All men gave written informed consent.
Intervention
In this trial, patients were treated for 12 months with six capsules per day, each containing 40 mg TU (Andriol Testocaps) or identical placebo capsules, in divided doses with breakfast, lunch and dinner. Eligible patients were randomized to one of four treatment groups: placebo, oral TU 80 mg/d (80 mg once daily), oral TU 160 mg/d (80 mg twice daily) or oral TU 240 mg/d (80 mg thrice daily). While calcium/Vitamin D supplementation was allowed during the study, this was not given systematically to placebo- or TU-treated patients as part of their daily study medication.
Screening
Participants in this study were identified through recruitment in local newspapers, in outpatient endocrine and urology departments, and in primary care settings. To investigate eligibility for inclusion, blood samples were collected in the morning (between 6 and 10 am) to determine levels of total testosterone and sex hormone-binding globulin (SHBG) for calculation of free testosterone using the formula by Vermeulen et al. [Citation20]. These screening assessments of total testosterone and SHBG were done by ABL (Assen, the Netherlands) using DELFIA, Time-Resolved Fluoroimmunoassay (Wallac Oy, Turku, Finland). The ADAM questionnaire was also administered at screening. A positive ADAM questionnaire score was defined as a ‘yes’ response to questions 1 or 7 or to any three of the other eight questions [Citation18].
Biochemical bone markers
Fasting blood samples (between 6 and 10 am) were taken at baseline and at Months 1, 3, 6 and 12, for determination by a central laboratory (Synarc, Lyon, France) of serum osteocalcin and serum type I collagen C-telopeptide. Serum osteocalcin, a marker of bone formation, was measured by a two-site immunoassay using two monoclonal antibodies raised against human osteocalcin on an automatic platform (N-Mid Osteocalcin, Roche Diagnostics GmbH, Mannheim, Germany). C-terminal cross-linked telopeptide of type I collagen (S-CTX), a marker of bone resorption, was measured by a two-site immunoassay using monoclonal antibodies raised against the b-isomerized CTX sequence on an automatic platform (Serum Crosslaps, Roche Diagnostics GmbH, Mannheim, Germany).
Bone mineral density
At baseline and Month 12, BMD of the lumbar spine (L1–L4) and hip (femoral neck, trochanter, intertrochanter, Ward’s triangle and total hip) were measured by means of dual energy X-ray absorptiometry (DEXA). The DEXA scanners used were Hologic types QDR-1000(W), −1500, −2000 or −4500 or comparable Lunar DEXA scanners. The DEXA scanners were calibrated by a third party (Bio-Imaging Technologies, Newtown, PA) in addition to the routine calibrations. All electronic data of the DEXA measurements for BMD were analyzed centrally by Bio-Imaging Technologies. Results were reported in measured density in g/cm2 and T-scores. A T-score indicates the number of standard deviations above or below the mean of a healthy, young, male adult population.
Body composition
For assessment of body composition, a whole-body DEXA was performed at baseline and at Months 6 and 12 (total mass, lean mass, fat mass: total and for arms, legs and trunk). All electronic data of the DEXA measurements for body composition were analyzed centrally by Bio-Imaging Technologies.
Waist circumference and hand grip strength
At baseline and Months 6 and 12, waist circumference was measured with subjects in a standing position, after exhaling, using a non-stretchable measuring tape that measured in mm and was at least 1.5 cm in width. The measuring tape was kept horizontal, and each measurement was performed in duplicate. Hand grip strength was measured at these times with subjects in a standing position using a hand grip dynamometer. The subjects were verbally coached to squeeze the inner grip of the dynamometer with maximum force over a 2 s period. For both hands, the highest of three attempts was recorded.
Patients who discontinued prematurely were to undergo the same assessments as at the Month 12 visit, but were only asked to undergo a DEXA measurement for BMD when a BMD assessment was not performed within the last 6 months prior to discontinuation.
Statistical analysis
A two-way analysis of variance was used to compare the change from baseline in each dose group with placebo. An outcome was considered statistically significant if p ≤ 0.05. For waist circumference, body composition, and handgrip strength, an analysis of covariance was performed including the baseline value as a covariate. Furthermore, descriptive statistics were calculated for each efficacy parameter by group and assessment.
Results
Patient demographics
A total of 1444 men were screened, and of these, 322 men were randomized. The most common cause of screening failure was a morning calculated free testosterone level above the cut-off level of 0.26 nmol/L (7.4 ng/dL), despite a positive ADAM score. Details on the disposition of patients are presented in .
At screening, patients had a mean ± SD age of 58.7 ± 5.8 years, a mean ± SD morning calculated free testosterone of 0.21 ± 0.04 nmol/L, a mean ± SD total testosterone level of 12.8 ± 4.2 nmol/L, a mean ± SD SHBG of 45.6 ± 19.8 nmol/L and a mean ± SD BMI of 27.3 ± 3.4 kg/m2. The mean ± SD baseline luteinizing hormone level was 4.2 ± 4.1 IU/L and mean ± SD baseline estradiol was 98.8 ± 20.9 pmol/L. There were no meaningful differences between the treatment groups in baseline characteristics. Dosing compliance was high, with a median of 98.7% of all capsules taken according to the study protocol. There were no differences in compliance among the four treatment groups with respect to morning, afternoon or evening intake.
Bone parameters
There was an initial increase relative to placebo in mean serum osteocalcin for the 240 mg/d oral TU group, which was statistically significant at Month 3 (). This initial increase was followed by a decrease relative to placebo, which was statistically significant at Month 12 (240 mg/d TU group): −9.9%; p < 0.001 (). Treatment with oral TU 80 mg/d had no effect on mean serum osteocalcin compared with placebo over 12 months. Treatment with oral TU 160 mg/d led to a decrease in mean serum osteocalcin compared with placebo, with the between-group difference being significant at Month 12 (−5.3%; p < 0.05) ().
Figure 2. Effect of oral TU on osteocalcin and type I collagen C-telopeptide (mean percent change from baseline in the intention to treat group).
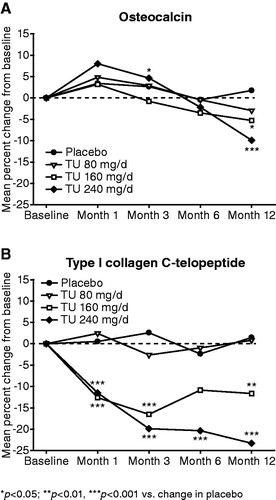
For mean serum type I collagen C-telopeptide, there were no changes in the placebo and oral TU 80 mg/d groups during the 12 months of treatment. Treatment with 160 and 240 mg/d oral TU led to a decrease in mean serum type I collagen C-telopeptide levels, with this decrease being statistically significant compared to placebo for all time points, except at Month 6 for the 160 mg/d oral TU group. After 1 month of treatment, the mean decrease from baseline in serum type I collagen C-telopeptide was 12.7% for oral TU 160 mg/d and 11.5% for oral TU 240 mg/d. The mean percentage decrease in serum type I collagen C-telopeptide seen with 160 mg/d oral TU at Month 1 was sustained through to Month 12, but the magnitude of the decrease with 240 mg/d oral TU continued to increase from Month 1 to Month 12. At Month 6, a statistically significant decrease was found for oral TU 240 mg/d: (−20.3%; p < 0.001), whereas at Month 12 serum type I collagen C-telopeptide levels were statistically significantly decreased with oral TU 160 mg/d (−11.7%; p < 0.01) and 240 mg/d (−23.3%; p < 0.001) ().
BMD values at baseline and the percentage change from baseline at Month 12 are presented in . For the lumbar spine (L1–L4), the mean T-score at baseline was −0.65, ranging from −0.48 in the placebo group to −0.75 in the oral TU 160 mg/d group. With respect to the total hip, the mean T-score at baseline was −0.23, ranging from −0.15 in the oral TU 80 mg/d group to −0.31 in the placebo group. After 12 months of treatment, lumbar spine BMD had increased, with a larger increase with increasing oral TU dose. In the 240 mg/d group, the difference in percent change from baseline compared to placebo was statistically significant (1.12% ± 3.16% for placebo versus 2.70% ± 3.39% for 240 mg/d oral TU; p < 0.05) (). After 12 months of treatment, total hip BMD had decreased with placebo, whereas dose-dependent increases were observed in all three oral TU groups. In the 240 mg/d group, the difference in percent change from baseline in total hip BMD compared to placebo was statistically significant (−0.44% ± 2.58% for placebo versus 0.84% ± 2.02% for 240 mg/d oral TU; p < 0.05) (). Oral TU had no meaningful effect relative to placebo on BMD at Month 12 at the site of the femoral neck (). The two highest oral TU doses (160 mg/d and 240 mg/d) produced similar, significant increases in BMD relative to placebo at Month 12 at the trochanter and inter-trochanter (). For Ward's triangle, there appeared to be numerical improvement in BMD at Month 12 relative to placebo in all three oral TU dose groups, but none of the differences with placebo was statistically significant ().
Figure 3. Effect of oral TU on BMD at the lumbar spine (L1–L4) and total hip at Month 12 (mean percent change from baseline in the intention to treat group).
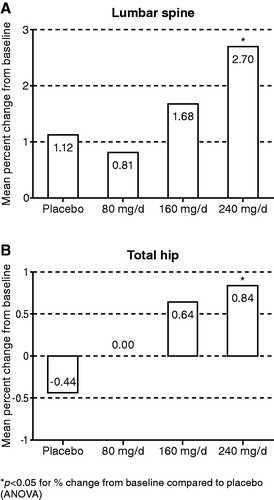
Table 1. Effects of oral TU and placebo on BMD (g/cm3) after 12 months of treatment (ITT-group, LOCF approach).
Body composition and muscle strength
There were no significant changes in total body mass during treatment in any of the four treatment groups (). After 6 months of treatment, the mean ± SD increase in total LBM was significantly greater in all active treatment groups compared with placebo: 0.98 ± 1.53 kg with oral TU 80 mg/d (p < 0.05 versus placebo), 0.97 ± 1.59 kg with oral TU 160 mg/d (p < 0.05 versus placebo) and 2.00 ± 2.20 kg with oral TU 240 mg/d (p < 0.001 versus placebo) compared with 0.27 ± 1.37 increase with placebo (). This effect was maintained after 12 months of treatment (, ). The increase in LBM was observed in various parts of the body, and was statistically significantly different from placebo for all three oral TU groups in the legs and trunk (). In the arms, a statistically significant difference from placebo was noted only with oral TU 240 mg/d ().
Figure 4. Effect of oral TU on LBM and BFM (mean change from baseline in the intention to treat group).
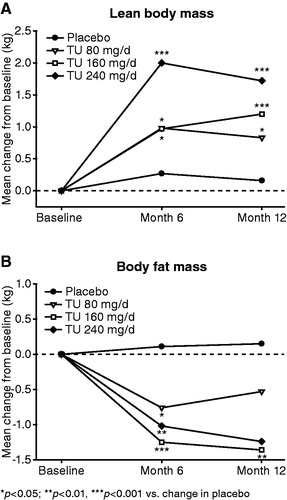
Table 2. Effects of oral TU and placebo on body composition after 12 months of treatment (ITT group, LOCF approach).
Compared with placebo, treatment with all three TU doses led to a statistically significantly decrease in BFM after 6 months: −0.76 ± 1.88 kg with oral TU 80 mg/d (p < 0.05 versus placebo), −1.25 ± 2.84 kg with oral TU 160 mg/d (p < 0.001 versus placebo) and −1.02 ± 2.38 kg with oral TU 240 mg/d (p < 0.01 versus placebo) compared with 0.11 ± 1.87 with placebo (). At Month 12, the difference from placebo was only statistically significant with oral TU 160 mg/d (p < 0.01) (, ). BFM was statistically significantly decreased in arms and legs with oral TU 160 and 240 mg/d, but not in the trunk (). There were no statistically significant differences in waist circumference () and hand grip strength (data not shown) between the active treatment groups and placebo after 6 or 12 months of treatment.
Discussion
This is the largest long-term (1 year), placebo-controlled trial of oral TU therapy in aging (≥50 years) men with symptomatic TD. In this study, oral TU treatment for 12 months led to dose-dependent improvement in BMD at the hip and lumbar spine, which was statistically significant for 240 mg/d in comparison to placebo. Oral TU had no effect relative to placebo on BMD at Month 12 at the site of the femoral neck. The two highest oral TU doses (160 and 240 mg/d) produced similar, significant increases in BMD relative to placebo at Month 12 at the trochanter and inter-trochanter. Consistent with the observed increases in BMD, treatment with oral TU led to a reduction in bone turnover biomarkers compared with placebo, with a significant, dose-dependent decrease in serum type I collagen C-telopeptide as well as a dose-dependent decrease by Month 12 in serum osteocalcin. Treatment with oral TU also led to dose-dependent improvement in body composition, with significant increases relative to placebo in LBM at all oral TU doses examined (at Months 6 and 12) and significant decreases relative to placebo in BFM at all oral TU doses at Month 6 and at the 160 mg/d dose at Month 12.
In the present study, the mean T-scores at baseline for lumbar spine and total hip were lower than that for a young adult male population, but on average considered normal since they were higher than 1 in all treatment groups. Despite baseline BMD being in the normal range, the increases in BMD at Month 12 observed in the present study were generally comparable to those reported in other long-term (≥1 year) placebo-controlled trials with testosterone therapy in hypogonadal men with low to low-normal bone mass. A 1 year, placebo-controlled study in 40 aging, hypogonadal men demonstrated a significant (p < 0.05 versus placebo) increase in lumbar spine BMD of 1.7% and a numerical increase of 0.7% in femoral neck BMD with oral TU 240 mg/d [Citation22]. A placebo-controlled study 12–24 months in duration in 131 elderly men with low-normal total testosterone, low bioavailable testosterone, history of fracture or BMD T-score less than −2.0, and frailty, examined the effects of 1% testosterone gel 5 mg/d on BMD [Citation3]. BMD in the testosterone group increased 1.4% at the femoral neck and 3.2% at the lumbar spine (p = 0.005 versus placebo) and decreased 1.3% at the mid-radius (p < 0.001) [Citation3]. Testosterone therapy had no significant effect on BMD compared with placebo at the femur, femoral neck and trochanter [Citation3]. Testosterone therapy also led to a significant reduction compared with placebo in deoxypyridinoline, a marker of bone resorption [Citation3]. A 1 year placebo-controlled study with a testosterone patch (two 2.5 mg patches per day) in 67 elderly men with low bioavailable testosterone showed a modest, but significant, gain in femoral neck BMD compared with placebo (0.3% increase with testosterone versus 1.6% decrease with placebo; p = 0.015) and no significant changes in bone biomarker levels [Citation3]. In a 2 year placebo-controlled study with a testosterone patch in elderly men with low bioavailable testosterone levels, there was a significant placebo-adjusted increase of BMD at the femoral neck of approximately 3.3% [Citation9]. A 3 year placebo-controlled study in men >65 years with low to low-normal serum testosterone demonstrated a significant increase in lumbar spine BMD (10.2% increase with testosterone versus 1.3% increase with placebo; p < 0.001) and hip BMD (2.2% increase with testosterone versus 0.2% increase with placebo; p < 0.02) with testosterone enanthate injections compared with placebo [Citation13]. Significant increases in BMD were also seen with testosterone enanthate in the intertrochanteric and trochanteric regions of the hip compared with placebo. No significant changes were seen in bone biomarker levels with testosterone enanthate compared with placebo. A linear regression analysis from a 3 year, placebo-controlled study with a testosterone patch in 108 men ≥65 years showed a mean placebo-adjusted increase in lumbar spine BMD of 0.9% in men with a baseline testosterone level of 400 ng/dL (13.9 nmol/L), 3.4% in men with a baseline total testosterone of 300 ng/dL (10.4 nmol/L), and 5.9% in men with a baseline total testosterone of 200 ng/dL (6.9 nmol/L) [Citation23]. In addition to improving bone mass in men with TD, long-term testosterone therapy has also been shown to improve bone mass in men with drug-induced hypogonadism and bone loss. Thus, a 1 year, placebo-controlled study in men on chronic glucocorticoid therapy who had low-normal total testosterone, low free testosterone and low-normal bone mass demonstrated a significant improvement in BMD relative to placebo of approximately 4% with testosterone 200 mg given IM every 2 weeks [Citation24].
It is well established that inhibition of BMD reduction/bone loss in aging men and women results in a reduction of fracture risk. Although bone pathophysiology in men is still poorly understood, an age-related decrease of circulating sex steroids appears to play a significant role in male osteoporosis [Citation25]. It has been reported that estrogens account for approximately 70% and androgens for approximately 30% of the total effects of sex steroids on bone resorption [Citation26]. Thus, estrogen deficiency [Citation27] as well as TD [Citation28–30] result in reduced BMD and contribute to an increased risk of osteoporosis and fractures. Two meta-analyses demonstrated that testosterone treatment in men with TD may increase BMD at the lumbar spine and hip [Citation31,Citation32], and testosterone therapy has generally been accepted as a viable option for the treatment of osteoporosis in men with TD [Citation33].
The increase in LBM with oral TU in this study showed a clear dose-response and was larger than that reported in a previous placebo-controlled trial with oral TU 160 mg/d in aging men with TD [Citation5], in which an increase in LBM of 0.7 kg was found after 12 months (compared with 1.2 kg in our study). The results of our study were similar to a previous study with oral TU 160 mg/day in healthy aging men that found a mean increase of LBM of 1.2 kg relative to placebo after 6 months of treatment [Citation10]. Our results are also similar to that of a 1 year study with oral TU 240 mg/d in aging, hypogonadal men, which reported a significant 2.2% increase in LBM compared with placebo [Citation22]. A 3 year, placebo-controlled study in men >65 years with low to low-normal total serum testosterone levels reported that testosterone enanthate injections led to a significant increase relative to placebo in LBM (3.7 kg increase with testosterone versus 0.21 kg increase with placebo; p < 0.0001) and a significant decrease relative to placebo in total fat mass (∼4.8 kg decrease with testosterone versus no change with placebo; p < 0.0001) [Citation7]. The increase of LBM in our study was also similar to that reported in aging, hypogonadal men treated with testosterone patches for 6–36 months [Citation3,Citation4,Citation9,Citation23], but less than observed after treatment for 6 months with testosterone ester injections of 125 mg/week or higher [Citation7]. Our study is the first to document body composition changes induced by oral TU in various body regions in men with symptomatic TD. The increase of LBM was found to be statistically significant in the legs, arms and trunk. In contrast, in a previous testosterone patch study, only a statistically significant increase of LBM in the trunk was reported, without increasing LBM in the extremities [Citation23]. It has been demonstrated that older men are as responsive as younger men with respect to the effects of testosterone on skeletal muscle mass, and that skeletal muscle remodeling with testosterone therapy is possible, even in older males. Our data are in agreement with this. Mechanisms involved in testosterone's action on muscle are only partly understood, but are thought to include an improvement of the net muscle protein balance caused by an increase of the fractional muscle protein synthesis and a reduction of muscle protein degradation [Citation34].
The preservation of LBM in aging men with TD receiving testosterone therapy may be clinically meaningful. In men, there is a progressive, age-related loss of LBM of 5% per decade, primarily affecting skeletal muscle [Citation35]. It has been estimated that 10–15% of the US male population <70 years of age has clinically relevant loss of LBM (sarcopenia), and this proportion increases to 25% in men >80 years of age [Citation36]. Sarcopenia has been associated with reduced muscle strength and frailty (diminished ability to carry out the important practical and social activities of daily living), falls, morbidity, disability and mortality in the elderly, and it is therefore important to develop strategies that prevent and/or reverse this condition. The cause of sarcopenia and resulting falls is multifactorial, but TD has been shown to be a clearly identified factor [Citation37]. Studies on the effect of testosterone therapy on muscle strength in aging men with TD have shown inconsistent results. The absence of a statistically significant effect on muscle strength as reported in some studies (including ours) may have been due to a combination of factors. A well-recognized problem in this regard is the lack of a precise and accurate measure of muscle strength. The variability in results may be due to differences in baseline testosterone level and muscle group tested, differences in standardization of measuring equipment, training effect, motivational effect, tolerance to pain, adequate blinding and intrinsic intra-subject variability. Nevertheless, in a meta-analysis of 11 randomized trials on the effects of testosterone therapy on muscle strength in aging men with TD, it was concluded that testosterone therapy produced a moderate increase in muscle strength, with this increase being more apparent for the lower extremities as compared to the upper extremities [Citation38]. In addition to testosterone therapy, exercise training may be required to show further improvements in physical function.
BFM was reduced by 1.4 and 1.2 kg after 12 months treatment with oral TU 160 and 240 mg/d, respectively. This finding is consistent with that of a previous 6 month placebo-controlled study with oral TU 160 mg/d in elderly men, which reported a similar magnitude of reduction of BFM [Citation10]. A 1 year study with oral TU 240 mg/d reported a significant 2.1% reduction in BFM compared with placebo [Citation22], whereas another 1 year study in elderly men showed virtually no change (approximately −0.2 kg) in BFM with oral TU 160 mg/d [Citation5]. The reduction of BFM in studies with testosterone in aging men with TD shows wide variability. Some studies show a modest change of BFM between 0 and 1 kg [Citation4,Citation5,Citation9], whereas in other studies, a more robust reduction ranging from −1.7 to ∼−4.8 kg has been reported [Citation3,Citation7,Citation23]. Similar to the effects on LBM, testosterone generally has a dose-dependent effect on BFM [Citation8].
The reduction of BFM with oral TU compared with placebo reported in the current study was statistically significant in the extremities, but not in the trunk. Our results are in agreement those of a study using a testosterone patch, which also demonstrated fat mass reductions in the extremities, but not in the trunk [Citation23]. One explanation for the absence of a statistically significant reduction of truncal fat may have been the large inter-individual variability of the treatment effect, requiring larger sample sizes to detect significant differences. With DEXA, the total amount of fat in the truncal region is measured including both subcutaneous and intra-abdominal (visceral) fat. Reductions in intra-abdominal fat are of particular interest, as an increased amount of fat in this region has been associated with increased cardiovascular disease risk. More accurate imaging techniques such as computed tomography or magnetic resonance imaging are needed in order to determine the effect of oral TU therapy on subcutaneous and intra-abdominal fat.
The inverse relationship between serum testosterone level and BFM in men has been known since the 1970s [Citation39], and TD has been associated with an excess of abdominal fat [Citation40]. A causal involvement of testosterone in fat metabolism was demonstrated in an experimental study, in which pharmacologically induced TD for 10 weeks in healthy men resulted in a statistically significant increase of BFM of 1.1 kg [Citation41]. Further evidence came from the Rancho Bernardo Study in which lower testosterone levels predicted central adiposity later in life [Citation42]. Conversely, in the Massachusetts Male Aging Study, development of obesity also appeared to predict TD [Citation43]. The exact mechanisms linking TD and increased BFM are unclear, but decreased testosterone-induced lipolysis, TD-induced insulin resistance and increased aromatization of testosterone into E2 in adipose tissue, are thought to play a role. In the present study, there were no statistically significant changes in waist circumference of the active treatment groups compared with placebo, presumably due to the net effect of oral TU therapy on LBM (increased by oral TU) and fat mass (decreased by oral TU) in the truncal region.
Among the major strengths of this study were its large sample size, relatively long duration (1 year), [Citation23] and its design, comparing three different doses of oral TU in a randomized, placebo-controlled setting. Additionally, our study enrolled aging men with baseline characteristics that are frequently encountered in the real-world clinic setting: TD symptoms, low-normal total testosterone levels and low free testosterone levels. The present report provides physicians with valuable data concerning the long-term (1 year) efficacy of oral TU therapy on BMD and body composition in such patients.
In summary, this 1 year, placebo-controlled trial in 322 aging men with symptomatic TD demonstrated that treatment with oral TU resulted in improvement of BMD and body composition (increased LBM and decreased BFM).
Author contributions
J. M. H. E. was responsible for the conception, design and execution of the study. J. M. H. E. and M. J. G. H. K. contributed to the statistical analysis and interpretation of the results and critically reviewed the manuscript. T. B. P. G. contributed to the interpretation of the results and prepared the manuscript. Study investigators P. M. G. B., J. J. L. and E. J. M. recruited and treated patients with trial medication, collected study results, contributed to the interpretation of the results and critically reviewed the manuscript. All authors have approved the manuscript for important intellectual content and are in the position to take public responsibility for this manuscript.
Declaration of interest
J. M. H. E., M. J. G. H. K., T. B. P. G. and A. G. M. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, sponsor of this study. P. M. G. B., J. J. L. and E. J. M. have received lecture fees from the sponsor. No other potential conflict of interest relevant to this article was reported.
This study was funded by Organon International (now Merck, Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.), Oss, the Netherlands (Protocol # 43203).
Clinical trial registry: This trial was registered at www.clinicaltrials.gov under number NCT00434824
Organon International bv, (now Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.), Oss (the Netherlands) provided funding, contributed to the study design, provided study medication and placebo, oversaw quality control at the clinical centers including periodic site visits and assembled the data collected by the centers. The database containing the findings of the individual investigator sites was maintained by the sponsor, and statistical analyses were performed by the sponsor.
Acknowledgements
The authors thank the study investigators and their staff for their assistance in conducting this trial, and the study subjects for their willing participation in this research. The authors also wish to thank Amy Johnson-Levonas, Kathleen Newcomb and Kristen Lewis of Merck Sharpe & Dohme Corp., a subsidiary of Merck & Co., Inc., for their assistance in preparing this paper for publication.
References
- Wang C, Nieschlag E, Swerdloff RS, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2009;12:5–12
- Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 1999;84:1966–72
- Kenny AM, Prestwood KM, Gruman CA, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 2001;56:M266–72
- Steidle C, Schwartz S, Jacoby K, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab 2003;88:2673–81
- Wittert GA, Chapman IM, Haren MT, et al. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 2003;58:618–25
- Haren MT, Wittert GA, Chapman IM, et al. Effect of oral testosterone undecanoate on visuospatial cognition, mood and quality of life in elderly men with low-normal gonadal status. Maturitas 2005;50:124–33
- Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 2005;90:1502–10
- Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 2005;90:678–88
- Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 2006;355:1647–59
- Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 2008;299:39–52
- Svartberg J, Agledahl I, Figenschau Y, et al. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res 2008;20:378–87
- Basurto L, Zarate A, Gomez R, et al. Effect of testosterone therapy on lumbar spine and hip mineral density in elderly men. Aging Male 2008;11:140–5
- Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 2004;89:503–10
- Horst HJ, Holtje WJ, Dennis M, et al. Lymphatic absorption and metabolism of orally administered testosterone undecanoate in man. Klin Wochenschr 1976;54:875–9
- Shackleford DM, Faassen WA, Houwing N, et al. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J Pharmacol Exp Ther 2003;306:925–33
- Anawalt BD, Amory JK, Wang C, et al. A pharmacokinetic study of oral testosterone undecanoate (Org 538), Abstract. J Androl 2002;23(S1):37
- Schnabel PG, Bagchus W, Lass H, et al. The effect of food composition on serum testosterone levels after oral administration of Andriol Testocaps. Clin Endocrinol (Oxf) 2007;66:579–85
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000;49:1239–42
- Legros JJ, Meuleman EJ, Elbers JM, et al. Oral testosterone replacement in symptomatic late-onset hypogonadism: effects on rating scales and general safety in a randomized, placebo-controlled study. Eur J Endocrinol 2009;160:821–31
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Vermeulen A. Androgen replacement therapy in the aging male–a critical evaluation. J Clin Endocrinol Metab 2001;86:2380–90
- Bebb R, Anawalt B, Wade J, et al. A randomized, double blind, placebo controlled trial of testosterone undecanoate administration in aging hypogonadal men: effects on bone density and body composition. Proc Annu Meet Endocrine Soc Endocrine Society Press; 100–1; 2001; Bethesda, MD
- Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 1999;84:2647–53
- Crawford BA, Liu PY, Kean MT, et al. Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab 2003;88:3167–76
- Rochira V, Balestrieri A, Madeo B, et al. Osteoporosis and male age-related hypogonadism: role of sex steroids on bone (patho)physiology. Eur J Endocrinol 2006;154:175–85
- Falahati-Nini A, Riggs BL, Atkinson EJ, et al. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 2000;106:1553–60
- Riggs BL, Khosla S, Melton LJ, III. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 1998;13:763–73
- Jackson JA, Riggs MW, Spiekerman AM. Testosterone deficiency as a risk factor for hip fractures in men: a case-control study. Am J Med Sci 1992;304:4–8
- Leifke E, Wichers C, Gorenoi V, et al. Low serum levels of testosterone in men with minimal traumatic hip fractures. Exp Clin Endocrinol Diabetes 2005;113:208–13
- Stanley HL, Schmitt BP, Poses RM, Deiss WP. Does hypogonadism contribute to the occurrence of a minimal trauma hip fracture in elderly men? J Am Geriatr Soc. 1991;39:766–71
- Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63:280–93
- Tracz MJ, Sideras K, Bolona ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab 2006;91:2011–6
- Eastell R, Boyle IT, Compston J, et al. Management of male osteoporosis: report of the UK Consensus Group. QJM 1998;91:71–92
- Bhasin S, Taylor WE, Singh R, et al. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci 2003;58:M1103–10
- Short KR, Vittone JL, Bigelow ML, et al. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 2004;286:E92–101
- Morley JE, Baumgartner RN, Roubenoff R, et al. Sarcopenia. J Lab Clin Med 2001;137:231–43
- Orwoll E, Lambert LC, Marshall LM, et al. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med 2006;166:2124–31
- Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, et al. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc 2006;54:1666–73
- Glass AR, Swerdloff RS, Bray GA, et al. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab 1977;45:1211–9
- Couillard C, Gagnon J, Bergeron J, et al. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE Family Study. J Clin Endocrinol Metab 2000;85:1026–31
- Mauras N, Hayes V, Welch S, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab 1998;83:1886–92
- Khaw KT, Barrett-Connor E. Lower endogenous androgens predict central adiposity in men. Ann Epidemiol 1992;2:675–82
- Mohr BA, Bhasin S, Link CL, et al. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. Eur J Endocrinol 2006;155:443–52