Abstract
Aim: To systematically review evidence on the efficacy and safety of mirodenafil treatment in erectile dysfunction (ED) from randomised controlled trials.
Methods: We searched PubMed, Embase and the Cochrane Library database up to March 2013. Two authors independently assessed study quality and extracted data. All data were analyzed using RevMan 5.0. Outcome measures assessed were the International Index of Erectile Function (IIEF), erectile function domain (EFD) score (primary), the Sexual Encounter Profile questions 2 and 3, and the response to the Global Assessment Questionnaire and adverse effects (secondary).
Results: A total of 374 participants from three randomized controlled trials were identified in this meta-analysis. After 12 weeks treatment, mirodenafil was found to be more effective than placebo, and tolerability was good. The pooled results showed that the IIEF EFD score for 100 mg mirodenafil group was higher than placebo group (MD = 8.13, 95%CI: 6.64–9.61, p < 0.00001) and the mirodenafil group was also higher than placebo group in the changes from baseline for the IIEF EFD score (MD = 7.32, 95%CI: 5.56–9.07, p < 0.00001), respectively. The most common drug-related adverse events were flushing and headache (mirodenafil versus placebo: 15.8% versus 3.2%, 3.1% versus 0%; respectively).
Conclusion: This meta-analysis suggested that mirodenafil is effective and well-tolerated therapy for ED.
Introduction
Erectile dysfunction (ED), also known as impotence, is the inability to attain or maintain an erection to permit satisfactory sexual activity. ED can have a significant impact on both patient’s quality of life and their partner’s. ED was classified as mild, moderate, or complete. According to the Massachusetts Male Aging Study, the total prevalence of mild, moderate and complete impotence was approximately 52%, and ED is a disease associated with age, the incidence of complete ED increases from 5% to 15% as age increases from 40 to 70 years [Citation1]. The prevalence rate of ED in Asia ranged from 2% to 88% [Citation2]. Moreover, the patients with ED will increase 170 million from 1995 to 2025 for men aged 40–79 years in the worldwide [Citation3]. Various conditions can cause ED. The risk factors of ED include diabetes mellitus, hypertension, cardiovascular diseases, cigarette smoking, nerve or spinal cord damage, low testosterone levels, psychological factors and drugs caused ED [Citation4,Citation5].
Currently, according to 2010 European Urology (EAU) guidelines, the treatments option for ED include first-line therapy, oral phosphodiesterase type 5 (PDE5) inhibitors and vacuum constriction devices; second-line therapy, intracavernous injections; and third-line therapy, penile prostheses [Citation6]. Men have reported a clear preference for oral PDE5 inhibitors who do not have a specific contraindication to their use. Three PDE5 inhibitors, sildenafil (Viagra®), vardenafil (Levitra®) and tadalafil (Cialis®) approved by the U.S. Food and Drug Administration, have been introduced in the management of ED. However, many patients are still dissatisfied with existing therapies owing to high cost, adverse events and lack of efficacy [Citation7–9]. Therefore, it is necessary for the development of novel PDE5 inhibitors to improve the management of ED.
Recently, one new oral selective PDE5 inhibitors, mirodenafil (SK Chemicals Life Science Biz., Korea) has been developed for the treatment of ED. Its pharmacokinetic profiles include a Tmax of 1.25 h and a T1/2 of 2.5 h [Citation10] for mirodenafil. As well known, the Nitric Oxide (NO)/cyclic guanosine monophosphate (cGMP)/cGMP-dependent protein kinase I pathway is considered as the principal regulatory basis for penile erection. Accordingly, by inhibiting the destruction of cGMP, PDE5 inhibitors can increase blood flow into the penis and decrease blood flow out of the penis, which is the essential mechanism of PDE5 inhibitors [Citation11]. The mechanism of action of mirodenafil is similar to other PDE5 inhibitors and has a higher selectivity for PDE5. Several randomized controlled trials (RCTs) have reported the clinical effectiveness and safety of mirodenafil for ED. However, there was no systematic review and meta-analysis including RCTs to determine the effectiveness and safety of the mirodenafil for ED. Therefore, this meta-analysis is to evaluate the efficacy and safety of mirodenafil for patients with ED so that providing more reliable evidence for further clinical use.
Methods
Search strategy
The following databases were searched to obtain the relevant RCTs: PubMed (1966–March 2013), Embase (1974–March 2013), and the Cochrane Library (2013 issue 2). The following search term were used: (“mirodenafil” OR “5-ethyl-2-(5-(4-(2-hydroxyethyl)piperazine-1-sulfonyl)-2-propoxyphenyl)-7-propyl-3,5-dihydro-4H-pyrrolo(3,2-d)pyrimidin-4-one” OR “SK3530”) AND (“Erectile Dysfunction” OR “Impotence”). We also searched the references of included studies to identify additional potentially relevant studies. No language restrictions were imposed. The titles and abstracts were screened independently by two reviewers, who discarded studies that were not applicable, and two reviewers independently assessed full article of all identified trials to confirm fulfillment of inclusion criteria. Data extraction was carried out independently by the same authors using standard data extraction forms. To reduce bias, one of the reviewers was blinded to the source of the publication and to the authors’ names. Disagreements were resolved in consultation with the third reviewers. The quality of included RCTs was assessed using the Cochrane Collaboration’s tool [Citation12].
Included criteria
Inclusion criteria were as follows: eligible patients were men with ED for at least 6 months according to the National Institutes of Health Consensus Development Panel on Impotence, who were >18 years of age, in a stable heterosexual relationship for at least 6 months, and who made at least four attempts at sexual intercourse on four separate days during the baseline period, of which at least 50% of the attempts were unsuccessful.
Outcome measures
Our primary outcome was the International Index of Erectile Function (IIEF) erectile function domain (EFD) score. The secondary outcomes were the Sexual Encounter Profile (SEP) questions 2 and 3, the response to the Global Assessment Questionnaire (GAQ), Life Satisfaction Checklist (LSC) and adverse effects.
Statistical analysis
We analyzed the data using Review Manager (version 5.0) and extracted and pooled data for summary estimates. A p value <0.05 was considered statistically significant. For meta-analysis, we combined data on dichotomous outcomes using the Mantel-Haenszel relative risk (RR) method. For continuous outcomes, we used the inverse variance weighted mean difference (MD) method and 95% confidence intervals (95% CI). Heterogeneity will be analyzed using a χ2 test on N-1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test [Citation13]. We used a fixed effect model for calculations of summary estimates and their 95% CI unless there was significant heterogeneity, in which case results were confirmed using a random effects statistical model. If the data were not depicted by mean ± standard deviation, the standard deviation was estimated using the statistical method [Citation14].
Results
According to the above search strategies, we identified five mirodenafil RCTs [Citation10,Citation15–18] that met the inclusion criteria (). All studies were multicenter, double-blind, randomized, placebo-controlled study. Five studies were from Korea. The study duration ranged from 8 to 12 weeks. However, only the study duration of one mirodenafil trial [Citation15] was 8 weeks and there was inadequate reporting in other study [Citation18], which did not include this meta-analysis.
Figure 1. Flow chart for selecting randomized controlled trials for analysis (RCTs = randomized controlled trials).
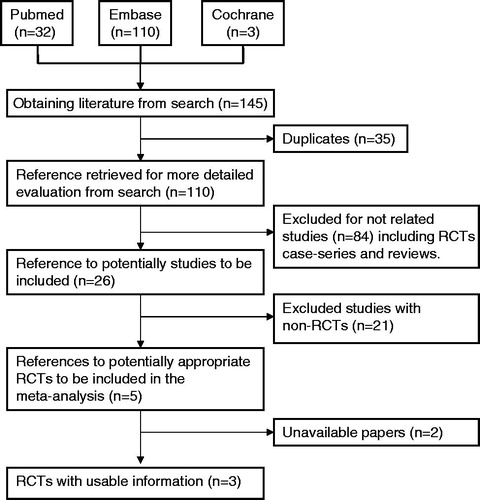
The characteristics and the quality assessment of the included studies are summarized in and . The results of meta-analysis for the efficacy and safety of mirodenafil in comparison with placebo for ED are summarized in .
Table 1. Baseline characteristics of the included studies.
Table 2. The methodological quality of the included studies.
Table 3. Compared to placebo, the pooled results of meta-analysis on the efficacy and safety of mirodenafil for erectile dysfunction.
Primary end points
The IIEF EFD score
For mirodenafil, three studies reported the IIEF EFD score after 12 weeks treatment. The pooled results showed that the 100 mg mirodenafil group was higher than placebo group in IIEF EFD score at follow-up endpoint (MD = 8.13, 95% CI: 6.64–9.61, p < 0.00001). Moreover, the mirodenafil group was also higher than placebo group in the changes from baseline for the IIEF EFD score (MD = 7.32, 95% CI: 5.56–9.07, p < 0.00001). In addition, the changes from baseline in each domain of the IIEF showed significant improvement in mirodenafil group compared with placebo (orgasmic function, sexual desire, intercourse satisfaction, overall satisfaction; p < 0.00001). Our studies also showed significant changes in mirodenafil group compared with placebo for IIEF Q3 and Q4 scores (Q3: MD = 1.11, 95% CI: 0.80–1.43, p < 0.00001; Q4: MD = 1.52, 95% CI: 1.22–1.81, p < 0.00001) ().
Secondary end points
The change from baseline for SEP questions 2
For mirodenafil, three studies reported the changes from baseline for SEP questions 2. The pooled results showed that the mirodenafil group was higher than placebo group (MD = 22.14, 95% CI: 14.77–29.51, p < 0.00001) ().
The change from baseline for SEP questions 3
For mirodenafil, three studies reported the changes from baseline for SEP questions 3. Compared to placebo group, the mirodenafil group showed a significantly greater increase in this case (MD = 43.78, 95% CI: 36.03–51.53, p < 0.00001) ().
The response to the GAQ
Three studies reported the response to the GAQ. The pooled results showed that the mirodenafil group was significantly higher than placebo group in the response to the GAQ (RR = 3.18, 95% CI: 2.48–4.08, p < 0.00001) ().
The change from baseline for LSC
Three studies reported the changes from baseline for LSC. The pooled results showed that there was no statistical difference between mirodenafil group and placebo group in the changes from baseline for LSC (RR = 0.70, 95% CI: −0.01 to 1.41, p = 0.05) ().
Adverse effects
All included studies indicated that most adverse events were mild or moderate in severity, and no serious adverse events were reported during the study period. The most common drug-related adverse events were flushing and headache in mirodenafil treatment. For mirodenafil, three studies reported the incidence of flushing and headache in the study duration of 12 weeks (mirodenafil versus placebo: 15.8% versus 3.2%, p = 0.0003, I2 = 0%; 9.2% versus 1.1%, p = 0.003, I2 = 0%; respectively) ().
Discussion
To our knowledge, this is first meta-analysis to evaluate the efficacy and safety of mirodenafil for ED. Mirodenafil (SK3530) is newly developed oral PDE-5 inhibitor for the treatment of ED [Citation15]. In this study, our pooled results demonstrated that mirodenafil can significantly improve the ED of patients including the IIEF erectile function domain score, SEP questions 2 and 3, and the response to the GAQ, and there was no obvious changes for LSC and serious adverse events occurred during the study period, the most common drug-related adverse events were flushing and headache compared with placebo.
It is well known that penile erection is caused through vascular pressure changes within the corpora cavernosa. The nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway serves as the principal regulatory basis for penile erection [Citation11]. Sexual stimulation or sexual arousal (such as, psychogenic, reflexogenic, or nocturnal tumescense) causes the nonadrenergic–noncholinergic nerves in penis to release NO, and then NO causes the relaxation of the smooth muscle cells of the trabeculae and arterioles of the corpora cavernosa, which increases penile blood flow and results in erection. The enzyme phosphodiesterase type 5 is a selective inactivator of cGMP in the cavernosal smooth muscle. However, PDE5 inhibitors can activate cGMP by inhibiting PDE5 enzyme, which makes cGMP more available and prolongs erection [Citation19]. Similarly, mirodenafil and avanafil, as PDE5-Is, is through the same way to treat ED. Preclinical studies demonstrated that the selectivity of mirodenafil toward PDE5 was 10-fold higher than that of sildenafil, whereas its inhibitory effects on other PDEs were much lower than those of sildenafil [Citation10,Citation20].
A previous meta-analysis have demonstrated that compared to placebo, sildenafil generated an obvious improvement about 9.65 points, tadalafil could be pooled into an effect of 8.52 points improvement, vardenafil leaded to an effect of 7.50 points improvement in IIEF EFD score and udenafil resulted in an effect of 8.62-point improvement [Citation21,Citation22]. In this study, 100 mg mirodenafil resulted in an effect of 7.32 points improvement. The other meta-analysis reported that the percentages of successful sexual intercourse attempts based on SEP Q3 was 34.3% in favor of sildenafil, 33.2% in favor of vardenafil and 35.1% in favor of tadalafil [Citation23]. Our study demonstrated that the percentages of successful sexual intercourse attempts based on SEP Q3 was 43.78% in favor of mirodenafil. These results indicated that the effects of mirodenafil are comparable to current PDE5-Is. Moreover, the changes of mirodenafil are obvious in primary and secondary outcomes.
In this meta-analysis, the most common drug-related adverse events were flushing and headache. A recent meta-analysis demonstrated that in short-term trials (<6 months), sildenafil-treated men had a higher risk for headache, flushing, dyspepsia, and visual disturbances compared with placebo-treated men [Citation24]. This indicated that mirodenafil is not inferior to sildenafil in drug-related adverse events.
Our meta-analysis also had several limitations. First, all included studies are moderate quality in this meta-analysis [Citation25], this might not allow for a reliable conclusion. Second, for mirodenafil, all participants came from Korea, more studies from other countries and race is required to evaluate the effectiveness in the future. Third, because of lacking data, subgroup analysis could not be performed by the duration of ED, the severity of ED and the etiology of ED – such as hypertension, diabetes mellitus. In this study, three mirodenafil trials reported the effectiveness of mirodenafil for ED with various causes, ED with hypertension and ED with diabetes mellitus. This may be the reason why there is no statistical difference in LSC. Based on the above analysis, we need more efficient performance of higher quality, various races and long-term RCTs to verify in the future.
Conclusions
This meta-analysis suggested that mirodenafil is effective and well-tolerated therapy for ED. Moreover, the changes of mirodenafil are obvious in primary and secondary outcomes. We need higher quality, various races, long-term randomised controlled trials to verify these findings in the future.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This paper was supported by the Fundamental Research Funds for the Central Universities (lzujbky-2012-225), China.
References
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54–61
- Park K, Hwang EC, Kim SO. Prevalence and medical management of erectile dysfunction in Asia. Asian J Androl 2011;13:543–9
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999;84:50–6
- Lam TH, Abdullah AS, Ho LM, et al. Smoking and sexual dysfunction in Chinese males: findings from men's health survey. Int J Impot Res 2006;18:364–9
- Kubin M, Wagner G, Fugl-Meyer AR. Epidemiology of erectile dysfunction. Int J Impot Res 2003;15:63–71
- Hatzimouratidis K, Amar E, Eardley I, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol 2010;57:804–14
- Hanson-Divers C, Jackson SE, Lue TF, et al. Health outcomes variables important to patients in the treatment of erectile dysfunction. J Urol 1998;159:1541–7
- Souverein PC, Egberts AC, Meuleman EJ, et al. Incidence and determinants of sildenafil (dis)continuation: The Dutch cohort of sildenafil users. Int J Impot Res 2002;14:259–65
- Gonzalgo ML, Brotzman M, Trock BJ, et al. Clinical efficacy of sildenafil citrate and predictors of long-term response. J Urol 2003;170:503–6
- Paick JS, Ahn TY, Choi HK, et al. Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. J Sex Med 2008;5:2672–80
- Beckman TJ, Abu-Lebdeh HS, Mynderse LA. Evaluation and medical management of erectile dysfunction. Mayo Clin Proc 2006;81:385–90
- Zhao E, Cui D, Yuan L, et al. MDM2 SNP309 polymorphism and breast cancer risk: a meta-analysis. Mol Biol Rep 2012;39:3471–7
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, version 5.0.2, updated September 2009. The Cochrane Collaboration 2009
- Paick JS, Choi HK, Kim SC, et al. Efficacy and safety of oral SK3530 for the treatment of erectile dysfunction in Korean men: a multicenter, randomized, double-blind, placebo-controlled, fixed dose, parallel group clinical trial. Asian J Androl 2008;10:791–8
- Paick JS, Kim JJ, Kim SC, et al. Efficacy and safety of mirodenafil in men taking antihypertensive medications. J Sex Med 2010;7:3143–52
- Park HJ, Choi HK, Ahn TY, et al. Efficacy and safety of oral mirodenafil in the treatment of erectile dysfunction in diabetic men in Korea: a multicenter, randomized, double-blind, placebo-controlled clinical trial. J Sex Med 2010;7:2842–50
- Chung JH, Kang DH, Oh CY, et al. Safety and efficacy of once daily administration of 50 mg mirodenafil in patients with erectile dysfunction: a multicenter, double-blind, placebo controlled trial. J Urol 2013;189:1006–13
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am 2005;32:379–95, v
- Lee J, Yoo HH, Rhim KJ, et al. Metabolism and excretion of 5-ethyl-2-{5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-7-propyl-3,5-dihydropyrrolo[3,2-d]-pyrimidin-4-one (SK3530) in rats. Rapid Commun Mass Spectrom 2007;21:1139–49
- Berner MM, Kriston L, Harms A. Efficacy of PDE-5-inhibitors for erectile dysfunction. A comparative meta-analysis of fixed-dose regimen randomized controlled trials administering the International Index of Erectile Function in broad-spectrum populations. Int J Impot Res 2006;18:229–35
- Ding H, Du W, Wang H, et al. Efficacy and safety of udenafil for erectile dysfunction: a meta-analysis of randomized controlled trials. Urology 2012;80:134–9
- Tsertsvadze A, Fink HA, Yazdi F, et al. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med 2009;151:650–61
- Tsertsvadze A, Yazdi F, Fink HA, et al. Oral sildenafil citrate (viagra) for erectile dysfunction: a systematic review and meta-analysis of harms. Urology 2009;74:831–6 e838
- Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6

