Abstract:
Prostate cancer is a common malignancy among men, and the current screening, imaging and sampling approaches aim to detect early-stage, organ-confined disease. In such scenario, focal prostate cancer therapy currently relies on the index lesion concept as the dominant lesion that drives the disease natural history. Focal therapy demands the essential imaging and sampling techniques to strategically locate and qualify the disease, but, despite advances in technology, prostate imaging and biopsy have several limitations that need to be overcome if focal therapy is to be developed further. The I Prostate Cancer Focal Treatment International Symposium was convened to foster discussion on this topic that sits at the crossroads of multiple disciplines (Urology, Pathology, Radiology, Radiation Oncology and Medical Oncology) all of which were represented for this comprehensive multidisciplinary review of the current literature.
Introduction
The multifocal nature of prostate cancer has demanded whole-gland therapy in the past; however, since the widespread use of Prostate-Specific Antigen (PSA) screening, patients are presenting with smaller disease burden. This lead time now allows us to consider a curative intent strategy that without whole gland radical treatment. It's hoped that such an approach will lessen the morbidity of prostate cancer treatments for the vast majority of men.
Focal prostate cancer therapy currently relies on the index lesion concept and demands the essential imaging and sampling techniques to advantageously locate and qualify the disease. Despite advances in technology, prostate imaging and biopsy of localized disease have several limitations that need to be overcome.
Progresses in imaging technologies, specifically magnetic resonance (MR) image, have improved lesion detection and local staging. The evolution of these sophisticated imaging techniques into prostate interventions (targeted biopsy and focal therapy) allows accurate sampling and minimally invasive targeted treatments of localized prostate cancer.
In this evolving scenario, the I Prostate Cancer Focal Treatment International Symposium Panel that occurred in Campinas, São Paulo, Brazil, on August 2013, through an International Consensus of Experts (“ICE”) joined multidisciplinary specialists in the intersection fields of urology, pathology, radiology, oncology and radiation oncology working together to review the current literature and to dissect the fundamental pieces from the index lesion concept and related anatomopathological supportive findings in radical prostatectomy surgical specimen to where we stand in terms of image and prostate biopsy techniques supporting prostate cancer focal therapy broadcasting and advancement.
For didactic purposes the issue is comprehensively organized in five topics:
The index lesion concept
Pathologic findings supporting focal therapy
Image for focal therapy: where do we stand?
Biopsy technique essentials for focal therapy
Take home messages
The index lesion concept
The index lesion (IL) concept in prostate cancer (PCa) was first proposed by Los Angeles Group [Citation1] and has becoming important, mainly because the emergence of focal therapies (FT) [Citation2] and the popularization of the image methods as Ultrasound and multiparametric MR.
There is not a consensual definition for IL in the literature. The multifocality of PCa, present in the majority (70–80%) of the PCa patients, configures an additional difficulty to determine exactly which is the IL among multiple malignant focuses [Citation3].
A meeting of uropathology experts from the International Society of Uropathology (ISUP) did not result in a consensus definition of IL; 24% of the participants voted that IL is the largest lesion, 26% voted that IL in the largest lesion with the higher grade, and 28% voted that IL is the largest and higher stage lesion [Citation4].
Aside from the precise academic definition, the majority of the authors accept that IL is the most important lesion, which usually is the biggest one in size [Citation3]. The IL is the dominant lesion that drives the disease evolution [Citation3,Citation2] and presented the higher Gleason grade in comparison with others, which are named secondary lesions.
A great collaboration in the comprehension of IL in human PCa was attained by the elegant study through a high-resolution genome-wide single nucleotide polymorphism and copy number survey, of Liu et al. [Citation5].
In this study, 30 men whose death was attributed to metastatic PCa (PELICAN – Project to Eliminate Lethal prostate Cancer) agreed to postmortem sampling of the metastatic sites. The authors showed that cells present at distinct metastatic lesions, are by and large derived from a sole cell clone (same clonal origin) from a specific lesion in the primary tumor, named IL, which contain the most aggressive phenotypic/genotypic lesions (higher Gleason Scores). The other multifocal lesions in the prostate did not originate metastases or in some specific cases can originate only non-lethal lesions [Citation5]. Since this knowledge, the IL concept and FT came to the center of scientific debate [Citation2].
The determination of IL is fundamental for the FT protocols, since the PCa focal approaches, intend to treat the IL with minimal security margins, and to preserve neighbor benign prostate tissues with the intent of causing less collateral sexual and urinary effects as the result of prostate cancer treatment [Citation2,Citation3,Citation6].
However, fundamental to this concept is the ability to localize the IL, for this, we must be based in anatompathologic information and image methods.
In a recent series of 300 radical prostatectomies specimens, Billis et al. found that the IL is associated with higher clinical stage, more surgical positive margins and higher PSA levels. Also, in multivariate analyses, extension of the IL significantly predicted worst biochemical recurrence free survival, but not the total tumor extension in comparison [Citation7].
In another series of 71 patients that underwent laparoscopic radical prostatectomy, 19 presented with positive surgical margins in surgical specimens and the IL were present 13/19 positive margins, reinforcing the clinical relevance of IL. In the 6 remaining positive margins cases, there were satellite lesions (size 0.6 cm) at the positive margin focus in addition to the IL [Citation8].
Confirming that IL usually is the more visible and largest lesion in the gland, the most significant core samples biopsies (largest length of cancer extension/core and higher percentage of tumor/core) were found in hypoechoic lesions target biopsied (mean and median size around 1.4 cm] visualized through high definition trans-rectal ultrasound, in comparison with the cores obtained from systematic target biopsy in a cohort of patients' candidates to focal HIFU [Citation9].
Nowadays, the MR has an impressive high negative predictive values to eliminate the diagnostic of clinical significant lesion with moderate sensitivity and positive predictive value to diagnose IL, although the sensitivity improved progressively in the setting of more aggressive pathologic features (Gleason score > 6, and lesion higher than 1.0 cm) and for identification of abnormal MR parameters [Citation10].
In a pathological study with 46 specimens of radical prostatectomies (analyzing tumor volume, grade and pathological stage), 58.5–67.5% of the cases were suitable for IL treatment, sparing secondary lesions. In this series there were no patients in whom the IL was insignificant and secondary lesion were significant (by upgrade or extra-capsular disease) [Citation11].
Meanwhile, there are some concerns about the IL and secondary lesions; secondary lesions may harbor significant lesions being not eligible for FT. Also non-index lesions can potentially originate metastasis [Citation3] once chromosomal abnormalities from IL may be absent in lymph nodal metastasis [Citation12].
The IL concept is actual and the future challenge is to accurately diagnose these seminal lesions in the glands. Advances in image methods, associated with strategies as perineal template biopsies and refined biomolecular studies are now being studied [Citation13] and will certainly contribute to the better understanding of the clinical role of IL. In this evolving scenario, large multicentric FT series with long follow-up are necessary and welcomed.
Pathologic findings supporting focal therapy
There are well-established pathologic findings in radical prostatectomy predictive of biochemical recurrence after surgery: Gleason score, positive surgical margins, extraprostatic extension and seminal vesicle invasion. However, it is controversial whether tumor extent on radical specimens is an independent prognostic factor of biochemical recurrence following surgery [Citation14].
For the purpose of focal therapy in patients with multifocal bilateral cancer, the index tumor or index lesion, defined as the largest volume lesion, is presumed to be the main driving factor for tumor progression and prognosis [Citation15,Citation16]. This indicates that patients with multifocal prostate cancer do not necessarily have a worse prognosis and outcome than those with unifocal or unilateral lesions [Citation17].
In a recent study, Billis et al. compared the total tumor extent versus dominant nodule (IL) extent as independent predictors of biochemical recurrence following radical prostatectomy [Citation7]. The surgical specimens were step-sectioned at 3 to 5 mm intervals and totally embedded in paraffin. A mean of 32 paraffin blocks were processed and 6 µm sections from each block were stained with hematoxylin and eosin. Each transverse section of the prostate was subdivided into two anterolateral and two posterolateral quadrants. Using the cone method, eight sections from the bladder neck and eight sections from the apex were obtained.
Tumor extent at radical prostatectomy was evaluated by a semi-quantitative point count method previously described [Citation18]. Briefly, drawn on a sheet of paper, each quadrant of the transverse sections contained eight equidistant points. During the microscopic examination of the slides, the tumor area was drawn on the correspondent quadrant seen on the paper. At the end of the examination, the amount of positive points represented an estimate of the tumor extent.
shows the drawing that is included in the pathology report with eight equidistant points in each quadrant. It is also used to visualize other findings such seminal vesicle invasion, extraprostatic extension, positive surgical margins and involvement of the bladder neck or apex.
Figure 1. Semiquantitative point count method for tumor extent evaluation. Total tumor extent was recorded as 18 positive points and index tumor (predominant nodule) in quadrant D4 as 7 positive points.
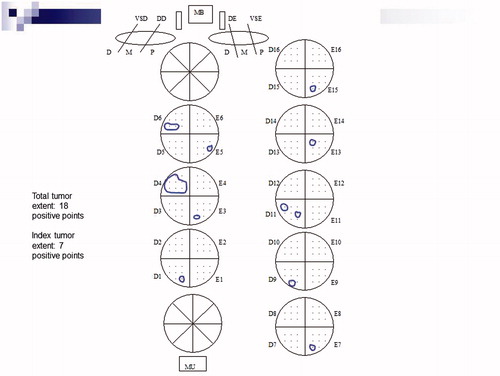
Total tumor extent was recorded as the total sum of positive points from all transverse quadrants. IL extent (dominant nodule) was recorded as the maximum number of positive points from the largest single focus of cancer present in the quadrants. In the total tumor extent was recorded as 18 positive points and the IL extent as seven positive points in quadrant D4.
The authors concluded in the study that both total and index tumor (IL) extent are significantly associated with higher preoperative PSA, clinical stage T2, pathological stage >T2, positive surgical margins, and higher radical prostatectomy Gleason score. Both total and index tumor (IL) extent were predictive of time to biochemical recurrence following radical prostatectomy in Univariate Analysis. However, only the dominant nodule (IL) extent was independent predictor of time to biochemical recurrence in multivariate analysis.
The study favors that any kind of tumor extent estimate in surgical specimens should be related to the dominant nodule (index lesion) and not the total tumor extent. The results also favor that the IL seems to be the driving force of prostate cancer progression and should be the target of focal therapy.
Image for focal therapy: where do we stand?
The role of imaging for prostate cancer can be divided into tumor localization, staging of disease, and the detection of recurrences. A critical challenge for imaging is to differentiate clinically significant disease from silent and indolent disease, a goal that has not yet been achieved. Ultimately, this determination will probably be made by tissue markers, but until the day arrives when we can reliably predict the biological behavior of prostate cancers, imaging will simply “point” to the lesion without further specificity as to its aggressiveness [Citation19]. This highlights the importance of prostate biopsy techniques development in association with image advances.
At present, MR is the only clinically available imaging method that depicts the zonal anatomy of the prostate in detail. Multiparametric MR imaging, including both anatomic and functional sequences, has been shown to be effective for the detection and local staging of prostate cancer [Citation20]; however, multiparametric MR imaging currently is not included in the decision-making algorithms or criteria for active surveillance (AS) or active treatment (AT) including focal therapy.
There are a limited number of studies incorporating MR imaging into clinical-pathologic decision algorithms. Guzzo et al. [Citation21] evaluated the ability of T2-weighted MR imaging findings to help predict adverse pathologic features in patients qualifying for AS on the basis of Epstein criteria and concluded that tumor identification at T2-weighted MR imaging was not predictive of adverse pathologic features in patients undergoing AS. However, this study did not include multiparametric MR imaging. Recently, Shukla-Dave et al. [Citation22] reported the results of a newly designed nomogram that incorporates T2-weighted MR imaging and MR spectroscopy findings in 181 patients and concluded that the model nomogram improved the predictive accuracy for clinically unimportant prostate cancer, with areas under the curve that increased from 0.56 to 0.77 (p < 0.001).
In another recent study Turkbey et al. [Citation23] demonstrated that multiparametric MR imaging provides useful additional information to existing clinicopathologic scoring systems of prostate cancer and improves the assignment of treatment (e.g. AS or AT). On the basis of this review of surgical outcomes, multiparametric MR imaging proved to be superior to the D’Amico, Epstein, and CAPRA scoring systems in correctly classifying patients for AS versus AT and greatly improved the accuracy of all of the scoring systems when it was combined with them.
Multiparametric MR image evaluation, incorporating an MR imaging scoring system with dominant tumor volume measurement, could be helpful in stratifying patients with prostate cancer for AS or AT in conjunction with existing guidelines.
Biopsy technique essentials for focal therapy
Paralleling the index lesion concept, the image techniques advancements and focal prostate cancer treatment, biopsy technique is also evolving, though the optimal template is to be defined.
While biopsy sample quality (length, percentage of cancer) and its influence in the treatment decision is under-reported in the literature. Reis et al. have pioneering showed that the mean core length in those presenting underestimated Gleason score on biopsy stage (42.7%, n = 76) was 11.61 mm (± 2.5, median 11.40) compared to 13.52 mm (± 3.2, median 13.70) in those with perfect Gleason score agreement between biopsy and radical prostatectomy (57.3%, n = 102), p < 0.001, CI 95% = 1.05–2.79. On stepwise multivariate analysis the mean core length was the only independent predictor of biopsy Gleason score underestimation. For each unit of core length increment in millimeters, the biopsy underestimation risk decreased 89.9%, p = 0.049, OR = 0.101, CI 95% = 0.010 – 0.990, highlighting the fact that core length is an important parameter that affects biopsy Gleason underestimation [Citation24].
Nevertheless, standard transrectal ultrasound (TRUS) guided biopsy with 12 systematic fragments routinely utilized to diagnose PCa might not be sufficient in all patients to efficiently locate the focus of disease to target therapy. Systematic random biopsy removes tissue only of the posterior peripheral zone, and sampling of the anterior peripheral and transition zones and apex are frequently missed [Citation25–27].
These facts can explain why 20—30% of patients can have PCa diagnosis in TRUS re-biopsies [Citation27] and in this regard, some studies suggest that transperineal biopsies can do better representation of longitudinal anterior and posterior areas improving detection rates in re-biopsies.
In this context different protocols recommend extended transrectal biopsies or grid template perineal biopsy to better locate and estimate tumor volume and index lesion before focal treatment [Citation9,Citation28].
Bargawi et al. observed that three-dimensional mapping transperineal biopsy revealed that a significant portion of men initially diagnosed with apparently low risk disease harbored clinically significant cancers requiring more aggressive therapy. They believe that this technique also enabled a number of men with low risk disease to elect less invasive therapy as surveillance or focal therapy [Citation28].
More recent studies suggest that the association of image methods and pathology reports can build a better characterization of index lesion to improve application of focal therapy.
Ukimura et al. studied potential candidates for focal cryotherapy that underwent grey-scale and Doppler TRUS-guided biopsy. All real-time TRUS images were recorded, allowing subsequent reviewing for the planning of targeted focal cryotherapy, and/or follow-up targeted biopsy. The spatial mapping of TRUS-visible lesions and targeted sampling areas were individually documented in schematic anatomic drawings of the prostate. Data from the baseline imaging-targeted biopsies were compared with systematic (non-targeted) biopsies. Of 93 patients, 73 with low- to intermediate-risk disease were eventually considered to be candidates for hemi-ablative focal cryosurgery. They showed that TRUS-guided targeted biopsies significantly improved the detection and staging of higher grade and larger volume cancers, compared with image-blind (non-targeted systematic) biopsies. Image visibility enhanced the precise targeting and accurate spatial mapping of cancer to help identify more appropriate candidates for focal therapy [Citation9].
Multiparametric MR target biopsy to suspicious areas could improve the detection of significant or aggressive cancers and the assessment of tumor grade and volume [Citation29–32]. Also, the MR target biopsy detection rate is good either for anterior and posterior cancers [Citation31–35]. After a negative TRUS-guided biopsy, MR-guided biopsy can detect cancer with fewer fragments than in the standard systematic biopsy [Citation30]. Furthermore, more than 90% of cancers detected by MR-guided biopsy were thought to represent clinically significant disease [Citation31].
Also, Diffusion-Weighted Imaging (DWI) may increase the efficacy of prostate biopsy by distinguishing localization of cancer foci from the benign ones, minimizing the need for third or more biopsies and the related morbidity [Citation36].
In this setting, contrast enhanced ultrasound guided biopsies, elastography and diagnostic algorithms like ultrasound guided prostate biopsy with MR image fusion are also promising techniques in the near future [Citation37] and for level one evidence, diagnostic performance of multi-parametric magnetic resonance imaging and prostate HistoScanning™ ultrasound against transperineal template prostate mapping biopsies as a reference test is underway [Citation38].
Take home messages
Prostate cancer is a common malignancy among men, and the current screening, imaging and sampling approaches end up detecting early-stage, organ-confined disease.
At the same time, it has been recently recognized that though multifocal, prostate cancer tends to manifest with dominant lesion(s) that determines the disease natural history, known as index lesion(s), validating focal therapy as a treatment option with sound oncological outcomes and less morbidity, warranting its endorsement and spread.
Though until nowadays no recommendation exists in terms of standardized chronology in diagnosis and focal therapy, many patients initially under active surveillance for long periods are candidates to be safely rescued by focal therapy, avoiding additional morbidity.
In such scenario, prostate biopsy technique advancements have tracked the expansion of index lesion concept and the image improvements, supporting the prostate cancer focal therapy; however, the optimal prostate biopsy template and also the ideal image technique are to be defined and large multicentric prostate cancer focal therapy trials with long term follow up are necessary and welcomed.
Declaration of interest
Authors declare no conflict of interest.
References
- Stamen TA, Freiha FS, MC Neal JE, et al. Localized Prostate cancer. Relationship of tumor volume to clinical significance fotr treatment of prostate cacer. Cancer 1993;71:933–8
- Ahmed H. The index Lesion and the origin of the prostate cancer. N Eng J Med 2009;361:1704--6
- Karavitakis M, Winkler M, Abel P, et al. Histological characteristics of the index lesion in whole mountain radical prostatectomy specimens: implications for focal therapy. Prostate Cancer Prostatic Dis 2001;14:46–52
- Van der Kwast TH, Amin MB, Billis A, et al. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of RP specimens. Working Group 2: T2 substaging and prostate cancer volume. Mod Pathol 2011;24:16--25
- Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med 2009;15:559–65
- De la Rosete, Ahmed H, Barentsz J, et al. Focal Therapy in prostate cancer-report from a consensus panel. J Endourol 2012;24:775–80
- Billis A, Meirelles LR, Freitas LL, et al. Prostate total tumor extent versus index tumor extent--which is predictive of biochemical recurrence following radical prostatectomy? J Urol 2013;189:99–104
- Karavitakis M, Ahmed HU, Abel PD, et al. Margin status after laparoscopic radical prostatectomy and the index lesion: implications for preoperative evaluation of tumor focallity in prostate cancer. J Endourol 2012;26:503–8
- Ukimura O, Abreu ALC, Gill IS, et al. Imagem visibility to enhance targeting precision and spatial mapping biopsy for focal therapy of prostate cancer. BJU Int 2013;111:E354–65
- Rosenkrantz AB, Deng FM, Kim S, et al. Prostate cancer: multiparametric MRI for index lesion localization--a multiple-reader study. AJR Am J Roentgenol 2012;199:830–7
- Simon RJ Bott, Hashim U Ahmed, Richard G Hindley, et al. The index lesion and focal therapy an analysis of the pathological characteristics of prostate cancer. BJU Int 2010;106:1607–11
- Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest 2013;123:4918–22
- Dickinsons L, Ahmed HU, Kirkham AP, et al. A multicenter prospective development study using high intensity focused ultrasound for localized prostate cancer. Contemp Clin Trials 2013;36:68–80
- Epstein JI. Prognostic significance of tumor volume in radical prostatectomy and needle biopsy specimens. J Urol 2011;186:790–7
- Eichelberger LE, Koch MO, Daggy JK, et al. Predicting tumor volume in radical prostatectomy specimens from patients with prostate cancer. Am J Clin Pathol 2003;120:386–9
- Mouraviev V, Villers A, Bostwick DG, et al. Understanding the pathological features of focality, grade and tumour volume of early-stage prostate cancer as a foundation for parenchyma-spearing prostate cancer therapies: active surveillance and focal targeted therapy. BJU Int 2011;108:1074–85
- Noguchi M, Stamey TA, McNeal JE, Nolley R. Prognostic factors for multifocal prostate cancer in radical prostatectomy specimens: lack of significance of secondary cancers. J Urol 2003;170:459–63
- Billis A, Magna LA, Ferreira U. Correlation between tumor extent in radical prostatectomies and preoperative PSA, histological grade, surgical margins, and extraprostatic extension: application of a new practical method for tumor extent evaluation. Int Braz J Urol 2003;29:113–9
- Turkbey B, Pinto PA, Choyke PL. Imaging techniques for prostate cancer: implications for focal therapy. Nat Rev Urol 2009;6:191–203
- Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011;186:1818–24
- Guzzo TJ, Resnick MJ, Canter DJ, et al. Endorectal T2-weighted MRI does not differentiate between favorable and adverse pathologic features in men with prostate cancer who would qualify for active surveillance. Urol Oncol 2012;30:301–5
- Shukla-Dave A, Hricak H, Akin O, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int 2012;109:1315–22
- Turkbey B, Mani H, Aras O, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology 2013;268:144–52
- Reis LO, Mendonça G, Menezes O, et al. Core biopsy length impacts gleason upgrading on radical prostatectomy. Urology 2013;82:S286
- Scattoni V, Zlotta A, Montironi R, et al. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol 2007;52:1309–22
- Fuganti PE, Tobias-Machado M, Pinto MA, et al. Twelve core biopsy versus six systematic sextant biopsies. Braz J Urol 2002;28::207–13
- Lawrentschuk N, Haider MA, Daljeet N, et al. Prostatic evasive anterior tumours: the role of magnetic resonance imaging. BJU Int 2010;105:1231–6
- Barqawi AB, Rove KO, Gholizadeh S, et al. The role of 3-dimensional mapping biopsy in decision making for treatment of apparent early stage prostate cancer. J Urol 2011;186:80–5
- Franiel T, Stephan C, Erbersdobler A, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding–multiparametric MR imaging for detection and biopsy planning. Radiology 2011;259:162–72
- Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol 2010;183:520–7
- Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 2011;258:488–95
- Villers A, Puech P, Mouton D, et al. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol 2006;176:2432–7
- Puech P, Potiron E, Lemaitre L, et al. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology 2009;74:1094–9
- Lemaitre L, Puech P, Poncelet E, et al. Dynamic contrast-enhanced MRI of anterior prostate cancer: morphometric assessment and correlation with radical prostatectomy findings. Eur Radiol 2009;19:470–80
- Puech P, Huglo D, Petyt G, et al. Imaging of organ-confined prostate cancer: functional ultrasound, MRI and PET/computed tomography. Curr Opin Urol 2009;19:168–76
- Kilinç R, Doluoglu OG, Sakman B, et al. The Correlation between diffusion-weighted imaging and histopathological evaluation of 356 prostate biopsy sites in patients with prostatic diseases. ISRN Urol 2012;2012:252846
- Salomon G, Schiffmann J. Real-time elastography for the detection of prostate cancer. Curr Urol Rep 2014;15:392
- Simmons LA, Ahmed HU, Moore CM, et al. The PICTURE study – Prostate Imaging (multi-parametric MRI and Prostate HistoScanning™) compared to transperineal ultrasound guided biopsy for significant prostate cancer risk evaluation. Contemp Clin Trials. 2014;37:69–83

