Abstract
Purpose: To describe the clinical-epidemiological features of male patients with breast cancer in Brazil.
Methods: Data from male patients with breast cancer treated from 2000 through 2009 were obtained from the Brazilian Hospital Cancer Register databases. Descriptive statistics were performed.
Results: A total of 1189 male patients were included. The mean age at diagnosis was 59.6 years (± 13.6). Tumours were categorised as clinical stage I (14.3%), stage II (38.3%), stage III (34.1%) and stage IV (13.3%). The most frequent histological type was invasive ductal carcinoma (83.7%). The first course treatment (alone or combined) consisted of chemotherapy in 53.2%, surgery in 49.2, radiation therapy in 36.8 and hormonal therapy in 21.0%; 3.4% of cases did not receive treatment. Treatment modality varies according to the tumor-node-metastasis (TNM) stage. The inadequate response rate was 15.9%, and 7.4% of patients died after the first course of treatment. Adequate response according to the first-course cancer treatment, after adjusted for clinical stage, was associated with being Caucasian (odds ratio (OR) = 2.50; 95% confidence interval (95% CI): 1.35–4.65) and submitted to chemotherapy (OR = 0.46; 95% CI: 0.28–0.74).
Conclusions: Male breast cancer diagnosis is often made in the advanced stage. Consequently, patients were subjected to more aggressive treatments, with poorer clinical response.
Background
Male breast cancer (MBC) is a rare disease accounting for less than 1% of all breast cancers and 1% of all male malignancies [Citation1]. In the United States, MBC deaths represent less than 0.5% of all cancer deaths [Citation2]. By contrast, in some parts of Africa, breast cancer may account for up to 6% of cancers in men [Citation3]. In Brazil, in the past decade (2001 to 2010), data from the Mortality Information System operated by Brazil’s Ministry of Health have shown that only 0.97% of 106 425 breast cancer deaths were in men. This percentage varies widely between 0.85 in the first triennium (2001–2003) and 1.12 in the last one (2008–2010), representing a relative increase of 31% [Citation4]. Although incidence data is not available for the entire country, data from the Sao Paulo Cancer Registry concerning different periods of time have shown that adjusted breast cancer incidence rates, per 100 000 males, increased three times in the past two decades: 0.5 (1988), 0.9 (1993) [Citation5], 0.97 (1997–1998) [Citation6], 1.21 (2001–2005) [Citation7] and 1.4 (1997–2008) [Citation8].
These cancers are biologically different from carcinomas of the female breast [Citation9]. However, little is known about its biological and histopathological features, epidemiology, causes, prognosis, ideal management and treatment. Generally, the knowledge is extrapolated from the experience with female patients or a relatively small number of studies in male, mainly case series. In addition, several recent studies show poor survival and suggest that the prognosis is worse than in females as a result of large tumour size, advanced stage, high grade and presence-positive nodes at the time of cancer diagnosis [Citation9,Citation10]. Besides, incidence is rising in the developed world, particularly in the urban US, Canada and UK [Citation11]. This study was carried out to examine the epidemiological and clinical features of male patients with breast cancer diagnosed and treated in Brazil.
Materials and methods
A retrospective cohort study was accomplished using information from Cancer Hospital Records in Brazil, obtained through the Integrator System (Brazilian National Cancer Institute) and Sao Paulo’s Hospital Cancer Registry (Oncocentro Foundation). These bases include information from 239 accredited cancer centres in Brazil involving 25 States and the Federal District. Between 2000 and 2009, it included men with breast cancer (International Classification of Diseases – Oncology – 3rd Edition – ICD-O C50), whose planning, treatment and follow-up were made in a cancer hospital.
Patients were followed up until the end of first course of treatment. The following variables were collected: age at diagnosis (in years), marital status (with partner versus no partner), race/skin colour (white versus non-white), level of education (less than middle school certificate versus middle school certificate or higher), family history of breast cancer (yes versus no), current alcohol consumption (more than three times per week, independent of amount consumed), habitual tobacco use and its derivatives at the time of hospital enrolment (yes versus no) and year of diagnosis and tumour site. TNM stage at diagnosis, histological type, first-course therapy and status at the end of the first course of treatment were classified using surveillance, epidemiology and end results (SEER) definitions [Citation12]. For all cases, the first course of therapy includes all cancer-directed treatment administered to the patient within four months after the initiation of therapy. All modalities of treatment were included regardless of sequence or the degree of completion of any component method. Two or more single agents given at separate times during the first course of cancer-directed therapy were considered a combination regimen.
A descriptive analysis of the study population was performed through measures of central tendency and dispersion to the age variable, and determination of frequency distribution to categorical variables, with intervals of 95% of confidence and p values. The crude and adjusted association between adequate response according to the first-course cancer treatment and select variables was estimated by odds ratios (OR) and its 95% confidence interval (95% CI). The statistical program used was SPSS, version 21.0 (São Paulo, Brazil).
This study was approved on 21 October 2011, by the Brazilian National Institute of Cancer (INCA) of Ethics and Research Committee (CAAE – 0104.0.007.000-11).
Results
During the 10-year study period, a total of 85 912 patients with breast cancer were registered, and a total of 1189 (1.38%) were male patients (mean 119 cases per year; minimum 98 – maximum 161).
In male patients with breast cancer, the mean age at diagnosis was 59.6 years (± 13.6); 23.9% were younger than 50 years. A family history of breast cancer was noted in 45.4% of cases. According to the TNM classification, tumours were categorized as stage I (14.3%), stage II (38.3%), stage III (34.1%) and stage IV (13.3%); 30.5% of patients presented with pT4 stage. The most frequent histological type was invasive ductal carcinoma (83.7%) ().
Table 1. Baseline demographics and clinical characteristics of study population (n = 1189).
The percentage of advanced stage (stage ≥ 2B) cancers ranged from 54.2 in the year 2005 to 71.1 in 2000. The first-course treatment consisted of chemotherapy (alone or combined) in 53.2%, surgery (alone or combined) in 49.2%, radiation therapy (alone or combined) in 36.8% and hormonal therapy (alone or combined) in 21.0%; 3.4% of cases did not receive treatment (data not shown).
Treatment modality varies according to the TNM stage (). At the end of the first treatment, 37.4% of patients had a complete response, 46.7% were in partial remission or had stable disease, 8.5% had progressive disease or relapsed and 7.4% died from breast cancer (data not shown). According to the TNM stage, adequate response was observed in 94.1% (95% CI: 87.8–97.3) of patients in stage I, 96.5% (95% CI: 93.6–98.1) in stage II, 82.3% (95% CI: 77.0–87.0) in stage III and 43.2 (95% CI: 33.7–53.2) in stage IV ().
Figure 1. First-course cancer treatment (alone or combined with other treatment), according to clinical stage (TNM).
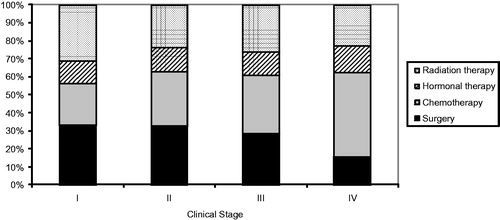
Table 2. Response classification of male breast cancer according to the first-course cancer treatment (n = 722)*.
The association between adequate response according to the first-course cancer treatment, after adjusted for clinical stage, showed that white men were 2.5 times more likely to have an adequate response (OR = 2.50; 95% CI: 1.35–4.65) and patients submitted to chemotherapy had 54% less chance (OR = 0.46; CI 95%: 0.28–0.74) ().
Table 3. Association between adequate response of male breast cancer according to the first-course cancer treatment and selected variables.
Discussion
Male breast carcinoma is similar to breast cancer in women, but there are some distinct features that should be highlighted. During the past few years, there has been an increase in the incidence and mortality in this cancer in Brazil. This can be caused by the coexistence of risk factors such as family history, obesity, low levels of physical activity, environmental exposures and genetic predisposition [Citation13].
The mean age of our case series was 59.6 years, confirming that median age of onset of breast cancer in men is approximately 5–10 years later than in women [Citation14–16]. A series from the same database involving 117 601 women has shown that age was slightly inferior (median: 55.5 years; SD: 13.5) (Author, unpublished data), supporting the conclusion that women are typically diagnosed with breast cancer at a younger age than men. In the United States of America in a study that compared 612 males with 2413 females who had breast cancer, the mean age at diagnosis was 67 years for male patients and 57 years for female patients (p < 0.005) [Citation17]. Other studies had lower mean ages, such as 58 years [Citation18] in Turkey, 55 in Africa [Citation19] and 59 years [Citation20] in Nigeria. This early presentation in these countries can be explained by, among other things, the lower life expectancy in those countries.
In women, family history of breast cancer is an important risk factor. In men, the prevalence of a positive family history in a first or second relative degree can range between 13% and 30% [Citation16,Citation21,Citation22]. Family history of cancer was observed in a high percentage (45.4%) of our cases, but it was not possible to know the type of cancer and the degree of kinship of these cases. Part of these results may be attributable to reporting bias, due to differential recall between breast cancer cases. The incidence of breast cancer among black people is approximately 15% less than in whites [Citation16]. Our results showed higher frequency of white patients (65.7%), and this variable was associated with an adequate response after the first course of cancer treatment (p = 0.004). Studies observed that black men tend to have poorer prognostic features (advanced-stage disease, tumour sizes, more nodal involvement and higher tumour grade) [Citation12,Citation21]. In Brazil, skin colour has been related to the access to treatment and care [Citation23].
In this study, the frequency of patients in stages III and IV was 47.4%, which was less than the findings of Bourhafour et al. [Citation24] in Morocco (80.3%) and Ahmed et al. [Citation20] in Nigeria (93.0%). On the other hand, in an Australian series, only 20.6% of MBC cases were diagnosed in stages III or IV [Citation25]; in a German study, 23.9% of cases were diagnosed [Citation26]. A significant proportion of advanced clinical stage in our series can be, at least in part, caused by the paucity of knowledge and public awareness regarding the existence of MBC, no recommendations for male breast care and difficult access to health care services [Citation27,Citation28].
Invasive ductal carcinoma was the predominant histological type (83.7%). This results confirms other studies, which have shown values ranging from 77.8 [Citation24] to 96.0% [Citation24]. This can be explained by the fact that the male breast does not have lobular elements [Citation29].
Due to the low incidence of MBC, few clinical trials are conducted to assess the effectiveness of cancer treatments in this population [Citation16]. As a general rule, breast cancer in men should be treated similarly to postmenopausal hormone receptor-positive disease in women [Citation21,Citation29]. With no evidence to support female-to-male data extrapolation, epidemiological comparisons become an alternative source of information [Citation27].
The primary therapy in man is modified radical mastectomy or simple mastectomy, with no differences in survival between these techniques [Citation16]. There is a little evidence that hormonal therapy and chemotherapy is as effective as in women in male patients with primary breast cancer [Citation29,Citation30]. In this study, patients in clinical stages I and II were predominantly treated with surgery (alone or combined) and those in stages III and IV with chemotherapy (alone or combined). After removing the effect of clinical staging, patients receiving chemotherapy had a poorer response in the first course of treatment (p = 0.002). According to Korde et al. [Citation21], it is difficult to detect a chemotherapy advantage in MBC because, in general, benefits are more apparent in endocrine non-responsive breast cancer, in high-risk groups and in younger patients. In males, most of the tumours are hormone receptor-positive in older patients and have multiple comorbidities.
A few patients’ at all clinical stages underwent hormonal therapy. However, it was not possible to identify the status of oestrogen and progesterone receptor. Others studies have shown that MBC was more likely to be hormone-receptor positive (oestrogen and progesterone) than female breast cancer [Citation16,Citation21,Citation31] and that breast cancer is biologically different between genders [Citation9]. Apart from hormonal status, the use of hormonal therapy in men appears to be less common due to low treatment adherence and an increased frequency of side effects [Citation28,Citation30,Citation32,Citation33].
Adjuvant radiotherapy has been show to decrease local recurrence in large tumours with lymph node and muscle involvement [Citation16]. This involvement is more frequent in men, subsequent to the small breast size and the proximity of the tumour to these structures [Citation29]. In our study, 36.8% of patients received radiotherapy, and it was not associated with an adequate response to the first-course cancer treatment. Other studies have shown similar frequency of radiotherapy in male patients with breast cancer with no impact on overall survival [Citation29].
A number of limitations must be considered to interpret our findings. First of all, studies based on population registries should be less susceptible to selection bias than hospital-based studies. The data used in this study come from 239 cancer hospitals, located in 25 states of Brazil, and were collected over 10 years. This may have caused a loss of quality of information and constraints on adherence to the protocols of the Brazilian Hospital Cancer Registry platforms. Over the years, INCA has promoted trainings of cancer registrars and has published several manuals in order to reduce these biases. Conversely, this is the first study in the country to consider such a large number of MBC cases. In addition, in this series, it was not possible to assess Oestrogen Receptor, Progesterone Receptor and Human Epidermal Growth Factor Receptor 2 expression. However, our findings may have implications for cancer control programs. Although treatment response is associated with earlier detection and the recommendation of more appropriate therapy, these two factors alone do not account for the entire prognosis of men with breast cancer. The quality and promptness of the services provided are critical to patients’ recovery.
In conclusion, MBC diagnosis is often made at advanced stages. Consequently, patients were submitted to more aggressive treatments, with poorer clinical responses.
Declaration of interest
The authors declare that they have no conflict of interest.
References
- White J, Kearins O, Dodwell D, et al. Male breast carcinoma: increased awareness needed. Breast Cancer Res 2011;13:219
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. Cancer J Clin 2011;61:212–36
- Sasco AJ, Lowenfels AB, Pasker-de Jong P. Review article: epidemiology of male breast cancer. A meta-analysis of published case-control studies and discussion of selected aetiological factors. Int J Cancer 1993;53:538–49
- Ministério da Saúde, Departamento de Informática do SUS (DATASUS) Sistema de Informação de Mortalidade. Available from: http://www.datasus.gov.br [last accessed 27 April 2013]
- Mirra AP. Incidência de câncer no Município de São Paulo, Brasil, 1983-1988-1993. Tendência no período 1969-1993. São Paulo: Registro de Câncer de São Paulo; 1998
- Instituto Nacional de Câncer (INCA); Ministério da Saúde. Câncer no Brasil: dados dos registros de base populacional. Vol 3. Rio de Janeiro (Brasil): INCA; 2003
- Instituto Nacional de Câncer (INCA); Ministério da Saúde. Câncer no Brasil – Registros de base populacional. Avalilable from: http://www.inca.gov.br/cancernobrasil/2010/docs/SaoPaulo/P437-440.pdf [last accessed 20 April 2012]
- Michels FAS, Simon A, Sconza IAC, et al. Câncer em São Paulo 1997-2008. Incidência, Mortalidade e Tendência de Câncer no Município de São Paulo. São Paulo: S.P. – Registro de Câncer de São Paulo; 2011
- Shaaban AM, Ball GR, Brannan RA, et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat 2012;133:949–58
- Agrawal A, Ayantunde AA, Rampaul R, Robertson JF. Male breast cancer: a review of clinical management. Breast Cancer Res Treat 2007;103:11–21
- Contractor KB, Kaur K, Rodrigues GS, et al. Male breast cancer: is the scenario changing. World J Surg Oncol 2008;6:58
- Cunningham J, Hankey B, Lyles B, et al. The SEER program code manual. Revised edition, June 1992. Bethesda, MD: National Cancer Institute; 1992
- Brinton LA, Richesson DA, Gierach GL, et al. Prospective evaluation of risk factors for male breast cancer. Natl Cancer Inst 2008;100:1477–81
- Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol 2010;28:232–9
- Gnerlich JL, Deshpande AD, Jeffe DB, et al. Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol 2011;18:1837–44
- Reis LO, Dias FGF, Castro MAS, Ferreira U. Male breast cancer. Aging Male 2011;14:99–109
- Nahleh ZA, Srikantiah R, Safa M, et al. Male breast cancer in the veterans affairs population: a comparative analysis. Cancer 2007;109:1471–7
- Yoney A, Kucuk A, Alan A, Unsal M. Male breast cancer: a retrospective analysis. Cancer Radiother 2009;13:103–7
- Ndom P, Um G, Bell EM, et al. A meta-analysis of male breast cancer in Africa. Breast 2012;21:237–41
- Ahmed A, Ukwenya Y, Abdullahi A, Muhammad I. Management and outcomes of male breast cancer in Zaria, Nigeria. Int J Breast Cancer 2012;2012:845143
- Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 2010;28:2114–22
- Bouchardy C, Rapiti E, Fioretta G, et al. Impact of family history of breast cancer on tumour characteristics, treatment, risk of second cancer and survival among men with breast cancer. Swiss Med Wkly 2013;143:w13879
- Guerra MR, Mendonça GAS, Bustamante-Teixeira MT, et al. Five-year survival and prognostic factors in a cohort of breast cancer patients treated in Juiz de Fora, Minas Gerais State, Brazil. Cadernos de Saúde Pública 2009;25:2455–66
- Bourhafour M, Belbaraka R, Souadka A, et al. Male breast cancer: a report of 127 cases at a Moroccan institution. BMC Res Notes 2011;4:219
- de Ieso PB, Potter AE, Le H, et al. Male breast cancer: a 30-year experience in South Australia. Asia Pac J Clin Oncol 2012;8:187–93
- Kowalski C, Steffen P, Ernstmann N, et al. Health-related quality of life in male breast cancer patients. Breast Cancer Res Treat 2012;133:753–7
- Foerster R, Foerster FG, Wulff V, et al. Matched-pair analysis of patients with female and male breast cancer: a comparative analysis. BMC Cancer 2011;4;11:335
- Xu S, Yang Y, Tao W, et al. Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Res Treat 2012;136:495–502
- Liu T, Tong Z, He L, Zhang L. Clinicopathological characteristics and survival analysis of 87 male breast cancer cases. Breast Care (Basel) 2011;6:446–51
- Fogh S, Hirsch AE, Langmead JP, et al. Use of tamoxifen with postsurgical irradiation may improve survival in estrogen and progesterone receptor-positive male breast cancer. Clin Breast Cancer 2011;11:39–45
- Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat 2004;83:77–86
- Pemmaraju N, Munsell MF, Hortobagyi GN, Giordano SH. Retrospective review of male breast cancer patients: analysis of tamoxifen-related side-effects. Ann Oncol 2012;23:1471–4
- Visram H, Kanji F, Dent SF. Endocrine therapy for male breast cancer: rates of toxicity and adherence. Curr Oncol 2010;17:17–21

