Abstract
Androgen deprivation therapy (ADT) for the treatment of prostate cancer (PCa) causes an increase in total body fat, leading to a net gain in body weight. Moreover, the use of the luteinizing hormone-releasing hormone agonists in ADT causes a decrease in serum androgen levels, leading to the development of metabolic syndrome (MetS). Androgen blockade significantly increases plasma adiponectin levels, which has some efficacy against MetS, whereas ADT increases fasting plasma insulin and decreases insulin sensitivity, suggesting that there are other mechanisms involved in the onset of MetS besides adiponectin activation. We investigated the effects of ADT on serum aP2 and adiponectin in PCa patients. Six months post-ADT, serum aP2 and adiponectin levels were significantly increased, although there were no changes in patient body weight and no correlation between the changes in serum aP2 and total adiponectin levels. The serum adiponectin and aP2 levels have independent implications in ADT for PCa; therefore, their combined measurement will clarify the impact on the development of obesity-related diseases during ADT. Contrary to adiponectin, high serum aP2 levels were correlated with the late development of MetS. Further studies are needed to investigate the future occurrence of metabolic diseases post-ADT.
Introduction
Obesity is associated with an increased risk for the development, progression and recurrence of prostate cancer (PCa) [Citation1,Citation2]. Although androgen deprivation therapy (ADT) post or combined with radiation therapy has been shown to prolong the overall survival of PCa patients [Citation3–5], the treatment leads to a decrease in lean body mass and increase in total body fat, often resulting in an overall gain in body weight [Citation6–8]. Moreover, the use of luteinizing hormone-releasing hormone agonists in ADT, such as leuprolide acetate and goserelin, can decrease serum levels of androgens leading to the development of metabolic syndrome (MetS), which is a risk factor for both type 2 diabetes mellitus and cardiovascular disease [Citation9–11]. Therefore, it is very important to control the body weight of the patients with PCa and monitor MetS-related serum parameters during ADT.
Low serum levels of adiponectin, which is mainly secreted by adipocytes, was reported to be strongly correlated with MetS onset [Citation12,Citation13]. During the progression of obesity, the adipose tissue becomes hypertrophied and the secretion of adiponectin decreases. Although androgens decrease serum adiponectin levels, androgen-induced hypoadiponectinemia may be related to an increased risk of insulin resistance and atherosclerosis in men [Citation14]. However, androgen blockade with leuprolide showed a significant increase in fat mass and serum adiponectin levels, whereas ADT was found to increase fasting plasma insulin and decrease insulin sensitivity [Citation15] suggesting other mechanisms besides the actions of adiponectin in MetS.
In addition to adiponectin, aP2 or fatty acid binding protein 4 is an abundant adipocyte cytoplasmic fatty acid-binding protein that is also secreted by adipocytes and binds to fatty acid ligands with high affinity [Citation16]. aP2 expression is transcriptionally regulated during adipocyte development by peroxisome proliferator-activated receptor (PPAR)-γ agonists, insulin and fatty acids [Citation17]. Studies of aP2-deficient mice have shown that aP2 plays an important role in regulation of glucose and lipid homeostasis [Citation18]. Therefore, serum aP2 levels may be useful to predict the development of adiposity-independent MetS, insulin resistance and systemic inflammation [Citation13,Citation19].
We demonstrated that aP2 is also expressed in macrophages and is induced by exposure to phorbol esters, oxidized low-density lipoproteins and PPAR-γ ligands [Citation20]. Furthermore, aP2 production by adipocytes and macrophages plays a significant role in protection from atherosclerosis [Citation20–22]. Due to its expression in adipocytes and macrophages, aP2 is now considered to robustly impact multiple components of MetS by integrating metabolic and inflammatory responses in adipocytes and macrophages, respectively.
Despite the increase in serum adiponectin levels, other MetS-related changes occur concomitantly with ADT. Therefore, in the present study, we investigated the influence on the serum aP2 and adiponectin levels during ADT for patients with PCa.
Materials and methods
Study design
Serum aP2 and adiponectin levels of all the subjects were evaluated at Osaka University Hospital (Suita, Osaka, Japan) at baseline and about 6 months post-ADT. While receiving ADT, all PCa patients were referred to a weight management clinic and received information on calorie restriction and exercise training from dieticians. Body weight, body mass index (BMI) and biochemical parameters, including serum levels of fasting blood sugar (FBS), insulin, hemoglobin A1c (HbA1c), adiponectin and aP2, were determined at baseline and about 6 months post-ADT, concurrent with clinical evaluations.
Patients
A total of 18 consecutive patients with locally advanced or recurrent PCa who received ADT and weight management information by a dietician were followed-up at Osaka University Hospital. The baseline stage of PCa ranged from B2 to D2 according to the Jewett staging system. The basal characteristics of these patients are shown in .
Table 1. Clinical characteristics of patients before ADT.
Anthropometric parameters
Body composition, height and weight were measured by standard procedures. BMI was calculated as the quotient of weight in kilograms divided by the square of the height in meters.
Biochemical assays
Serum total adiponectin levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit specific for human adiponectin according to the manufacturer’s instructions (total: Otsuka Pharmaceutical Co., Ltd., Tokushima, Japan). Serum aP2 levels were measured using an ELISA kit specific for human aP2 according to the manufacturer’s instructions (SPI Bio, Montigny le Bretonneu, France). Other MetS-related biochemical parameters were measured at the Laboratory for Clinical Investigation (AST and ALT; Wako chemical, Osaka, Japan, PSA and Testosterone; Beckman Coulter, Inc., Brea, CA, Glucose; Shino-Test Corporation, Tokyo, Japan, Insulin; FUJIREBIO Inc., Tokyo Japan, Triglyceride, Total cholesterol, HDL cholesterol and LDL cholesterol; Sekisui Medical, Tokyo, Japan, HbA1c was analyzed by HPLC, TOSHO corporation, Tokyo, Japan; Osaka University Hospital, Osaka, Japan).
Statistical analysis
All the analyses were performed using StatView statistical software (version 5.0; SAS Institute, Inc., Cary, NC). All data were presented as the mean ± SD. Longitudinal changes between values at baseline and post-ADT for all outcome measures were examined using the non-parametric Wilcoxon matched pairs test. Spearman’s rank correlation analyses were performed to examine the linear relationships between changes in total adiponectin and aP2 levels. A probability (p) value of <0.05 was considered statistically significant.
Ethical statement
All experimental procedures were approved by the Ethics Review Committee of Osaka University Hospital (approved No. 09220-3).
Results
ADT
Of the 18 patients enrolled in this study, nine received leuprorelin acetate by subcutaneous injection (SI) (initial dose, 3.75 mg/4 weeks; maintenance dose, 11.25 mg every 12 weeks; SR Luplin, Takeda Pharmaceuticals Co., Ltd., Osaka, Japan) and nine others received goserelin acetate by SI (initial dose, 3.6 mg/4 weeks; maintenance dose, 10.8 mg every 12 weeks; Zoladex LA Depot, AstraZeneca, London, UK). As an anti-androgenic, 16 patients also received bicalutamide orally (80 mg/d; Casodex, AstraZeneca) and one received flutamide orally (375 mg/d; Odyne, Nippon Kayaku, Tokyo, Japan), which prevents potential flare-ups associated with the first administration of a gonadotropin-releasing hormone agonist and blocks the action of adrenal androgen.
ADT increased circulating adiponectin and aP2 levels in subjects with PCa
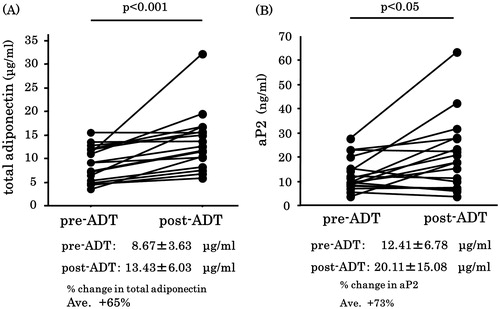
Post-ADT, there were no significant changes in the body weight, BMI or serum levels of FBS, HbA1c () total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides (data not shown). The mean serum adiponectin levels increased significantly (by 65%) post-ADT compared to that at baseline (8.67 versus 13.43 μg/mL). The serum total adiponectin levels increased post-ADT in all patients included in this study (). The mean serum aP2 levels significantly increased by 73% post-ADT compared to that at baseline (12.41 versus 20.11 ng/mL). In 11 (61%) of the 18 patients, the serum aP2 levels increased post-ADT ().
Figure 1. Changes in body weight (BW), body mass index (BMI), fasting blood glucose and haemoglobin A1c (HbA1c) post-ADT as compared to that of the baseline values. There were no changes by ADT. Values are expressed as the mean ± SD.
Figure 2. Changes in total serum total adiponectin (A) and aP2 (B) levels during ADT. Both adiponectin and aP2 levels were significantly increased post-ADT. Each black circle signifies a prostate cancer patient. Values are expressed as mean ± SD.
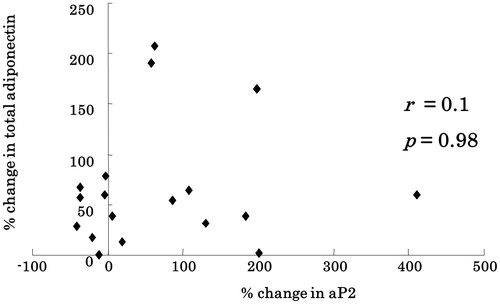
Spearman’s rank correlation analyses were performed to assess the linear relationships among the relative changes in BMI and serum levels of adiponectin and aP2. There was no correlation between the changes in BMI and total adiponectin levels (r < 0.01, p = 0.98) (data not shown). Moreover, there was no correlation between the changes in aP2 and total adiponectin levels (r = 0.126, p = 0.262; ).
Discussion
Previous studies have reported that ADT can cause obesity, hyperglycemia and triglyceridemia; thereby, predisposing patients to a higher risk of cardiovascular disease. Therefore, management of body weight is important to reduce ADT-associated complications, so that patients can continue cancer treatment and achieve improved survival.
An in vitro study by Gupta et al. [Citation23] showed that dihydrotestosterone (DHT) down-regulated mRNA expression levels of aP2, leptin and PPAR-γ in human mesenchymal stem cells and adipocytes. Therefore, DHT decreased the differentiation of stem cells into adipocytes and reduced fat mass. In contrast, ADT promotes the differentiation of adipocytes by decreasing testosterone production, resulting in increased serum adiponectin and aP2 levels, both of which are enhanced during the differentiation of adipocytes. As expected, both mean serum levels of adiponectin and aP2 were increased post-ADT in the current study, although no changes in patient body weight were observed.
Serum adiponectin levels increased in all patients, while aP2 levels increased in 11 (61%) of 18. Consequently, there were no correlations between the relative changes in serum adiponectin and aP2 levels. However, the precise reason for this difference remains unclear, because the current study included patients with variable stages of PCa, which may have affected the aP2 levels. However, one possible mechanism is the production of aP2 by macrophages [Citation20]. Adiponectin is specifically expressed in adipocytes, whereas aP2 is expressed not only in adipocytes but also in macrophages [Citation20]. To date, there is no report describing the effect of DHT on macrophages. However, androgen receptors are expressed on the cellular membrane of macrophages [Citation24] and previous studies have reported that testosterone reduced toll-like receptor 4 expression by these cells [Citation25]. Moreover, recently it was reported that in addition to macrophages and adipocytes, aP2 is expressed in a subset of endothelial cells (ECs) in several normal tissues and promotes angiogenesis [Citation26–28]. Androgen receptor is also expressed in ECs and mediates the biological effects of androgens in a wide range of physiological and pathological processes in ECs [Citation29]. Considering the co-expression both of aP2 and androgen receptor in macrophages and ECs, these cells might be influenced by variations in DHT levels, leading to be possible sources of aP2 secretion.
ADT with weight management did not worsen metabolic parameters and positively regulated serum adiponectin levels in all PCa patients. Much evidence from experimental studies indicates that adiponectin protects against the development of diabetes mellitus, hypertension, cardiovascular disease and cancer [Citation30]. Contrary to adiponectin levels, high serum aP2 levels were associated with later development of MetS and progression of type 2 diabetes and atherosclerosis [Citation13]. In the current study, ADT increased serum levels of both adiponectin and aP2. Consequently, further studies are needed to investigate the future occurrence of metabolic diseases in PCa patients receiving ADT.
In present study, weight management might have preventive effects against MetS in this population; however, there was no control group. Importantly, many nutrients, including n-3 fatty acids, lycopene, vitamin E and isoflavone, have been shown to decrease the onset and progression of PCa and extend patient survival [Citation31–35]. Thus, PCa patients should receive more intensive intervention during ADT. Moreover, it could not justify whether the time allocated (6 months) was sufficient for MetS to develop or for the changes in serum aP2 and adiponectin to manifest in these patients by present study. Further studies regarding the effect of weight management for this population and long-term follow-up are needed.
In summary, serum adiponectin and aP2 levels have independent significance in ADT for PCa. Therefore, the combined measurement of adiponectin and aP2 will clarify the impact on the development of obesity-related diseases.
Acknowledgements
We owe our deepest gratitude to Miki Sakaue and Megumi Matsumoto (Osaka University, Osaka, Japan) for their tremendous supports.
Declaration of interest
The authors report no conflicts of interest associated with this study.
References
- MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Cause Contr 2006;17:989–1003
- Strom SS, Wang X, Pettaway CA, et al. Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clin Cancer Res 2005;11:6889–94
- Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010;11:1066–73
- Hanks GE, Pajak TF, Porter A, et al; Radiation Therapy Oncology Group. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol 2003;21:3972–8
- Warde P, Mason M, Ding K, et al.; NCIC CTG PR.3/MRC UK PR07 investigators. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 2011;378:2104–11
- Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2002;87:599–603
- Berruti A, Dogliotti L, Terrone C, et al.; Gruppo Onco Urologico Piemontese (G.O.U.P.), Rete Oncologica Piemontese. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol 2002;167:2361–7
- Braunstein LZ, Chen MH, Loffredo M, et al. Obesity and the odds of weight gain following androgen deprivation therapy for prostate cancer. Prostate Cancer 2014;2014:230812
- Inaba M, Otani Y, Nishimura K, et al. Marked hyperglycemia after androgen-deprivation therapy for prostate cancer and usefulness of pioglitazone for its treatment. Metabolism 2005;54:55–9
- Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol 2006;24:3979–83
- Kintzel PE, Chase SL, Schultz LM, O'Rourke TJ. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy 2008;28:1511–22
- Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des 2010;16:1896–901
- Maeda K. Role of adiponectin and adipocyte fatty acid binding protein in the metabolic syndrome. Diabetes Res Clin Pract 2007;77:S17–22
- Nishizawa H, Shimomura I, Kishida K, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 2002;51:2734–41
- Smith MR, Lee H, Fallon MA, Nathan DM. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology 2008;71:318–22
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 2008;7:489–503
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 2008;77:289–312
- Hotamisligil GS, Johnson RS, Distel RJ, et al. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 1996;274:1377–9
- Tso AW, Xu A, Sham PC, et al. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care 2007;30:2667–72
- Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 2001;7:699–705
- Boord JB, Maeda K, Makowski L, et al. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler Thromb Vasc Biol 2002;22:1686–91
- Boord JB, Maeda K, Makowski L, et al. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation 2004;110:1492–8
- Gupta V, Bhasin S, Guo W, et al. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol 2008;296:32–40
- Lai JJ, Lai KP, Chuang KH, et al. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest 2009;119:3739–51
- Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 2008;78:432–7
- Elmasri H, Karaaslan C, Teper Y, et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J 2009;23:3865–73
- Elmasri H, Ghelfi E, Yu CW, et al. Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis 2012;15:457–68
- Iso T, Maeda K, Hanaoka H, et al. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler Thromb Vasc Biol 2013;33:2549–57
- Torres-Estay V, Carreno D, San Francisco IF, et al. Androgen receptor in human endothelial cells. J Endocrinol 2015;224:R131–7
- Kishida K, Funahashi T, Shimomura I. Adiponectin as a routine clinical biomarker. Best Pract Res Clin Endocrinol Metab 2014;28:119–30
- Aronson WJ, Glaspy JA, Reddy ST, et al. Modulation of omega-3/omega-6 polyunsaturated ratios with dietary fish oils in men with prostate cancer. Urology 2001;58:283–8
- Hoenjet KM, Dagnelie PC, Delaere KP, et al. Effect of a nutritional supplement containing vitamin E, selenium, vitamin c and coenzyme Q10 on serum PSA in patients with hormonally untreated carcinoma of the prostate: a randomised placebo-controlled study. Eur Urol 2005;47:433–9
- Kucuk O, Sarkar FH, Djuric Z, et al. Effects of lycopene supplementation in patients with localized prostate cancer. Exp Biol Med (Maywood) 2002;227:881–5
- Heinonen OP, Albanes D, Virtamo J, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Nat Cancer Inst 1998;90:440–6
- Hussain M, Banerjee M, Sarkar FH, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer 2003;47:111–17