Abstract
Introduction: Testosterone treatment has evolved rapidly over the past 25 years as new, more effective and convenient methods have become available. This study reports experience with seven different methods, introduced on the market in the UK.
Aim: To establish the symptom response when testosterone treatment was initiated on the basis of clinical features and symptoms of androgen deficiency, and the resulting endocrine, biochemical and physiological responses.
Methods: Of 2693 patients attending the 3 Men’s Health Centers – The UK Androgen Study (UKAS), 2247 were treated. Treatments included pellet implants, oral testosterone undecanoate (Testocaps), mesterolone (Proviron), testosterone gel (Testogel), testosterone scrotal cream (Andromen) and scrotal gel (Tostran).
Results: There was no correlation between initial testosterone level, initial symptom score or the success of treatment as defined by adequate resolution of symptoms. Despite the diverse endocrine patterns produced, the testosterone preparations appear equally safe over prolonged periods, with either no change or improvement of cardiovascular risk factors, especially in lowering cholesterol and diastolic blood pressure.
Conclusions: It is suggested that because of excessive reliance on laboratory measures of androgens and undue safety concerns, many men who could benefit from symptom relief, improvement in related clinical conditions and given preventive medical benefits remain untreated.
Introduction
There is great debate about the indications for testosterone treatment, its safety and effectiveness. Many generalizations are made often without reference to the particular form used, the endocrine changes they produce, their effects on cardiovascular risk factors and their relative suitability for different clinical conditions.
Testosterone treatment has evolved greatly over the past 25 years becoming both safer and more effective. Not all testosterone preparations are equal in terms of their endocrine actions and side effects. Such factors need to be taken into consideration in selecting an appropriate formulation for testosterone replacement treatment (TRT).
Cost can also substantially influence prescribing practice in some countries. This was illustrated in a paper earlier this year on the “Population-based patterns of prescription androgen use, 1976–2008” which found that the commonest form of testosterone treatment in Canada during that period was methyl testosterone at 36.2% [Citation1]. This was similar to the prescription rate seen in Russia, despite its long-recognized hepatotoxicity and cardiotoxicity which caused it to be declared “obsolete” and taken-off the European market in the 1980s [Citation2], and yet it is still cheaply and widely available via the web in all countries.
A similar lack of practical clinical information about another oral preparation being considered for marketing in the US, testosterone undecanoate (Rextoro – Clarus Pharmaceuticals), was shown when the FDA recently rejected a new drug license for this, while calling for more data on efficacy and safety, despite virtually the same formulation being available in Europe, Canada and Australia for the last 30 years.
The present study was designed as a retrospective audit to investigate the indications for treatment, symptomatic responses together with the endocrine, metabolic and cardiac risk factor changes on seven different testosterone preparations, illustrating the evolution of this treatment over 25 years.
Methods
The UK Androgen Study (UKAS) is an ongoing analysis of patients attending the Centre for Men’s Health clinics in London, Manchester and Edinburgh since they were established in 1989. Ethical permission for this audit was obtained from the St. Mary’s Hospital Local Research Ethics Committee (LREC), with informed consent from the patients for the use of their anonymized data.
Though treatment within this clinical practice could not be blinded, and bias in the subjective reporting cannot therefore be excluded, every effort has been made to make the observations as objective as possible. First visit data served as the control in each patient, for comparison with data from subsequent visits, which were usually at 6 monthly intervals once treatment had been optimized by dosage adjustment according to symptomatic response.
The study was initiated 25 years ago, before diagnostic guidelines for testosterone treatment had been established. Patients were diagnosed with androgen deficiency based on history, clinical assessment, Andropause Check List (ACL) and Aging Male Symptoms (AMS) [Citation3] scores. The ACL is a detailed testosterone deficiency symptom scale comprising 20 questions rated on a five-point scale of 0 – none, 1 – mild, 2 – moderate, 3 – severe, 4 – very severe or total [Citation4]. It is based on the characteristic symptoms described nearly 70 years ago by Dr August Werner and correlates closely with other descriptions of the symptoms and rating scales, including the AMS scale developed 15 years ago, with which many items overlap (r = 0.731). The ACL was used at each subsequent visit to monitor symptom response to treatment. With a maximum score of 80, a total of 10 or less was rated as normal and was the target for treatment. Seven different testosterone preparations were used, and some patients changed treatment groups to achieve symptom remission.
Full endocrine, biochemical and hematological profiles were available to guide treatment and monitor its safety, together with the clinical and physiological data recorded at each visit. The laboratory data are taken according to the hospital and major independent laboratories reporting them, all using full quality control procedures and standard guidelines. The laboratory data were entered directly into an Access database via secure electronic data link, to prevent transcription errors. Fasting blood samples were taken between 9 and 11 am to minimize the variables which can otherwise invalidate androgen assays and to get a clearer picture of changes in lipid and carbohydrate metabolism with treatment. They were also taken prior to digital or ultrasound trans-rectal examination of the prostate.
The endocrine profile included total testosterone (TT), sex hormone binding globulin (SHBG), calculated free testosterone (cFT), estradiol (E2), luteinizing hormone (LH), follicle stimulating hormone (FSH), and cFT was determined using the Vermeulen formula [Citation5]. The biochemical profile included total and free prostate specific antigen (PSA), free/total PSA ratio, standard renal function tests and estimated glomerular filtration rate (eGFR), liver function tests, uric acid, iron, fasting glucose, triglycerides and total cholesterol, together with HDL, total cholesterol/HDL ratio and LDL. The hematology profile included hemoglobin and indices to exclude polycythemia, white cell count and differential platelets, and erythrocyte sedimentation rate (ESR). Routine urine chemistry was also performed at each visit.
The data were collated using a computerized practice management system called the Global Andrology Assessment and Treatment Tracking program (GAATT), developed specifically for use in the Centre for Men’s Health. This program assembled the information on all patients in an Access (Microsoft) database, and then extracted it using a data mining program, by which individual or group responses to treatment could be assessed.
Statistical tests were performed using the PASW (SPSS) 22 statistics program. Results for the endocrine data were log-transformed for analysis, because it is now recognized that as with most endocrine data, a skewed distribution must be normalized prior to analysis. The before and after treatment logged endocrine and PSA data, and other measures were analyzed by paired Student’s t tests. Logistic regression analysis compared baseline and final testosterone, estrogen and SHBG levels with treatment response failure. The failure event was defined by persistence of symptoms with an ACL symptom score over 10 on testosterone replacement when that particular treatment was discontinued. A Cox regression model was used to calculate adjusted hazard ratios (HRs) for response failure, comparing the different testosterone preparations. These analyses were performed using STATA version 11 (StataCorp, College Station, TX).
Exclusion criteria
As the intention was to study the diagnosis and treatment of secondary testosterone deficiency, certain groups of patients were excluded:
Primary testosterone deficiency, e.g. cases with a history of non-descent of testes, bilateral orchidectomy or patients diagnosed as Klinefelter’s Syndrome.
Prostate or breast cancer, or suspected prostate cancer.
Asymptomatic men attending for general medical screening.
Men seeking physical fitness training. Anabolic steroid treatment is not offered at the clinic to young men seeking improvement in athletic performance or physique
Young men with anxiety symptoms described as “locker room syndrome”.
Men diagnosed as “Male Mid-life Crisis”.
Patents with a primary diagnosis of depression.
Results
2693 patients attended the three centers, being were either self-referred or sent by their physicians the UK Androgen Study (UKAS). On the basis of their symptoms, history and clinical findings, 2247 men were diagnosed as androgen deficient and given testosterone treatment. Their mean age was 54 years, range 24–90, 93% being Caucasian, and median follow-up was 1 year (mean 1.6 years). Often symptoms of testosterone deficiency, including loss of libido and energy, erectile dysfunction, loss of morning erections, night sweats, joint pains, depression, irritability and impaired memory had been present for 3–5 years prior to attending the clinics for the first time.
The distribution of the initial pre-treatment testosterone levels are shown in and together with the other variables baseline levels served as controls for the later values. This audit enabled total and free testosterone to be evaluated as criteria for the diagnosis of androgen deficiency and as predictors of ability to achieve a full symptomatic response on treatment.
Figure 1. (a) Pre-treatment total testosterone levels, showing marked skewing to the left of the normal distribution curve. (b) The log-normal distribution of all pre-treatment blood total testosterone levels.
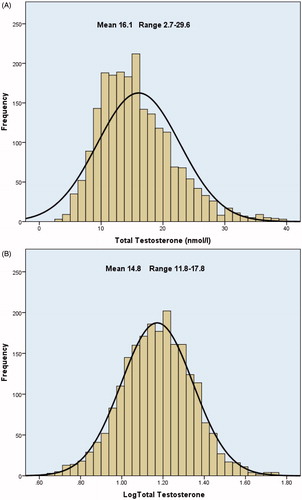
As the initial diagnosis was made on the basis of clinical features and symptoms alone, all symptomatic patients were given a therapeutic trial of testosterone treatment. Many of these patients had previously been declined treatment by their doctors on the basis of a testosterone level in the “normal” range even though this varies between laboratories, and reliability is susceptible to many factors [Citation6]. Their “normal ranges” are poorly defined not only because of the diurnal variation and age-related decline in testosterone levels, but particularly because they do not take account of the log-normal distribution. This changes both the mean and range, as shown by the distribution of the patient’s pre-treatment testosterone levels ().
In this study, TT and cFT are totally unrelated to the initial symptom score, which was the reason for patients attending the clinic for treatment. This is shown in . Also, there is no association between initial symptom score and baseline estradiol or SHBG. These findings are consistent with those in other studies showing failure of endocrine assays to correlate with symptoms [Citation7,Citation8].
Figure 2. Lack of relationship between pre-treatment total ACL symptom scores and levels of total testosterone.
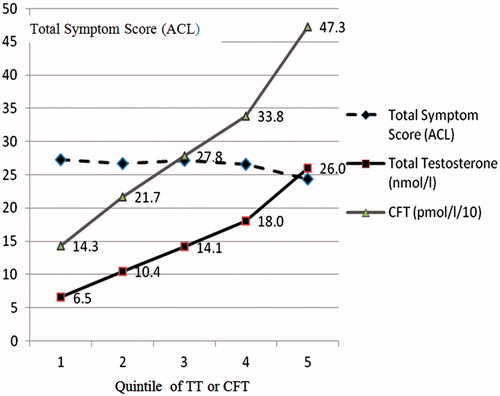
In this series, overall only 2% of pre-treatment TT levels were below 6 nmol/l (173 ng/dl), 6% below 8 nmol/l (230 ng/dl), 17% below 10 mol/l (288 ng/dl), 31% below 12 nmol/l (346 ng/dl), 45% below 14 nmol/l (404 ng/dl) and 58% below 16 nmol/l 460 ng/dl). These are all commonly used cut-off points required for the diagnosis of “hypogonadism”, though the higher levels are increasingly accepted [Citation9].
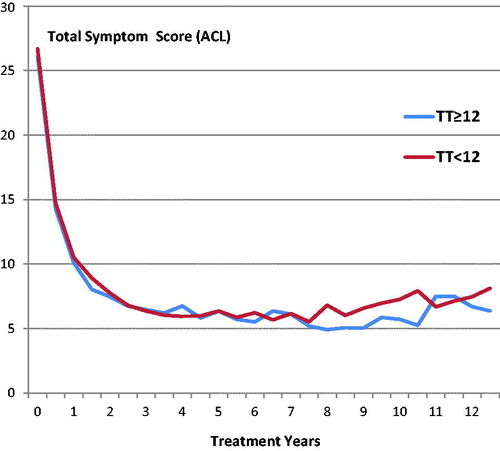
Comparing the symptomatic responses to treatment for the 31% of patients with initial TT levels below 12 (mean 9.6 nmol/l) with those greater than or equal to 12 (mean 19.1 nmol/l), there was no significant difference found between either the initial symptom scores (mean 26.7 and 26.1, respectively) or their response to treatment for up to 12 years for all forms of treatment ().
Figure 3. Symptomatic response over 12 years for patients presenting with TT ≤12 nmol/l and those >12 nmol/l.
Logistic regression analysis of failure to achieve a full symptomatic response also found no association with pre-treatment TT. Also, no statistically significant association is found between final TT, cFT, oestradiol or SHBG and the failure to achieve a full symptomatic response on any particular treatment. Unadjusted 1-year symptom failure is, however, strongly associated with the severity of presenting symptoms, increasing from only 12% for patients with the least severe symptom score prior to commencing testosterone replacement therapy to 59% for patients with the most severe symptoms prior to replacement therapy, a finding that was highly statistically significant (HR 4.6, 95% CI 2.6–8.0, p < 0.001). In those patients who did not achieve complete symptom response, many nevertheless continued on treatment with significant symptomatic benefit compared to baseline.
Treatment groups
As seen in , there were markedly different changes in endocrine, metabolic and cardiac risk factor changes with the various preparations, so each is considered separately for the seven treatment groups. Some patients changed dosage or treatment groups to achieve optimal remission of symptoms, which was the goal of treatment.
Table 1. Symptom score and endocrine changes between the initial visit and after 1 year, on treatment with seven different testosterone preparations: ACL Total Symptom Score, TT total testosterone (nmol/l), SHBG sex hormone binding globulin (nmol/l), E2 estradiol (pmol/l), LH luteinizing hormone (IU/l), FSH follicle stimulating hormone (IU/l).
Table 2. Glucose and lipid changes between the initial visit and after 1 year, on treatment with seven different testosterone preparations: Gluc (glucose, mmol/l), TG (triglycerides, mmol/l), Tchol (total cholesterol mmol/l), HDL Chol (high-density lipoprotein, mmol/l) and ratio (total cholesterol/HDL ratio).
Table 3. Changes in BMI (body mass index, kg/m2), systolic and diastolic blood pressure (mm Hg), pulse rate (BPM), Hb (hemoglobin, g/dl) and HCT (hematocrit) between the initial visit and after 1 year on treatment with seven different testosterone preparations.
The treatments are considered in the order that they appeared on the UK market. Their short-term endocrine, biochemical and physiological effects are shown in after 1 year of treatment, and thereafter there were no progressive changes. Long-term endocrine effects are shown in .
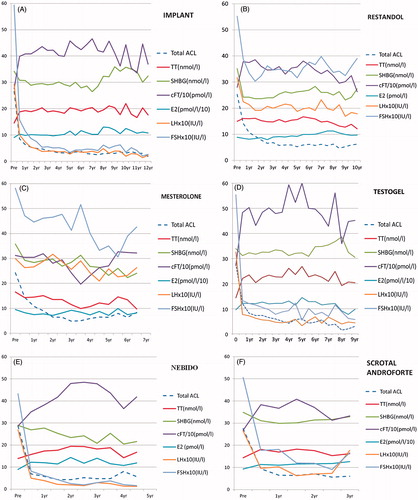
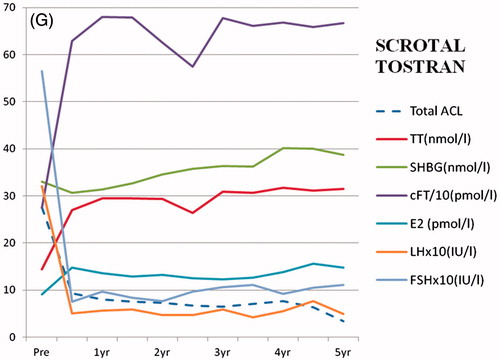
Figure 4. Symptom response and endocrine changes on treatment with: (a) testosterone implant (TI) over a period of 12 years, (b) oral testosterone undecanoate (TU) over a period of 10 years, (c) proviron (ME) over a period of 7 years, (d) Testogel (TG) over a period of 9 years, (e) Nebido (TJ) over a period of 5 years, (f) Scrotal AndroForte (SA) over a period of 3 years and (g) Scrotal Tostran (ST) over a period of 5 years.
Pellet implants (TI)
This is considered first because it is the oldest form of treatment studied, having been in use for over 70 years, though it now being replaced in most clinical practices in countries where it is available by testosterone undecanoate injections (Nebido), which last 2–3 months. An improved system using smaller pellets (Testopel) is available in the USA [Citation10].
This group received testosterone pellet implants (TI) (Organon, 325 patients, 806 treatment years). These patients had 4–10 of the 200-mg pellets of the fused crystals implanted into alternate buttocks every 6 months. There was a marked reduction in ACL symptom scores (; ), under 10 being considered a satisfactory response. The dose needed to maintain freedom from symptoms of testosterone deficiency for a 6-month period varied widely between individuals, but was relatively constant for each person, with different thresholds of TT and cFT at which symptoms returned.
The endocrine measurements were taken at the end of the implant period and are shown after 1 year, which includes two implant cycles for dose stabilisation, as seen in , and for up to 12 years as seen in . Peak testosterone levels are known to occur within a month of each implantation, but the trough TT levels as measured here were still significantly raised over baseline at 6 months, when the next implant was due.
The SHBG was slightly decreased for up to 7 years, rising to pre-treatment values after 9 years perhaps due to aging. cFT was markedly raised, with both LH and FSH reduced to very low levels throughout the implant cycle. There was only a slight but significant increase in E2 throughout the 12 years of treatment, also more marked after 8 years.
There were slight but statistically significant decreases in fasting glucose and triglyceride levels, but highly significant reductions in total cholesterol, HDL and LDL but no change in total cholesterol/HDL ratio ().
There was a slight increase in body mass index () and reduced diastolic blood pressure, with a raised pulse rate, suggesting the vasodilatation seen with all the testosterone preparations except for mesterolone. There was a highly significant increase in hemoglobin and hematocrit again seen with all preparations except mesterolone. These, however, remained within the normal range in over 90% of cases, but being included in the routine screen, polycythemia was detected early and could be corrected by reducing the dose or using another preparation.
Oral testosterone undecanoate (TU)
The largest treatment group was those receiving oral testosterone undecanoate (Restandol–Andriol Organon, 1016 patients, 2147 treatment years). The original preparation was found to be temperature unstable and had to be refrigerated to remain fully active for more than a month, but was replaced by a bioequivalent stable form in castor oil (Testocaps) in 2005. When taken with a meal containing fat, absorption of 40–120 mg doses (1–3 40 mg capsules) via the chylomicra in twice daily doses provided good clinical results in most cases (). As with other preparations, the dosage was varied to sustain optimum symptom relief, while not exceeding the physiological range in non-fasting samples. The endocrine changes on this preparation at 1 year are seen in and for up to 10 years in .
Though the increase in TT measured was only small in these fasting samples because of lack of absorption, cFT rose significantly, mainly due to a large reduction in SHBG. There was a significant decrease in E2, as well as LH and FSH, which, however, usually remained within the physiologically normal ranges.
Glucose did not change, but all lipid levels were decreased (), though the total cholesterol/HDL ratio increased. The BMI, systolic and diastolic blood pressures were decreased, but pulse rate increased. Hemoglobin and hematocrit were increased but less than with parenteral treatments and again usually stayed within the normal range.
Mesterolone (ME)
The other oral treatment group was mesterolone (Proviron–Bayer, 317 patients, 477 treatment years). For many patients, this preparation, being converted entirely to DHT and therefore only a partial form of androgen replacement, often provided inadequate symptom relief, and was abandoned after the first 5 years of the study.
The endocrine results are shown in and . The paradoxical reduction in TT (p .012), with no change in cFT, reflects a significant reduction in SHBG due to the suppression of endogenous testosterone synthesis by this form of treatment, as indicated by the slight decrease in LH and FSH. Also, since this preparation cannot be aromatized, there was a marked reduction in E2 which may partly account for its weak clinical action.
Though BMI increased slightly, there were no changes in glucose or triglyceride levels and smaller reductions in total cholesterol, HDL, the ratio and LDL (). There was a slight reduction in diastolic blood pressure and rise in pulse rate, and as with the other preparations, hemoglobin and hematocrit were unchanged.
Testosterone gel (TG)
When it became available 10 years ago, testosterone gel (Testogel–Androgel–Bayer, 343 patients, 740 treatment years), being a once-daily transdermal treatment with 5 G of 10% gel, was soon found to be highly acceptable and clinically effective. Application to the axilla more than doubled the absorption and when needed could avoid the use of two sachets.
Large increases in TT and cFT, measured an average of 2 h after the application of the gel, were maintained for up to 24 h, as shown by the occasional patient who failed to apply the gel on the day of the test, as well as by previous studies [Citation11] (; ).
There was only a slight reduction in SHBG, while LH and FSH levels were markedly suppressed, often to below the limits of measurement. As well as the reported increase in DHT [Citation12], aromatization also produced a slightly greater increase in E2 at 1 year than the other preparations ().
There was no change in glucose levels, but all lipids were decreased (). Unlike other reports on the treatment with non-genital testosterone gel [Citation11], there was an increase in BMI (), probably due to an increase in muscle mass. As with the majority of other preparations studied, there was a significant decrease in diastolic blood pressure and an increase in pulse rate. Hemoglobin and hematocrit were increased, again usually within the normal ranges.
Testosterone undecanoate injections (TJ)
Nebido (Reandron–Bayer, 49 patients, 85 treatment years; 1 g injections in 4 ml castor oil) was used in this study over 5 years. The androgen levels measured here are trough values 6–12 weeks after injection, when symptoms were beginning to return. This treatment has been shown to be safe and effective in large-scale international studies over 8 years [Citation13] and has recently been approved in the USA as 0.75 g testosterone undecanoate in 3 ml castor oil injections (Aveed). Symptom relief was excellent and rapid, and there were no adverse reactions apart from mild pain at the injection site lasting 1–6 h.
Endocrine values as measured after 1 year, when symptoms were returning at 6–12 weeks after the initial injection and progressively longer intervals thereafter, showed the trough levels of testosterone to be only moderately raised from pre-treatment values, with no change in SHBG, but a significant rise in CFT. E2 was slightly raised and there was a highly significant suppression of LH and FSH (; ).
Glucose and triglyceride levels were unchanged, and there were slight reductions in total and HDL cholesterol, but no change in LDL or the cholesterol/HDL ratio ().
There was no change in BMI or systolic blood pressure, and a slight decrease in diastolic pressure, but unlike the other preparations, no change in pulse rate. There were highly significant increases in hemoglobin and hematocrit, but again usually within the normal range ().
Scrotal AndroForte (SA)
Testosterone creams applied to the scrotum are found to be over 10 times better absorbed through the scrotum than through the skin anywhere else in the body [Citation14]. This makes it an extremely economic route for treatment, with a good safety record over 10 years when applied as a patch [Citation15].
AndroForte cream 2% Lawley Pharmaceuticals (84 patients, 141 treatment years) was applied to the scrotum as a 1–2-cm length on a measuring stick once or twice daily according to the clinical response. It proved convenient to use and was non-irritant.
There was good symptom relief, with a moderate rise in TT, which combined with a slight reduction in SHBG produced a marked rise in CFT (; ). E2 was raised, and LH and FSH markedly suppressed.
Glucose and TG were unchanged, cholesterol slightly reduced, with no change in the HDL or ratio (). There was no change in BMI, blood pressure or pulse rate, but the hemoglobin and hematocrit were raised ().
Scrotal tostran (ST)
Unlike testogel which is irritant when applied to the thin skin of the scrotum, Tostran 2% testosterone gel (Prostrakan, 113 patients, 118 treatment years) can be safely applied in that region as one or two 10 mg pump applications once or twice daily and gave good symptom relief (; ). As absorption of the gel was rapid, blood samples taken usually 1–2 h after application showed levels well up in the physiological range or briefly higher. Though the SHBG was unchanged, this gave significantly increased cFT and estrogen levels, with suppression of LH and FSH.
Glucose and triglyceride levels were unchanged, but total cholesterol, HDL, the LDL and cholesterol/HDL ratio were significantly reduced (). BMI was slightly raised but the systolic blood pressure was unchanged, while the diastolic was reduced and pulse rate increased (). Hemoglobin and hematocrit increased significantly, but again were usually within the normal range.
Treatment group comparison
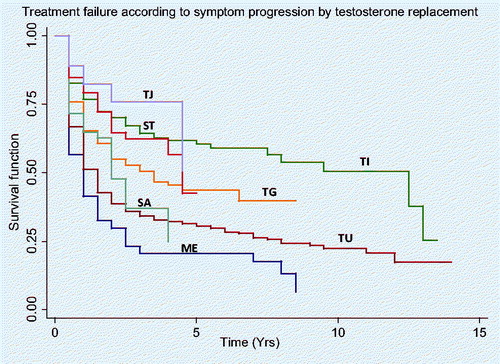
Comparing the different testosterone replacement therapies, the unadjusted rates for failure to achieve complete symptom response with TJ, TI, ST, TG, SA,TU and ME are 18, 23, 21, 35, 35, 49 and 59% at 1 year, and 24, 30, 35, 45, 52, 61 and 70% at 2 years, respectively. Hazard ratios by Cox Regressions for failure relative to ME are 26, 34, 38, 53, 64 and 76% for TJ, TI, ST, TG, SA and TU, respectively (p < 0.02). The differences in treatment failure between treatment groups are shown by the Kaplan–Meier analysis, as seen in .
Discussion
This study showed that good symptomatic relief can be obtained by a wide variety of testosterone preparations, but there are significant differences in a full symptomatic response to treatment between preparations.
Overall, safety factors monitored using these preparations, including renal and liver function tests, remained normal. In few cases, generally on pellet implants or Testogel, where polycythemia with excessive increases in hemoglobin or hematocrit occurred, this could be reversed by phlebotomy, reducing the dosage or switching to another preparation. The absence of adverse prostate changes or increases in PSA in this study has been reported previously [Citation16].
This study showed that good relief of the symptoms of testosterone deficiency could be safely obtained over extended periods by all seven of these preparations in spite of the wide variations in the resulting endocrine profiles. However, the more recently introduced creams and injections are not only convenient but, with the scrotal route of application Androforte and Tostran, also can be extremely economic, greatly improving the cost/benefit ratio of testosterone treatment [Citation17,Citation18] ().
Table 4. % Absorption, theoretical benefits, practical benefits, average monthly cost of product (£UK), average monthly cost of application, e.g. implant or injection (£UK) and total monthly cost of treatment (£UK) of seven different treatments.
With the exception of the mesterolone group, who were younger and had slightly fewer symptoms of androgen deficiency, the pre-treatment endocrine, glucose, lipid and cardiac risk factor values were similar, making the effects of the different treatments more comparable.
The reductions in LH are taken as representing the suppression of endogenous testosterone synthesis, while those in FSH reflect the variable reduction in spermatogenesis caused by the individual preparations. This may explain the varied effects of different preparations on fertility. While the pre-treatment LH levels were generally in the normal range, the corresponding FSH levels tended towards the upper end of the reference range, with some markedly raised levels in those cases where there was long-standing testicular damage.
It is appreciated that there are several unavoidable potential sources of bias in this study, most of which are inherent in long-term studies in clinical practice. Firstly, the findings could be biased towards cases which respond well clinically and therefore continued on treatment. However, as far as possible, patients who did not respond to their initial treatment in doses titrated against symptoms were provided with another preparation which gave the required relief.
Secondly, a general medical approach to treatment was used, with advice and encouragement where needed in relation to reducing stress, alcohol and weight, as well as increasing physical activity and other life-style modifications. Some of these goals were often difficult for the patient to achieve and maintain even with the improved mood and energy induced by testosterone treatment. Also, where there were other clinical conditions needing intervention, such as hypertension, hyperlipidemia or diabetes [Citation19], the additional treatment given may have distorted the response attributable to testosterone alone.
Thirdly, the patients did not live in a controlled and stable experimental setting, and over the many years of treatment patients and their partners could be subject to major life events which may, in some cases, have influenced their responses to the particular form of testosterone being taken at the time. Such major life events included relationship break-ups, job-losses, retirement, major illnesses and bereavement. In general, however, the large number of cases studied would tend to even out effects due to these extraneous influences.
Despite the very different endocrine profiles produced by the seven preparations studied, a good symptomatic response could be produced and maintained by all of them, though mesterolone had the weakest action and tended to be used in younger patients who wished to retain fertility and avoid gynecomastia during treatment.
This was seen by sustained decreases in the symptom checklist score that the patients noticed the return of symptoms when they discontinued treatment, and the fact that many remained on the treatment for periods up to 20 years. This is not pattern of response which would be expected of a placebo, which is typically transient. Their sustained reversal also refutes the notion that the symptoms are an untreatable effect of aging or are to be dismissed as normal.
Though the diagnosis and treatment of testosterone deficiency are often mainly based on TT, the key fact clearly demonstrated in this study is that there is virtually no relationship between diagnostic symptoms and initial total testosterone, or any other endocrine variable such as cFT or LH. Nor is any level of these hormones predictive of failure to relieve the symptoms of testosterone deficiency by any of the androgen preparations.
In other published studies reporting symptom response to testosterone implants, “time of return of androgen deficiency symptoms and the blood total and free testosterone concentrations are highly reproducible within individuals, but vary markedly between men, indicating that each person had a consistent testosterone threshold that may differ significantly between individuals” [Citation20] The same workers also observe that “Some androgen-dependent biological functions require higher plasma T levels than others, and these thresholds differ among men”. This later observation has been confirmed by other researchers [Citation21] who state “There is no evidence that a uniform structure of testosterone concentrations and complaints exists within the cohort of elderly male patients” and would again suggest that regulation of treatment according to symptomatic response is preferable to that based on achieving target TT levels.
A similar approach has recently been advocated by Maganty et al. who suggest “the search for a discrete threshold may be futile given emerging evidence. Recent studies suggest that testosterone threshold varies by symptoms and among individuals. In addition, thresholds may vary between young and old men. Therefore, initiation of treatment should rely more on symptoms and less on a discrete numerical threshold” [Citation22].
The study was initiated before guidelines were started and is unique in that the diagnosis was made predominantly on history, clinical findings and symptoms, even when initial androgen levels were within contemporaneous so-called normal ranges. This remarkable finding is explained by the theory of androgen resistance where, as with insulin in diabetes, there can be a relative rather than absolute deficiency [Citation23].
It is the complexities in the production and action of testosterone, its metabolites, especially dihydrotesterone (DHT) and oestradiol (E2), together with the variable tissue levels and potential for local synthesis, which suggests the case for the diagnosis and treatment of this condition predominantly on the basis of the assessment and relief of symptoms.
This suggestion is in accordance with an emerging consensus that “expert opinions differed from some published guidelines by the emphasis on symptoms as paramount, recognition of the limitations of total T as a diagnostic test, and the potential utility of a therapeutic trial in symptomatic cases with normal total T concentrations” [Citation9].
While TT and cFT are markedly and consistently raised by treatment with implants and testosterone gels and are easily measurable, treatment with oral preparations may rely more on reductions in SHBG in the case of oral testosterone undecanoate (TU) or a rise in DHT and reduction in estrogens with mesterolone. Both oral TU and transdermal testosterone gels cause doubling or trebling of DHT levels.
The increase in E2 due to aromatization, which is greatest on Testogel, reduces testosterone synthesis and may explain the “honey-moon” effect on beginning treatment. This is sometimes seen when, after an excellent initial response, the effectiveness of this treatment decreases within a month or two, and cannot be restored, or may even be worsened, by raising the dose. In some cases, efficacy can be restored by giving an aromatase inhibitor, such as anastrazole, which a few clinicians are using on its own to treat testosterone deficiency.
There was no clinical evidence of either increased cardiovascular events or venous thrombosis in this series, and an adverse effect on coagulation factors was not found even with high dosage of testosterone in early studies of TRT used as a male contraceptive [Citation24].
Zinc supplements are also reputed to decrease aromatization, possibly through reduced circulating luteinizing hormone and testosterone concentrations, alterations in hepatic steroid metabolism, and modification of sex steroid hormone receptor levels and the activity of their zinc fingers, though evidence for this is limited [Citation25].
Human chorionic gonadotrophin (hCG) has been shown to be an effective form of treatment and has the benefits of promoting physiological increase in endogenous testosterone production, without suppression of gonadotrophins with concomitant decrease in fertility seen with other forms of androgen replacement. This approach may come to be the standard form of treatment when the problem of the frequent injections required has been resolved and could even lead to “androversion” in some patients, which is when other forms of treatment are no longer required.
Conclusions
This long-term study of the evolution of different forms of testosterone treatment has shown widely different endocrine patterns between the seven preparations used, but also demonstrated their inherent safety overall. Safety in relation to the prostate has been shown in a previous publication from this series [Citation16], with no significant rise in PSA other than that due to age, and no increase in the incidence of prostate cancer.
In this study, the overall effects of all the treatments on metabolic and cardiac risk factors are shown to be neutral or even beneficial, especially in relation to total cholesterol and diastolic blood pressure. The FDA recognizes the issues relating to biochemical threshold, and in relation to the limitations of currently available published information, the authors are pleased to be able to contribute to the debate and present new informative data, without presuming to challenge the position of the FDA.
It is hoped that this data, together with the favorable clinical reports over the last 20 years [Citation26], will prove reassuring to the FDA and other doctors world-wide who have been alarmed by the alleged but unsubstantiated cardiovascular risks of testosterone treatment [Citation26].
In particular, this study gives further evidence against the use of very low-testosterone levels to deny TRT to men with obvious symptoms. In Australia, this year the Pharmaceutical Benefits Scheme has introduced regulations to dictate that under their rules, TRT shall not be provided to men whose total testosterones are above 6 nmol/l (173 ng/dl) in two samples, half the levels suggested by ISSAM [Citation27] and ISSM guidelines, and then treatment can only be initiated by a hospital consultant endocrinologist or urologist. The UK is following similar restrictive policies on prescribing. Apart from inflating the cost of diagnosis and treatment, this conflicts with findings published by the instigators of these guidelines on three important issues.
These are firstly that seven different Australian laboratories produced results that differ by up to 100%, i.e. a sample containing 8 nmol/l TT as measured by the reference measure of gas chromatography/mass spectrometry, can give readings of between 5 and 12 nmol/l by far commoner and cheaper immunoassays [Citation28], i.e. “major differences exist between commercial T immunoassays as well as divergence from the GC/MS standard” [Citation29] and between reference ranges according to arithmetic, geometric and quoted laboratory values. Secondly, the same authors have shown that “each person had a consistent testosterone threshold for androgen deficiency symptoms that differed markedly between individuals” [Citation20], and finally “in older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality” [Citation30].
However, the findings in the present study suggest that with appropriate and necessary monitoring of safety parameters, testosterone treatment appears safe and economic. Many men who could benefit in terms of symptom relief, with improvement in related clinical conditions and prevention of the long-term effects of testosterone deficiency, may remain untreated because of excessive reliance on laboratory measures of androgens for diagnosis and treatment alongside unwarranted safety concerns.
Acknowledgements
Thanks are due to our late colleague Hugh Welford who created the Practice Management System for collecting the patient data, and the Data Mining Program, which together enabled us to collate and analyze it, making the study possible. Jim McGrew office manager did huge amount of work coordinating the patients and organising their data. We would also like to thank Dr Doug Savage of the Centre for Men's Health for the use of his recent patients' data.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Hall SA, Ranganathan G, Tinsley LJ, et al. Population-based patterns of prescription androgen use, 1976–2008. Pharmacoepidemiol Drug Saf 2014;23:498–506
- Nieschlag S, Behre HM. Testosterone: action, deficiency, substitution. 4th edn. Cambridge, UK: Cambridge University Press; 2012
- Heinemann LAJ, Zimmermann T, Vermeulen A, et al. A new aging males’ symptoms (AMS) rating scale. Aging Male 1999;2:105–14
- Carruthers M. Androgen deficiency in the adult male – causes, diagnosis and treatment. Boca Raton, FL: CRC Press; 2004
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Carruthers M, Trinick TR, Wheeler MJ. The validity of androgen assays. Aging Male 2007;10:165–72
- Morales A, Spevack M, Emerson L, et al. Adding to the controversy: pitfalls in the diagnosis of testosterone deficiency syndromes with questionnaires and biochemistry. Aging Male 2007;10:57–65
- Miwa Y, Kaneda T, Yokoyama O. Correlation between the Aging Males’ Symptoms Scale and sex steroids, gonadotropins, dehydroepiandrosterone sulfate, and growth hormone levels in ambulatory men. J Sex Med 2006;3:723–6
- Morgentaler A, Khera M, Maggi M, Zitzmann M. Commentary: who is a candidate for testosterone therapy? A synthesis of international expert opinions. J Sex Med 2014;11:1636–45
- McCullough AR, Khera M, Goldstein I, et al. A multi-institutional observational study of testosterone levels after testosterone pellet (Testopel(R)) insertion. J Sex Med 2012;9:594–601
- Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 2004;89:2085–98
- Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab 2000;85:4500–10
- Zitzmann M, Mattern A, Hanisch J, et al. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1438 men. J Sex Med 2013;10:579–88
- Atkinson LE, Chang Y, Snyder PJ. Long-term experience with testosterone replacement through scrotal skin. In Testosterone: Action, deficiency, substitution. 2 edn. Berlin. Springer Verlag; 365--88
- Behre HM, von Eckardstein S, Kliesch S, Nieschlag E. Long-term substitution therapy of hypogonadal men with transscrotal testosterone over 7–10 years. Clin Endocrinol (Oxf) 1999;50:629–35
- Feneley MR, Carruthers M. Is testosterone treatment good for the prostate? Study of safety during long-term treatment. J Sex Med 2012;9:2138–49
- Moskovic DJ, Araujo AB, Lipshultz LI, Khera M. The 20-year public health impact and direct cost of testosterone deficiency in U.S. men. J Sex Med 2013;10:562–9
- Arver S, Luong B, Fraschke A, et al. Is testosterone replacement therapy in males with hypogonadism cost-effective? An analysis in Sweden. J Sex Med 2013;11:262–72
- Hackett G, Cole N, Bhartia M, et al. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med 2014;11:840–56
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004;89:3813–17
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 2006;91:4335–43
- Maganty A, Shoag JE, Ramasamy R. Testosterone threshold – does one size fit all?. Aging Male 2015;18:1–4
- Carruthers M. The paradox dividing testosterone deficiency symptoms and androgen assays: a closer look at the cellular and molecular mechanisms of androgen action. J Sex Med 2008;5:998–1012
- Anderson RA, Ludlam CA, Wu FC. Haemostatic effects of supraphysiological levels of testosterone in normal men. Thromb Haemost 1995;74:693–7
- Om AS, Chung KW. Dietary zinc deficiency alters 5 alpha-reduction and aromatization of testosterone and androgen and estrogen receptors in rat liver. J Nutr 1996;126:842–8
- Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not!. J Sex Med. 2014;11:624–29
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:1–11
- Yeap BB, Alfonso H, Chubb SA, et al. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab 2012;97:4030–9
- Sikaris K, McLachlan RI, Kazlauskas R, et al. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab 2005;90:5928–36
- Yeap BB, Alfonso H, Chubb SA, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab 2014;99:E9–18