Abstract
Objectives: Erectile dysfunction (ED) and lower urinary tract symptoms (LUTS) are common in diabetic men. The aim of this study was to investigate hormonal determinants, the prevalence and severity of ED and LUTS in middle-aged and elderly men with prediabetes (PD).
Methods: We investigated 176 men with PD and 184 healthy peers. PD was defined according American Diabetes Association. ED according IIEF scale and LUTS according IPSS scale were assessed. Total testosterone (TT), calculated free testosterone (cFT), dehydroepiandrosterone sulfate (DHEAS) and insulin-like growth factor 1 (IGF-1) were measured.
Results: The prevalence of ED in patients with PD was higher than in control group (30 versus 24%) as well as the prevalence and severity of ED and LUTS in elderly (60–80 years) and middle-aged (40–59 years) men with PD was higher than in healthy peers. In middle-aged pre-diabetic men, the more severe LUTS symptoms were associated with low TT and DHEAS, while in elderly men with low cFT and DHEAS. The higher prevalence of ED in middle-aged men with PD was associated with cFT and DHEAS, while in elderly pre-diabetic men with TT and IGF-1.
Conclusions: The prevalence and severity of LUTS and ED symptoms were higher in pre-diabetic men than in healthy peers. Hormonal determinants of these symptoms are different in middle-aged and elderly patients with PD.
Introduction
Aging of male population is associated with an increase in the incidence of metabolic, hormonal and urological disturbances among middle-aged and elderly men [Citation1]. One of the conditions associated with aging male is decreasing of testosterone (T) levels by 1–2% per year after the age of 40 years [Citation2]. Some aging men develop symptomatic hypogonadism, which is associated with overall dissatisfaction, atherosclerosis, metabolic syndrome (MetS), hypertension, diabetes mellitus type 2 (T2DM), lower urinary tract symptoms (LUTS) and erectile dysfunction (ED). There is a growing awareness that these entities are not disparate and, to improve the health of the ageing male, require an integral approach. A new concept of the role of testosterone in male physiology suggests that testosterone plays also a significant role in the development and maintenance of bone and muscle mass and is a determinant of glucose homeostasis and lipid metabolism [Citation3]. Male aging is also characterized by metabolic and psychosomatic symptoms attributed to the age-related decline in circulating dehydroepiandrosterone sulfate (DHEAS), and insulin-like growth hormone 1 (IGF-1) [Citation4].
Prediabetes (PD) is diagnosed in patients with impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and/or glycated hemoglobin (HbA1c) from 5.7% to 6.4% [Citation5], and is considered risk factor for the further development of diabetes mellitus type 2 (T2DM) as well as cardiovascular disease (CVD) [Citation6]. The prevalence of PD in Poland is one of the highest in the world, about 16% population is estimated to have IGT and by the year 2035, the number of people with IGT is projected to increase to about 19% [Citation7].
Low testosterone levels in T2DM are present in 25–40% of patients [Citation8,Citation9] and associated with symptoms of hypogonadism. In our previous study we described the high prevalence of late-onset hypogonadism (LOH) also among middle-aged and elderly men with PD in Polish population [Citation10]. T2DM is a chronic metabolic condition that is associated with numerous complications. LUTS are common in adults with T2DM and cause a considerable health burden for patients [Citation11], as well as ED occurs in up to 75% of men with T2DM [Citation12] and are known to be associated with a significantly reduced quality of life. The association between LUTS and ED was shown in [Citation13] and T2DM may influence on the lower urinary tract is multifactorial pathway and lead to dysfunction of the smooth muscle, urothelium and neuronal components of the bladder [Citation14]. It must be pointed, that ED in diabetic men can be a symptom of hypogonadism and both coexist in up to 54% of men with ED and T2DM [Citation15]; however, it is well recognized, that there are several pathologies that can contribute to ED in men with T2DM apart from hypogonadism, like: vasculopathy, neuropathy, venous leak and multiple causes may coexist [Citation16]. It was also shown, that in diabetic men increasing severity of ED positively correlated with low testosterone levels [Citation15]. Taken together, LUTS and ED are highly prevalent among men with T2DM; however, it seems difficult to explain the relationship between these two conditions, especially taken into considerations anabolic hormones levels.
The association between LUTS, ED and anabolic hormones deficiencies might be important because these conditions affect ageing men and the management of one condition may have an improving effect on the other. Only few studies have demonstrated relationships between sex hormones, sexual dysfunctions and IFG [Citation17] or PD [Citation9,Citation18] but the prevalence and impact of other anabolic hormones deficiency in patients with PD is unknown and probably may be associated with low quality of life, ED and LUTS.
Therefore, in this study we aimed to compare the International Index of Erectile Functions (IIEF) and the International Prostate Symptom Score (IPSS) between patients with PD and control group, and to investigate the clinical and hormonal determinants of the severity of these symptoms in middle-aged and elderly men with PD.
Material and methods
This study was performed in Department of Internal Diseases, Diabetology and Endocrinology, Medical University of Warsaw, Poland in patients attending the outpatient clinic for glucose metabolism disorders. The inclusion criteria were (1) a laboratory improved PD (2) age 40–80 years, (3) steady sexual relationship for the past 6 months. The exclusion criteria were as follows: (1) diabetes mellitus type 1 or 2, (2) conditions that contribute to sexual function, such as history of transuretheral resection of prostate, hyperprolactinemia, thyroid function disturbances, neurological diseases, (3) recent or current testosterone replacement, androgen deprivation therapy or any hormonal treatment, either during the study or in history as well as taking medication for LUTS or ED, (4) lack of informed written consent. We recruited 176 consecutive patients with PD (aged between 40 and 80 years) and as a control group, 184 healthy men, matched by age and with a fasting plasma glucose (FPG) <5.55 mmol/l (100 mg/dl) and HbA1c <5.7%. This study was approval by the local Research Ethics Committee and was conducted in accordance with Declaration of Helsinki; informed consent was obtained from all participants.
PD was diagnosed in patients with IFG from 100 to 125 mg/dl (5.6–6.9 mmol/l) and 2 h glucose concentration in oral glucose tolerance test (OGTT <140 mg/dl (<7.8 mmol/l) or in patients with IGT – 2-h glucose concentration in OGTT from 140 to 200 mg/dl (7.8–11.0 mmol/l) or in patients with HbA1c from 5.7 to 6.4% [Citation5]. Because PD is a transitory state with many individuals on repeated testing may show blood glucose in normal range, FPG and OGTT measurements were repeated after 2–3 weeks and reevaluated. The diagnosis of MetS was based on the following criteria: waist circumference ≥94 cm and any two of the following: triglycerides ≥150 mg/dl, HDL- cholesterol <40 mg/dl, blood pressure ≥130/85 mmHg and FPG ≥100 mg/dl [Citation19]. Height, weight and waist circumference were measured and body mass index (BMI) was calculated. Obesity was defined as a BMI of 30 or more. CVD was defined as coronary artery disease, congestive heart failure or arrhythmia. Hypertension and chronic obstructive pulmonary disease (COPD) were considered to be present if the participant reported having received the diagnosis or if he was receiving medication for the condition.
Assessment of LUTS and ED symptoms
Patients were interviewed prior to the beginning of any treatment and before any specific diagnostic procedures using the IIEF for ED questionnaire previously validated for the screening of EDs [Citation20] and LUTS were assessed using the IPSS [Citation21]. The severity of erectile dysfunction was classified as none (22–25), mild (12–21), moderate (8–11) and severe (1–7). ED was diagnosed when the total of the IIEF domain scores was <22 points. The IPSS includes seven LUTS indicators, including four voiding symptoms (straining, intermittency, weak stream and incomplete emptying) and three storage symptoms (frequency, urgency and nocturia). The subjects were asked to indicate the frequency of each of the seven symptoms over the preceding 6 months. Each symptom is graded from 0 (not at all) to 5 (almost always) according to the frequency of occurrence. Scores from the individual symptoms were aggregated to obtain a total IPSS score, which ranges from 0 to 35 and is categorized as either mild (0–7), moderate (8–19) or severe (20–35).
Laboratory measurements
In all men venous blood samples were obtained between 8.00 and 10.00 A.M. After centrifugation, the serum was collected and frozen at −70°C until analysis. FPG was measured with enzymatic method using BIOSEN 5040 analyzer (EKF-Diagnostic GmbH, Germany), glycated hemoglobin (HbA1c) with HPLC method using Variant analyzer (Bio-Rad Laboratories Inc, USA). HbA1c values were expressed as % according National Glycohemoglobin Standardization Program (NGSP). The serum levels of TT, DHEAS, estradiol (E2) and insulin-like growth factor 1 (IGF-1) were measured with immunometric assays (Immulite 2000 and RIA CAC; Siemens Medical Solution, Malvern, PA).and expressed in nmol/l for TT, pg/ml for E2 and ng/ml for DHEAS and IGF-1 (to convert the values for DHEAS to μmol/l, multiply by 0.00271; IGF-1 to nmol/l, multiply by 0.131 and E2 to pmol/l, multiply by 3.671). To estimate the circulating fraction of FT we measured the serum level of sex hormone-binding globulin (SHBG) using an immunoassay (Diagnostic Products Corp, San Francisco, CA), and SHBG was expressed in nmol/l. The serum level of calculated (cFT) expressed in nmol/l; was calculated with the validated equation of Vermeulen et al. [Citation22].
Statistical analysis
Statistical analyses were performed using the Statistica 9.1 data analysis software system (StatSoft, Tulsa, OK). Most continuous variables had a normal distribution, and were expressed as a mean ± SD of the mean. The intergroup differences were tested using the t test for unpaired samples. Serum DHEAS had a skewed distribution so were log-transformed to normalize their distribution, expressed as a median with lower and upper quartiles, and the intergroup differences were tested using the t test for unpaired samples for normalized values. Categorized variables were expressed as a number and a percentage, and the intergroup differences were tested using the χ2 test. The relationships between IIEF and LUTS scores and various factors including age, BMI and HbA1c in men with PD were examined by Pearson’s correlation analyses. Univariable and multivariable linear and logistic regression analyses were applied to establish variables determining the severity of ED and LUTS and associated with the higher prevalence of ED symptoms. Regression analyses were performed separately in younger (40–59 years) and older (60–80 years) age groups of men with PD. In the univariable analyses, as the potential determinants of the severity of ED and LUTS and as the potential risk factors for the higher prevalence of ED symptoms we included: age, BMI, HbA1c, total cholesterol, TT, cFT, DHEAS, IGF-1 and E2 as well as comorbidities, such as: CVD, hypertension and MetS. During the construction of multivariable models, we included all variables that had been shown to be significant (p < 0.05) determinants in the univariable analyses. A p value <0.05 was considered statistically significant.
Results
A total of 176 men with PD, mean age 59 ± 6 years (102 men from 40 to 59 years and 74 men from 60 to 80 years old), and 184 healthy men, mean age 61 ± 5 years (98 men from 40 to 59 years and 86 men from 60 to 80 years old) were evaluated in this study. The baseline characteristics of both groups are shown in . Patients with PD had lower TT and cFT levels than the control group (13.5 ± 3.9 versus 216.9 ± 4.3 mmol/l; p < 0.02, and 0.326 ± 0.08 versus 0.374 ± 0.11 nmol/l; p < 0.05, respectively). The levels of other anabolic hormones – DHEAS and IGF-1 – did not differ significantly between groups. We also observed significantly higher LH and estradiol concentrations in men with PD (p < 0.05 and 0.02, respectively), as well as BMI was higher in men with PD (p < 0.05), but WC did not differ between groups. FPG, HbA1c and total cholesterol were significantly lower in control group then in men with PD (p < 0.01, 0.001 and 0.05, respectively). In men with PD we observed the higher prevalence of obesity (68 versus 60%, p < 0.02) and MetS (80 versus 61%, p < 0.02) than in control group.
Table 1. Baseline characteristics of all patients men with PD, in the two age groups patients with PD, and in control group.
Among patients with PD we observed significant differences of hormonal and metabolic parameters – . Elderly patients with PD had lower TT levels (11.6 ± 3.7 versus 15.9 ± 3.5 mmol/l, p < 0.001), lower cFT levels (0.313 ± 0.08 versus 0.359 ± 0.09 nmol/l, p < 0.005), and also lower DHEAS levels (238 [159–739] versus 675 [229–921], p < 0.01) when compared with middle-aged men with PD, but IGF-1 levels did not differ between groups of patients with PD. In elderly men with PD we showed higher E2 and LH concentrations (p < 0.02 and 0.05, respectively), as well as higher BMI (p < 0.05). Men with PD in elderly group had higher HbA1c, FPG and glucose levels in 2-h of OGTT (p < 0.02, 0.01 and 0.05, respectively) then middle-aged men with PD. Also total-cholesterol levels were higher and HDL-cholesterol levels were lower in elderly prediabetic patients (all p < 0.05). In elderly men with PD we observed also higher prevalence of hypertension, COPD and CVD (p < 0.01, 0.05 and 0.001, respectively) than in middle-aged patients with PD.
The prevalence of ED in men with PD was significantly higher than in control group (30 versus 24%; p < 0.02). Analysis of groups after dividing by the age revealed, that the prevalence of ED in elderly patients with PD was higher than in healthy peers (38 versus 26%; p < 0.001) as well as the prevalence of ED in middle-aged men with PD was significantly higher than in healthy peers (20% versus 16%; p < 0.05) – . Among patients with PD, the prevalence of ED was significantly higher in elderly than in middle-aged men (38 versus 20%; p < 0.001) ().
Figure 1. Prevalence (%) of ED in men with PD (n = 176) and healthy men (n = 184). ED was diagnosed according to the IIEF [Citation19] when the total of the IIEF domain scores was <22 points.
![Figure 1. Prevalence (%) of ED in men with PD (n = 176) and healthy men (n = 184). ED was diagnosed according to the IIEF [Citation19] when the total of the IIEF domain scores was <22 points.](/cms/asset/9c046aa7-cf9b-4b01-b867-1332b3098c59/itam_a_1083972_f0001_b.jpg)
Analysis of the severity of ED symptoms revealed, that in patients with PD the mean score of ED according IIEF scale was significantly lower than in control group (11.8 ± 6.2 versus 15.5 ± 4.7 points; p < 0.01), so these results means, that the severity of ED in patients with PD was higher than in control group (). Analysis of both groups after dividing by the age revealed, that the severity of ED symptoms in elderly men with PD was significantly higher than in healthy peers (9.9 ± 5.5 versus 11.5 ± 5.8 points; p < 0.02) as well as the severity of ED symptoms among middle-aged men with PD also was higher than in healthy peers 12.4 ± 5.9 versus 13.9 ± 6.2 points; p < 0.05) (). Among patients with PD the severity of ED symptoms was significantly higher in elderly than in middle-aged men (9.9 ± 5.5 versus 13.9 ± 6.2 points; p < 0.001).
Figure 2. Severity (expressed as points) of ED symptoms according IIEF scale (A), and LUTS symptoms according IPSS scale (B) in men with PD aged 40–59 years (n = 102) and aged 60–80 years (n = 74), compared with healthy peers (n = 184). Higher score on the IIEF scale is associated with less severe symptoms; lower score on the IPSS scale is associated with less severe symptoms.
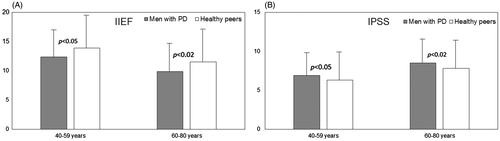
Analysis of the severity of LUTS symptoms according IPSS score revealed, that in patients with PD the mean score of IPSS scale was significantly higher than in control group (7.7 ± 4.2 versus 6.8 ± 4.2 points; p < 0.01) (). Analysis of both groups after dividing by the age revealed, that the severity of LUTS symptoms among elderly men with PD was significantly higher than in healthy peers (8.5 ± 3.9 versus 7.8 ± 4.1 points; p < 0.02) as well as the severity of LUTS symptoms among middle-aged men with PD was also significantly higher than in healthy peers (6.9 ± 3.7 versus 6.3 ± 3.7 points; p < 0.05) (). Among patients with PD the severity of LUTS symptoms was higher in elderly than in middle-aged men (8.5 ± 3.9 versus 6.9 ± 3.7 points; p < 0.002).
Analysis of correlations between IIEF and IPSS score, and clinical and laboratory variables in all patients with PD revealed, that there were negative, significant correlations between HbA1c and IIEF score (r = −0.3236; p = 0.02), positive correlation between HbA1C score and IPSS (r = 0.3786; p = 0.01), and positive correlation between IPSS score and BMI (r = 0.3782; p = 0.01). We did not observe any additional significant correlations between analyzed variables, including age ().
Table 2. Correlations between IIEF and IPSS scores and clinical characteristics in 176 men with PD.
In middle-aged patients with PD in multivariable linear regression models, the more severe ED symptoms were independently associated with serum low cFT, low DHEAS, high HbA1c and with the presence of MetS (all p < 0.05). The more severe LUTS symptoms were associated with low TT, low DHEAS, and the presence of obesity (all p < 0.05) (). In multivariable logistics regression models, the following variables were independently associated with the higher prevalence of ED in middle-aged men with PD: cFT (OR = 0.64, CI: 0.45–0.74; p < 0.05) and DHEAS (OR = 1.26, CI: 1.07–1.38; p < 0.05) ().
Table 3. Parameters associated with the severity of ED (according IIEF scale) and LUTS (according IPSS scale) symptoms in men (n = 176) with PD.
Table 4. Risk factors associated with the higher prevalence of ED in men with PD.
In elderly patients with PD in multivariable linear regression models, the more severe ED symptoms were independently associated with: low TT, low IGF-1, low DHEAS and with the presence of CVD and obesity (all p < 0.05) (). The more severe LUTS symptoms were associated with low cFT, low DHEAS and the presence of MetS (all p < 0.05) (). In multivariable logistics regression models, the following variables were independently associated with the higher prevalence of ED in older men with PD: TT (OR = 1.21, CI: 0.85–1.31; p < 0.02) and IGF-1 (OR = 1.66, CI: 1.23–3.11; p < 0.03) ().
Discussion
There is worldwide an epidemic of glucose metabolism disorders – T2DM and PD, and if we consider that urologic diseases are very prevalent in these populations, it is very important to know epidemiology and pathophysiologic associations of both, in order to diagnose and treat them early. LUTS and ED symptoms in patients with PD do not cause any life threatening disease; however, it does cause severe discomfort and affects their quality of life.
In this study, we investigated the prevalence and severity of ED and LUTS symptoms in men with PD as well as the clinical and hormonal determinants of the severity of these symptoms in middle-aged and elderly men with PD. In our opinion, there are three major findings arising from the present study. Firstly, ED was very common in male patients with PD aged 40–80 years. The prevalence of ED in patients with PD was about 25% higher than in control group (30 versus 24%) as well as the prevalence of ED in elderly and middle-aged men with PD was higher than observed in healthy peers (38 versus 26% and 20 versus 16%). Among the men with PD, the prevalence of ED was significantly higher in elderly when compared with middle-aged men (36 versus 20%). These results allow to propose that the occurrence of ED may be accelerated both in the middle-aged as well as in elderly patients with PD.
Second, the severity of ED and LUTS symptoms in patients with PD was higher than in healthy peers. Among patients with PD the severity of ED symptoms was significantly higher in elderly than in middle-aged men. We showed significant negative correlation between IIEF score and HbA1c and positive correlation between IPSS score and HbA1c in patients with PD but we did not observed correlation between IPSS or IIEF scores and age. These results means, that the severity of ED and LUTS symptoms was higher in men with PD than in control group, and these results also allow to propose, that even a small degree glucose metabolism disturbances may be associated with ED and LUTS symptoms in prediabetic men.
Third, we observed significantly lower TT and cFT levels in men with PD in both age groups but regression analysis showed that hormonal determinants of ED and LUTS symptoms are different in middle-aged and elderly men with PD. In middle-aged men, the more severe ED symptoms were independently associated with low cFT and low DHEAS, while the more severe LUTS symptoms were associated with low TT and low DHEAS. In elderly patients with PD, the more severe ED symptoms were independently associated with: low TT, low IGF-1 and low DHEAS, while the more severe LUTS symptoms were associated with low cFT and low DHEAS. In multivariable logistics regression models, the higher prevalence of ED in middle-aged men with PD was associated with cFT and DHEAS, while in elderly prediabetic men with TT and IGF-1. These results allow to propose, that hormonal determinants of the prevalence and severity of ED symptoms as well as the severity of LUTS symptoms differ significantly between middle-aged and elderly men with PD.
The prevalence of PD in Poland is one of the highest in the world, about 16% of population is estimated to have IGT or IFG [Citation7], so any disorders associated with PD, especially those that may affect negatively the quality of life, are a serious public health problem in Poland. The pathophysiology and possible mechanisms by which patients with PD have more urologic complications are not clear. The genitourinary organs have a multicellular make it unlikely that a single mechanism, including anabolic hormones, underlies ED and LUTS in men with PD. We showed previously [Citation14], that in prediabetic patients LOH was diagnosed in about 30% of patients and only in 14% of control group [Citation10]. As ED is one of the most prominent symptom of hypogonadism, these results indicated, that PD may have influence on sexual health in men and overall quality of life as well as these relationships may be associated with sex hormones levels. The influence of others anabolic hormones, such as DHEAS and IGF-1 on erectile function and LUTS in patients with PD is still unknown.
In addition to our observations [Citation10], only few studies have demonstrated relationships between androgens and PD in men. Corona et al. [Citation17] in 1687 men with sexual dysfunction showed, that 19% of men were classified as IFG and these men more often had severe ED or hypogonadism when compared with normoglycemic men. In MEST study Colangelo et al. [Citation23] showed, that IFG was associated inversely with TT and DHEA and E2 and these correlations were significant after adjustment for age and BMI. Ho et al. [Citation18] demonstrated in 1306 men, that PD was diagnosed in about 41% of patients and was associated with an increased risk of subnormal TT levels (age-adjusted OR = 1.87; 95% CI), and was still significant after adjusting for age, BMI and MetS. Fukui et al. [Citation24] showed that IIEF scores in 296 diabetic men were statistically higher than in healthy peers. These results clearly indicate, that low testosterone levels in male patients with glucose metabolism disorders are often and significant, while the results of this study clearly show, that not only the prevalence but also severity of ED in patients with PD is higher than in healthy peers.
In our study we observed, that in middle-aged men, the more severe ED symptoms were associated with low cFT and low DHEAS levels, while in elderly patients with PD, the more severe ED symptoms were associated with: low TT, low IGF-1 and low DHEAS. These relationships were age-independent. These results confirm hypothesis, that in prediabetic patients ED is associated with anabolic hormones levels, but these relations vary depending on the age. However, in both age groups we showed week but significant relationships between HbA1c and IIEF scores, and this fact might be an argument that poor glycemic control, besides hormonal mechanisms, may play crucial role in pathogenesis of ED.
The possible pathophysiological mechanism between PD, anabolic hormones and ED is multifactoral and represented by vasculopathy, neuropathy, visceral adiposity, insulin resistance and hypogonadism [Citation25]. Low testosterone levels in men are associated with insulin resistance [Citation26] and it has been suggested, that excessive estrogen secretion in the obese man may suppress secretion of testosterone [Citation27], and indeed, in our prediabetic cohort estradiol concentration was significantly higher in men with ED and PD. Chronic hyperglycemia results in endothelial dysfunction, decreased bioavailability of nitric oxide (NO) and insufficient relaxation of the vascular smooth muscle of the corpora cavernosa as well as proinflammatory state that results in the decreased availability and activity of NO [Citation28].
LUTS are common in adults with T2DM [Citation14]. The impact of glucose metabolism disturbances on the lower urinary tract is multifactorial and includes osmolarity diuresis, metabolic perturbations and microvascular damage as well as neuropathy observed in diabetic men lead to dysfunction of the smooth muscle and neuronal components of the bladder [Citation9–10]. The development of LUTS, and the underactivity or overactivity of the detrusor muscle are common symptoms associated with diabetes progression [Citation29]. The prevalence of moderate to severe LUTS in diabetic patients is estimated at about 30% in male <45 years [Citation30]. In the recently published observational study performed in patients with T2DM mean age 68.7 years, authors observed, that IPSS score was higher than in normoglycemic, control group (p < 0.0005), and they concluded, that T2DM was positively associated with the increasing of the LUTS symptoms independently from age [Citation31]. In our study we showed, that the severity of LUTS among middle-aged men, as well as in elderly men with PD was significantly higher than in healthy peers. These results demonstrate, that PD increases the risk of LUTS in all prediabetic men >40 years.Sarma et al. [Citation32] presented that nocturia and weak urinary stream were the highest LUTS in 170 men with T2DM. This fact may have a severe impact on an individual’s sleep quality and overall quality of life in these patients.
A few studies have evaluated the relationship between testosterone and LUTS symptoms. Schatzl et al. [Citation33] found, that low TT was observed in ∼30% of elderly men with LUTS, but it had no impact on symptom status. Litman et al. [Citation34] showed a relationship between symptoms of LUTS and TT but this relationship disappeared after statistical adjustment for age [Citation34]. Kaplan et al. [Citation35] evaluated the prevalence of low TT in 1896 men with LUTS associated with benign prostatic hyperplasia, who were enrolled in the Medical Therapy of Prostatic Symptoms Study. They found, that 25.7% of men had TT below 300 ng/dl and the prevalence of low TT increased with increasing BMI. Also, Kim et al. [Citation36] investigated the relationship between sex hormone levels and LUTS in 924 patients with benign prostate hyperplasia. They showed, that the total IPSS was associated with TT level, and TT level was decreased in patients with ≥four episodes of nocturia. Nevertheless, so far no clear relationship between LUTS and testosterone in prediabetic men could be demonstrated. In our study we showed, that hormonal determinants of LUTS are different in middle-aged and elderly men with PD. In middle-aged men, the more severe LUTS were associated with low TT and low DHEAS, while in elderly patients with PD, the more severe LUTS were associated with low cFT and low DHEAS. As in the case of ED, the severity of LUTS was higher in elderly prediabetic men and in men with PD when compared with healthy peers, and we did not observed correlation of LUTS severity with age but significant correlation with HbA1c was found. So, similarly to ED symptoms, poor glycemic control in men may negatively influence on the severity of LUTS in patients with PD.
The effect of testosterone therapy on LUTS and sexual functions in men with symptomatic LOH was presented in several studies. Shigehara et al. [Citation37] evaluated men with hypogonadism and BPH, who were randomly assigned to receive 250 mg of testosterone enanthate every 4 weeks or placebo. They showed a significant decrease of IPSS after 12 months of testosterone treatment and improvement in maximum flow rate and voided volume. Also Amano et al. [Citation38] and Karazindiyanoğlu et al. [Citation39] presented in men with LOH, that testosterone replacement therapy had beneficial effects on IIEF, total IPSS scores and bladder functions after testosterone treatment.
Our methodological model has enabled us to established a causal link between anabolic androgen deficiencies, ED and LUTS. The results of our study showed, that different hormonal mechanisms are involved in the regulation of these symptoms in middle-aged and elderly patients with PD. These conditions might simply overlap and they have probably separate pathophysiologic pathways. This study had also several limitations. The anabolic androgens measurements were not repeated in our sample set, total and free testosterone might not accurately describe the subject’s bioavailable testosterone. It is also not clear whether an improvement in glycemic control in patients with PD can lead to significant reduction in LUTS and or ED symptoms. However, it seems reasonable to diagnosed hormones deficiencies in men with PD [Citation40], because evidence derived from clinical studies supports the use of replacement therapy in hypogonadal patients with glucose metabolism disorders, although the benefit–risk ratio is uncertain in advanced age. Testosterone replacement therapy may improve insulin sensitivity and glycemic control in men with T2DM [Citation41–44] as well as body composition and quality of life [Citation45] but long-term influence of testosterone in men with PD is still unknown.
In conclusion, the prevalence and severity of LUTS and ED symptoms are higher in patients with PD as compared with healthy men. Hormonal and clinical determinants of these symptoms are different in middle-aged and elderly prediabetic men.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Saad F, Gooren LJ. The Role of testosterone in the etiology and treatment of obesity, the metabolic syndrome, and diabetes mellitus type 2. J Obes 2011;pii:471584
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal. Results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–98
- Yassin AA, Saad F, Gooren LJ. Metabolic syndrome, testosterone deficiency and erectile dysfunction never come alone. Andrologia 2008;40:259–64
- Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med 2007;167:2249–54
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37:S81–90
- Bergman M. Pathophysiology of prediabetes and treatment implications for the prevention of type 2 diabetes mellitus. Endocrine 2013;43:504–13
- Cho NH, Whiting D, Guariguata L. IDF diabetes atlas. 6th ed. Belgium: International Diabetes Federation; 2013:39–49
- Dhindsa S, Prabhakar S, Sethi M, et al. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004;89:5462–68
- Kapoor D, Aldred H, Clark S, et al. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007;30:911–17
- Rabijewski M, Papierska L, Piątkiewicz P. Late-onset hypogonadism among old and middle-aged males with prediabetes in Polish population. Aging Male 2015;18:16–21
- Liu RT, Chung MS, Chuang YC, et al. The presence of overactive bladder wet increased the risk and severity of erectile dysfunction in men with type 2 diabetes. J Sex Med 2012;9:1913–22
- Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease in men with type 2 diabetes. Diabetes Care 2011;34:1660–75
- Vallancien G, Emberton M, Harving N, van Moorselaar RJ; Alf-One Study Group. Sexual dysfunction in 1.274 European men suffering from lower urinary tract symptoms. J Urol 2003;169:2257–61
- Kebapci N, Yenilmez A, Efe B, et al. Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn 2007;26:814–19
- Kapoor D, Clarke S, Channer KS, Jones TH. Erectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetes. Int J Androl 2007;30:500–7
- Phe V, Roupret M. Erectile dysfunction and diabetes – a review of current evidence-based medicine and a synthesis of the main available therapies. Diabetes Metab 2012;38:1–13
- Corona G, Rastrelli G, Balercia G, et al. Hormonal association and sexual dysfunction in patients with impaired fasting glucose: a cross-sectional and longitudinal study. J Sex Med 2012;9:1669–80
- Ho CH, Yu HJ, Wang CY, et al. Prediabetes is associated with an increased risk of testosterone deficiency, independent of obesity and metabolic syndrome. PLos One 2013;8:e74173
- Athyros GV, Ganotakis ES, Mikhailidis DO. The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr Med Res Opin 2005;21:1157–64
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–26
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549–57
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Colangelo LA, Ouyang P, Liu K, et al. Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: Multi-Ethnic Study of Atherosclerosis. Diabetes Care 2009;32:1049–51
- Fukui M, Tanaka M, Toda H, et al. Andropausal symptoms in men with Type 2 diabetes. Diabet Med 2012;29:1036–42
- Yassin AA, Saad F, Gooren LJ. Metabolic syndrome, testosterone deficiency and erectile dysfunction never come alone. Andrologia 2008;40:259–64
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008;93:1834–40
- Piteous N, Dwyer AA, Decius S, et al. The relative role of gonad sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab 2008;93:2686–92
- Maiorino MI, Bellastella G, Esposito K. Diabetes and sexual dysfunction: current perspectives diabetes, metabolic syndrome and obesity. Targets and Therapy 2014;7:95–105
- Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int 2005;95:733–8
- Wang CC, Chancellor MB, Lin JM, et al. Type 2 diabetes but not metabolic syndrome is associated with an increased risk of lower urinary tract symptoms and erectile dysfunction in men aged <45 years. BJU Int 2010;105:1136–40
- Ferreira FT, Daltoé L, Succi G, et al. Relation between glycemic levels and low tract urinary symptoms in elderly. Aging Male 2015;18:34–7
- Sarma AV, Burke JP, Jacobson DJ, et al. Associations between diabetes and clinical markers of benign prostatic hyperplasia among community-dwelling black and white men. Diabetes Care 2008;31:476–82
- Schatzl G, Madersbacher S, Temml C, et al. Serum androgen levels in men: impact of health status and age. Urology 2003;61:629–33
- Litman HJ, Bhasin S, O’Leary MP, et al. An investigation of the relationship between sex-steroid levels and urological symptoms: results from the Boston Area Community Health survey. BJU Int 2007;100:321–6
- Kaplan SA, Lee JY, O’Neill EA, et al. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013;16:169–72
- Kim MK, Zhao C, Kim SD, et al. Relationship of sex hormones and nocturia in lower urinary tract symptoms induced by benign prostatic hyperplasia. Aging Male 2012;15:90–5
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomized controlled study. Aging Male 2011;14:53–8
- Amano T, Imao T, Takemae K, et al. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male 2010;13:242–6
- Karazindiyanoğlu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male 2008;11:146–9
- Lunenfeld B, Mskhalaya G, Kalinchenko S, Tischova T. Recommendations on the diagnosis, treatment and monitoring of late-onset hypogonadism in men – a suggested update. Aging Male 2013;4:143–50
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010;7:3495–503
- Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol 2014;2014:683515. doi: 10.1155/2014/683515
- Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 2011;34:828–37
- Saad F, Yassin A, Almehmadi Y, et al. Effects of long-term testosterone replacement therapy, with a temporary intermission, on glycemic control of nine hypogonadal men with type 1 diabetes mellitus – a series of case reports. Aging Male. [Epub ahead of print]. doi: 10.3109/13685538.2015.1034687
- Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med 2014;11:1567–76

