Abstract
This study examined the effect of Testofen, a specialised Trigonella foenum-graecum seed extract on the symptoms of possible androgen deficiency, sexual function and serum androgen concentrations in healthy aging males. This was a double-blind, randomised, placebo-controlled trial involving 120 healthy men aged between 43 and 70 years of age. The active treatment was standardised Trigonella foenum-graecum seed extract at a dose of 600 mg/day for 12 weeks. The primary outcome measure was the change in the Aging Male Symptom questionnaire (AMS), a measure of possible androgen deficiency symptoms; secondary outcome measures were sexual function and serum testosterone. There was a significant decrease in AMS score over time and between the active and placebo groups. Sexual function improved, including number of morning erections and frequency of sexual activity. Both total serum testosterone and free testosterone increased compared to placebo after 12 weeks of active treatment. Trigonella foenum-graecum seed extract is a safe and effective treatment for reducing symptoms of possible androgen deficiency, improves sexual function and increases serum testosterone in healthy middle-aged and older men.
Introduction
Testosterone concentrations decrease in men at a rate of 1–2% per year after the age of 40 [Citation1–3]. This decrease varies considerably between individuals, relates to the accumulation of co-morbid conditions including weight gain [Citation4–6] and may be due to a combination of reduced hypothalamic gonadotropin releasing hormone outflow, alterations in androgenic negative feedback and decreased responsiveness of testicular tissue [Citation7,Citation8]. This decline in testosterone is associated with decreased albumin and increased sex hormone binding globulin (SHBG), resulting in higher levels of serum testosterone binding to SHBG, which together decreases free (bioavailable) testosterone [Citation9].
The clinical significance of a low or low-normal testosterone level in men is not well understood [Citation10]. There is an association between decrease in serum testosterone in older men with reduction in sexual activity and desire, although causality is as yet undetermined [Citation11]. There is also evidence that lower testosterone levels are linked with an increased risk of developing metabolic syndrome and cognitive decline [Citation12,Citation13]. In addition, other symptoms of deficiency may include muscle weakness, weight gain, increased abdominal fat, musculoskeletal changes and joint pain, hot flushes or sweating, sleep disturbances, insomnia, fatigue, depression, decreased body hair and skin alterations, and low bone mineral density. Depending upon the set of symptoms being experienced, a negative effect on quality of life as well as an impact on health and relationships may occur [Citation14,Citation15].
Testosterone also decreases in response to age-related physical changes such as weight gain, increased waist circumference and deterioration of general health associated with chronic disease such as diabetes, liver disease, and cardiovascular disease [Citation16,Citation17]. Medications such as opioids, glucocorticoids and anti-depressants may directly inhibit the hypothalamic-pituitary-testicular axis also resulting in decreased testosterone [Citation18–20].
The combination of low testosterone and the array of the above symptoms is variably referred to as aging male syndrome (AMS), andropause, partial androgen deficiency of the aging male (PADAM), and late-onset hypogonadism (LOH) [Citation15,Citation21,Citation22]. The prevalence is unknown, large-scale studies suggest it may be as little as 2.1% in men aged 40–70 [Citation23,Citation24] but other clinical studies have reported that androgen deficiency affects 39% of men aged greater than 45 years [Citation25].
Currently, testosterone replacement therapy is prescribed to men when they are clinically defined as androgen deficient. Testosterone replacement in men with established hypogonadism significantly increases lean body mass and decreases fat mass without an overall change in body weight and improves sexual function and cognition [Citation15,26–28]. Overall, there are few large-scale clinical trials on the efficacy of testosterone therapy in the older male age group. Some studies suggest there are potential health risks including erythrocytosis and prostate cancer [Citation22,Citation29] while others have shown some beneficial, though short-lived effects [Citation30].
There is limited research on the use of herbal medicines to improve male health, particularly to increase testosterone levels and support healthy sexual function. To date, there are no published human clinical studies using herbal medicines for symptoms associated with the age related decrease in androgens.
Earlier research suggested that Trigonella foenum-graecum seed extract had a positive effect on sexual health and quality of life [Citation31], as well as demonstrating anabolic and androgenic activity in younger men [Citation32]. It is thought that this positive effect was, at least, partly, due to increased testosterone, including free testosterone and that Trigonella foenum-graecum seed extract may also be an effective treatment for the symptoms of possible androgen deficiency in aging men. The basis for this androgenic activity may be due to the fact that Trigonella foenum-graecum seeds contain soluble steroidal saponins, specifically furostanol glycosides responsible for complexing cholesterol in the cell membrane [Citation33,Citation34]. Other studies that have shown that Trigonella foenum-graecum increases testosterone, and free testosterone suggest it may be an incomplete 5-alpha reductase and aromatase inhibitor [Citation35].
The hypothesis was that the use of Trigonella foenum-graecum seed extract for 12 weeks as a therapy would reduce the symptoms of possible androgen decline, improve reported sexual function and increase serum testosterone in healthy middle-aged and older males.
Methods
Study design
This was a single-site, double-blind, randomised, short-duration (12 week) clinical trial utilising active and placebo arms to assess (i) the factors that influence testosterone concentrations and (ii) the effect of Trigonella foenum-graecum seed extract on symptoms of possible androgen deficiency, sexual function and serum hormone concentrations, particularly testosterone, in healthy aging males. It was conducted between February and November 2014 in Brisbane, Australia.
Study population
The study population included 120 healthy male subjects, aged 43–75 years, recruited through a trial recruitment database and public media. After preliminary screening via telephone, subjects were required to attend a pre-trial interview and provide written informed consent. Comprehensive screening was performed after an initial health assessment including lifestyle questions, current medications, medical history and a physical examination.
Potential subjects were excluded if they had any of the following: any condition which in the opinion of the investigator made the subject unsuitable for inclusion, diagnosed erectile dysfunction or any physical disability that may limit sexual function, had received any treatment/therapy (including testosterone or anabolic steroids) for any sexual disorder during last 6 months, were being prescribed anticoagulation therapy, were receiving levodopa for Parkinson’s disease or calcipotriene for psoriasis, were diagnosed with severe renal and/or hepatic insufficiency, were diagnosed with genital anatomical deformities, uncontrolled diabetes mellitus, had a history of spinal cord injury, major psychiatric disorder, and/or had any abnormal secondary sexual characteristics. They were also excluded if they were diagnosed with prostate cancer or benign prostatic hypertrophy, had an acute genitourinary disorder or history of genital surgery, if they had a current or past history of chronic alcohol and/or drug abuse or had a suspected or diagnosed chickpea allergy. If a potential subject was currently participating in another trial or had been in any other clinical trial during last 30 days they were also excluded from the present study.
Outcomes
Primary
Symptoms of possible androgen deficiency
The primary outcome measure was efficacy of treatment for symptoms using the Aging Male Symptom (AMS) questionnaire [Citation36]. It consists of 17 questions in 3 sub-scales, psychological, somatic and sexual. These sub-scales as well as the total score were used to assess symptoms. Subjects completed the questionnaires pre-trial (baseline data), upon completion of weeks 6 (mid-trial) and 12 (completion).
Secondary
Sexual function
Sexual function was assessed using the Derogatis Interview for Sexual Functioning-Self Report (DISF-SR) questionnaire at baseline and week 12 (completion). It was designed to measure the quality of sexual function. The DISF-SR is a set of 21 questions, with 4 sub-domains: sexual fantasy (SC), sexual arousal (SA), sexual behaviours (SB) and orgasm (O) [Citation37]. The DISF-SR Sexual sub-domain asks specifically about the frequency of morning erections and the Sexual Drive/Relationship sub-domain contained a question regarding the frequency of sexual intercourse.
Serum sex hormone concentrations
Secondary outcomes included serum total and calculated free testosterone (Sodergard equation, [Citation38]), sex hormone binding globulin (SHBG), DHEA-S, androstenedione, oestradiol and prolactin. The subjects had blood drawn in the morning after an overnight fast at baseline, 6 weeks (mid-trial) and 12 weeks (completion). Analysis was conducted at Queensland Medical Laboratories (QML), Brisbane, Australia by chemiluminescent immunoassay (CLIA).
Other outcomes
Sleep quality was assessed using the Pittsburgh Sleep Index (PSI) [Citation39]. The Multi-dimensional Fatigue Index (MFI-20) was used to assess fatigue. The MFI-20 provides a total score for fatigue and also includes the individual domains of general, emotional, physical, mental health and vigour as separate aspects to fatigue [Citation40]. Physical activity was measured using the International Physical Activity questionnaire (IPAQ) [Citation41].
Other physical health parameters and anthropometric measurements (weight, height, waist, hip, grip strength) were also performed. Red and white blood cells, electrolyte and liver function and lipid profile were also measured at baseline and 12 weeks.
Randomisation and blinding
Randomisation of the products was performed independently of the investigators using Random Allocation Software, version 1.0, May 2004. The investigational products were delivered to the investigators in trial product containers that were identical in function and appearance, marked from 001 to 0120. Once enrolled in the trial, subjects were randomly allocated to the next available number in the sequence, either the placebo comparator group (n = 60) or the active intervention group (n = 60). Investigators were blinded to the randomisation and therefore blinded to which subjects were allocated to the active and treatment arms.
Subjects were monitored for compliance with the protocol by a combination of telephone and email communications. The doses taken were assessed by number of returned tablets at completion of the study.
Statistical methods
Sample size
Sample size was calculated on the primary outcome, the AMS total score. Based on a power of 80%, a sample size of 48 subjects in each treatment group was required, with 60 subjects recruited to account for potential withdrawals.
Analysis
The primary outcome endpoint (AMS questionnaire total and domain scores) at baseline, week 6 and week 12 was analysed using a two way repeated measures ANOVA for treatment and time. The DISF-SR total and domain scores were assessed for statistical difference within-groups (change from baseline) and between groups by t-tests. Change in pathology data was analysed with ANCOVA using the covariates of BMI and age. The correlations were calculated using the Pearson Correlation Co-efficient. Effect sizes are reported as Eta squared (η2).
The study was carried out according to the principles expressed in the Declaration of Helsinki and was approved by the Queensland Clinical Trials Network Human Research Ethics Committee (QCTN) No: 2013002. The trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR) No: 12613000925741.
Results
Demographics
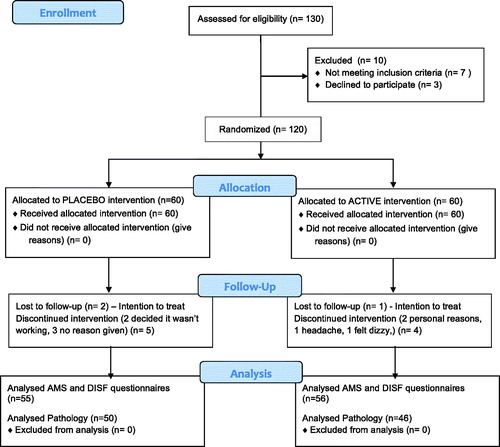
Initially, 120 men were enrolled and commenced treatment in the study, with 111 completing the study; 55 in the active treatment group and 56 in the placebo group (). There were no significant differences between the active treatment and placebo groups for age, anthropometric measures and the lifestyle factors (). All men had a sexual partner for the duration of the study.
Table 1. Subject demographics at baseline.
Total cholesterol, triglycerides, LDL and HDL were not significantly different between the two groups. All men had normal full blood count, renal and liver function and prostate specific antigen (PSA) levels within healthy reference range for their age. The mean serum total testosterone and free testosterone concentrations represented the lower end of the healthy reference range for men in this age group ().
Relationship between age, BMI and health indices
There was no correlation between age and total testosterone although there was a weak negative correlation between age and free testosterone (r= −0.196, p= 0.042). A negative correlation was found between BMI and both total testosterone (r= −0.304, p = 0.001) and free testosterone (r= −0.279, p = 0.003). Total testosterone was positively correlated with HDL cholesterol (r = 0.237, p = 0.017) but not with total or LDL cholesterol. There was no correlation between testosterone and the AMS score except on the somatic sub-domain (r= −0.310, p = 0.001). There was no correlation between total or free testosterone, sexual function (measured by the DISF-SR), sleep or physical activity in this group of men.
Treatment with Trigonella foenum-graecum seed extract
Effect on general health parameters
There was no change in BMI, waist/hip ratios or grip strength during the course of the study for either the active treatment or the placebo groups ().
Table 2. Anthropometric parameters in the active treatment and placebo groups at baseline, week 6 and week 12.
There were no significant changes in sleep patterns, the type or duration of physical activity or fatigue score in either the active treatment or the placebo group.
Effect on symptoms of possible androgen deficiency
The mean AMS total score and subscores for the active and placebo treatment groups at baseline, 6 weeks and 12 weeks are shown in . There was no significant difference between the groups at baseline.
Figure 2. Total and sub-domain scores of the aging male symptom questionnaire (AMS) for active treatment and placebo groups at baseline, week 6 and week 12. Data are expressed as mean ± SD.
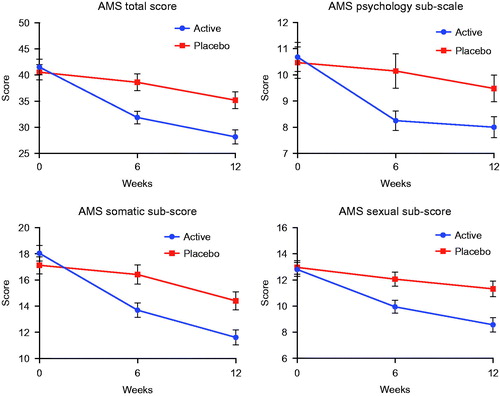
Repeated measures ANOVA showed a significant difference across time F(2,216) = 8.6, p < 0.001, η2 = 0.073 and a significant difference between groups F(1,108) = 6.4, p = 0.013, η2 = 0.056 for total AMS Score.
There were also significant differences for the somatic and sexual sub-scores across time F(2,216) = 9.1, p < 0.001, η2 = 0.078 and F(2,216) = 6.3, p = 0.002, η2 = 0.055 respectively and between treatment groups F(1,108) = 4.8, p = 0.03, η2 = 0.043 and F(1,108) = 7.1, p = 0.009, η2 = 0.062. There was a significant difference across time for the psychology sub-score F(2,216) = 4.9, p < 0.008, η2 = 0.043 but no treatment effect F(1,108) = 2.6, p < 0.11.
Effect on sexual function
The effect of Trigonella foenum-graecum seed extract on sexual function was assessed using the DISF-SR at baseline and at week 12. There was a significant change in the total score in active treatment group (p = 0.006) after treatment. Analysis of the sub-domains showed a significant difference in the sub-domains of Sexual Arousal (p = 0.001) and Sexual Drive/Relationship (p = 0.007), but there were no changes in the sub-domains of Sexual Cognition, Sexual Behaviour or Orgasm. There were no changes observed in the placebo group before or after treatment in the total score or any sub-domain ().
Table 3. Assessment of changes in sexual function in active treatment group and placebo group at baseline and 12 weeks.
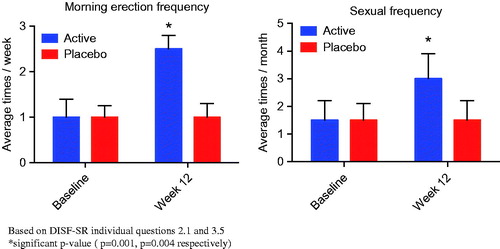
At baseline, both groups reported an average of one erection per week but this increased significantly to 2–3 per week (p = 0.001) in the active treatment group. At baseline, both groups reported sexual activity of approximately 1–2 times per month, this increased significantly to almost once a week (p = 0.004) in the active treatment group by week 12. These results were consistent with the positive results reported in the sexual function sub-domain of the AMS questionnaire “Which of the following symptoms apply to you at this time”, in particular, questions 16 “Decrease in the number of morning erections” and question 17 “Decrease in sexual desire/libido” ().
Effect on sex hormones, liver function and lipids
The results of this study showed that there were no changes in levels of DHEA-S, androstenedione, oestradiol, SHBG or prolactin in either group during the 12 weeks of the study (). There were no significant changes in liver function, total, LDL or HDL cholesterol, or triglycerides, in either group after 12 weeks.
Table 4. Hormone levels for active treatment and placebo groups at baseline, week 6 and week 12.
Total testosterone levels were similar between both groups at baseline. There was a small but significant difference in the change from baseline (Δ) values between the active treatment and placebo groups for testosterone and calculated free testosterone at week 12. Further, bivariate analysis of covariance was calculated with a Bonferroni adjustment using BMI and age as the covariate factors and both total testosterone F(1,91) = 13.8, p < 0.001, η2 = 0.134 and free testosterone F(1,91) = 10.8, p = 0.002, η2 = 0.106 remained significant ().
Safety data
The routine hematological and biochemical parameters were similar at baseline and after 12 weeks of treatment. The treatment was well tolerated. There were no serious adverse effects. There were five minor adverse reactions reported, three in the active treatment group (increased frequency of headaches (2), dizziness (1) and two in the placebo group (nausea (1), increased asthma symptoms (1), however these may not have been attributed to the treatment.
Discussion
This is the first published study of a Trigonella foenum-graecum seed extract in the treatment of symptoms of possible androgen deficiency in aging men. Symptom severity was measured by the aging males symptoms scale (AMS), which was developed as an indication of the severity of symptoms [Citation42]. The results of this study demonstrate that Trigonella foenum-graecum (Testofen) seed extract significantly reduces symptom severity in the aging male. The most significant changes observed were in the somatic and sexual function domains of the AMS.
Sexual function was also assessed using the DISF-SR sexual function questionnaire, and this was found to compare well with the sexual function domain of the AMS questionnaire. It showed that treatment with Trigonella foenum-graecum seed extract resulted in a significant improvement in sexual functioning, including desire, arousal and frequency of morning erections. These results are supported by an earlier study of younger men using the same Trigonella foenum-graecum seed extract which was found to have a positive effect on sexual function in men who were experiencing low libido [Citation31].
Furthermore, this study has demonstrated that this particular Trigonella foenum-graecum seed extract results in small but statistically significant increases in serum total and free testosterone. This effect was independent of age, as increases were observed in all age brackets from 40 years to 70 years. These results provide a potential mechanism for the positive effects on somatic and sexual function observed in this study. It is hypothesised that the reduction in severity of symptoms and increased somatic and sexual function was directly or indirectly related to the increased serum testosterone.
This is supported by an earlier study of another extract in younger exercising men that demonstrated that Trigonella foenum-graecum decreased body fat and increased total and free testosterone [Citation32]. Potential mechanisms by which Trigonella foenum-graecum may increase serum testosterone includes stimulation of pulsatile GnRH/LH, increased testicular sensitivity to LH, and increased testosterone synthesis or reduced testosterone catabolism. However, further study still needs to be undertaken before a comprehensive mechanism of action can be proposed.
A similar pattern of reduced symptoms and improved sexual function is also observed in men treated directly with testosterone. Behre et al. [Citation15] demonstrated that 6 months treatment with testosterone in men with low serum total testosterone (< 15.0 nmol/L) and bioavailable testosterone (6.68 nmol/L) and symptoms of androgen deficiency (using the same AMS scale) resulted in progressive improvement in quality of life as well as an improvement in lean body mass and decreased fat mass. This is also supported by a more recent study using testosterone in men aged 50–65 years for 24 months, where the AMS questionnaire was used to show a marked improvement in the items related to sexual function and mood, as well as significant improvements in both total and free testosterone after 3 months, and an increase in lean muscle and decrease in fat mass by 12 months. Interestingly, as seen with this study as well, SHGB levels remained relatively unchanged, despite the increased testosterone levels [Citation9]. It is also supported by previous research indicating that serum free testosterone is significantly correlated with the libido, erectile, and orgasmic function domains on International Index of Erectile Function (IIEF) questionnaire [Citation43].
While the AMS score consists of age-related and testosterone-associated decline in health parameters in males, it has been considered as a potential predictor of androgen status in men. Previous research had found a significant correlation of AMS scores with lowered free testosterone [Citation44,Citation45]. However, a number of other studies failed to reveal a correlation of the questionnaire results with serum testosterone levels [Citation46,Citation47]. A recent study by Zengerling et al. [Citation48] demonstrated that AMS total score is not a predictor of total or free testosterone levels or hypogonadism but there was a correlation with the sexual and somatic sub-scores, a result that was also observed in this current study. This suggests that the changes in testosterone, while linked to sexual function and mood in men, is not responsible for all aspects of aging.
There was only a weak correlation between free testosterone levels and age. Instead, total testosterone levels and free testosterone levels were inversely related to BMI. This is consistent with recent studies indicating that in generally healthy men, there was no decrease in the mean total testosterone level with increasing age, yet statistically and clinically negative correlation between testosterone and BMI and positive correlation between testosterone and fitness [Citation49]. While it has been suggested that there is an age-related decline in testosterone due to altered function both centrally (at the level of the hypothalamic-pituitary unit) and at the level of the testis [Citation8], other data indicate that the decline in testosterone is more associated with the accumulation of co-morbidities, particularly weight gain [Citation4–6]. The EMAS study of 3320 men aged between 40 and 79 years, showed that the aging-related decline of testosterone has great inter-individual variability, with about 20% of men over 60 years having serum testosterone in the upper normal range of young men, and about 20% being below the reference range. This research has also established that testosterone in a man with BMI >30 is, on average, 30% lower than that of a man with BMI <25, at any age, which is more than the purely age-dependent decrease between 40 and 80 years [Citation50]. The increase in serum testosterone observed across our study in the active treatment group occurred independently of any change in BMI.
Neither AMS nor serum testosterone was correlated with regular exercise (deemed more than 3 days per week). Interestingly, a previous study on a larger population of 421 men had shown that psychological, somatic and general scores symptoms, as well as their severity were lower among those subjects who reached the current recommendations for physical activity during leisure and commuting time [Citation51].
The MFI scale was used to assess individual components of fatigue including physical fatigue, mental fatigue, motivation and activity. As with physical activity, there were no changes in fatigue noted as a result of the treatment. The smaller sample size, time frame and possibly the subject age group may have been limitations to observing any differences. Earlier studies with testosterone gel have shown that sex hormone levels, physical complaints, depression, sexuality and life satisfaction, and low total and free testosterone are associated with reduced motivation [Citation52] and in an observational 6 month study using testosterone gel, significant improvements in fatigue scores were observed [Citation53].
Furthermore, in this study cohort, testosterone levels were positively correlated with HDL cholesterol. There were no changes observed in other hormones, DHEA-S, androstenedione, oestradiol or liver function after treatment with Trigonella foenum-graecum seed extract. The herbal medicine was well tolerated, although was associated with headache in <5% of subjects, an effect also observed in the earlier study [Citation31].
Recommendations have been published for the diagnosis, treatment and monitoring of hypogonadism in men [Citation54]. In this document, a relatively high threshold for total testosterone of <12.1 nmol/L was suggested to classify hypogonadism. Further long-term studies are awaited to establish clear indications for and long-term safety of testosterone therapy in men with low or borderline testosterone concentrations in the absence of organic hypothalamic-pituitary or testicular pathology [Citation8]. The use of herbal medicine such as extract of Trigonella foenum-graecum which has in this study shown efficacy in improving symptoms, may become an alternative for symptomatic men where low or borderline testosterone is associated with obesity, chronic disease and mood disorder rather than organic hypothalamic–pituitary–testicular axis pathology. However, further studies are needed to establish its long-term safety and efficacy.
In conclusion, this extract of Trigonella foenum-graecum seed was shown to be safe and effective, reducing symptoms of possible androgen deficiency, improving sexual function and increasing serum testosterone in healthy middle-aged and older men.
Trial registration
The trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR) with Trial ID. ACTRN No: 12613000925741 and can be accessed on the ANZCTR website.
Acknowledgements
We would like to thank Queensland Clinical Trials Network (QCTN) for providing the ethics committee review.
Declaration of interest
The investigational products and funding were supplied by Gencor Pacific, Hong Kong.
References
- Morley JE, Kaiser FE, Perry HM III, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metab Clin Exp 1997;46:410–13.
- Matsumoto AM. Andropause: Clinical implications of the decline in serum testosterone level with aging in men. J Gerontol a Biol Sci Med Sci 2002;57:76–99.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–98.
- Sartorius G, Spasevska S, Idan A, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol (Oxf). 2012;77:755–63.
- Camacho EM1, Huhtaniemi IT, O'Neill TW, et al. EMAS Group. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013 Feb 20;168:445–55.
- Shi Z, Araujo AB, Martin S, et al. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013 Aug;98:3289–97.
- Keenan DM, Takahashi PY, Liu PY, et al. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology 2006;147:2817–28.
- Golan R, Scovell JM, Ramasamy R. Age-related testosterone decline is due to waning of both testicular and hypothalamic-pituitary function. Aging Male. 2015;18:201–4.
- Rodriguez-Tolrà J, Torremadé Barreda J, del Rio L, et al. Effects of testosterone treatment on body composition in males with testosterone deficiency syndrome. Aging Male 2013;16:184–90.
- Yeap BB1, Araujo AB, Wittert GA. Do low testosterone levels contribute to ill-health during male ageing? Crit Rev Clin Lab Sci. 2012;49:168–82.
- Hsu B, Cumming RG, Blyth FM, et al. The longitudinal relationship of sexual function and androgen status in older men: the concord health and ageing in men project. J Clin Endocrinol Metab 2015;100:1350–8.
- Antonio L, Wu FC, O'Neill TW, et al. EMAS Study Group. Associations between sex steroids and the development of metabolic syndrome: a longitudinal study in European men. J Clin Endocrinol Metab 2015;100:1396–404.
- Hsu B, Cumming RG, Waite LM, et al. Longitudinal relationships between reproductive hormones and cognitive decline in older men: the concord health and ageing in men project. J Clin Endocrinol Metab 2015;100:2223–30.
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol 2009;5:673–81.
- Behre M, Tammela T, Arver S, et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male 2012;15:198–207.
- Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 2007;116:2694–701.
- Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004;27:1036–41.
- Cicero TJ. Effects of exogenous and endogenous opiates on the hypothalamic-pituitary-gonadal axis in the male. Fed Proc 1980;39:2551–4.
- Elliott JA, Opper SE, Agarwal S, Fibuch EE. Non-analgesic effects of opioids: opioids and the endocrine system. Curr Pharm Des 2012;18:6070–8.
- Corssmit EP, Endert E, Sauerwein HP, Romijn JA. Acute effects of interferon-alpha administration on testosterone concentrations in healthy men. Eur J Endocrinol 2000;143:371–4.
- Singh P. Andropause: current concepts. Indian J Endocrinol Metab 2013;17:621–9.
- Ullah MI, Riche DM, Koch CA. Transdermal testosterone replacement therapy in men. Drug Design Dev Therapy 2014;8:101–12.
- Liu PY, Beilin J, Meier C, et al. Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab 2007;92:3599–603.
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–35.
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762–9.
- Miner M, Canty DJ, Shabsigh R. Testosterone replacement therapy in hypogonadal men: assessing benefits, risks, and best practices. Postgrad Med 2008;120:130–53.
- LeBlanc ES, Wang PY, Lee CG, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab 2011;96:3855–63.
- Auyeung TW, Lee JS, Kwok T, et al. Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross-sectional study in 1489 older men. Eur J Endocrinol 2011;164:811–17.
- Gooren LJ. Androgens and male aging: current evidence of safety and efficacy. Asian J Androl 2010;12:136–51.
- Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2010;95:639–50.
- Steels E, Rao A, Vitetta L. Physiological aspects of male libido enhanced by standardized Trigonella foenum-graecum extract and mineral formulation. Phytotherapy Res 2011;25:1294–300.
- Wankhede S, Mohan V, Thakurdesai P. Beneficial effects of fenugreek glycoside supplementation in male subjects during resistance training: a randomized controlled pilot study. J Sport and Health Sci 2015; ‘in press.’
- Price KR, Johnson IT, Fenwick GR. The chemistry and biological significance of saponins in foods and feedingstuffs. Crit Rev Food Sci Nutr 1987;26:27–135.
- Aswar U, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of furostanol glycosides from Trigonella foenum-graecum on the reproductive system of male albino rats. Phytother Res 2010;24:1482–8.
- Wilborn C, Taylor L, Poole C, et al. Effects of a purported aromatase and 5 a-reductase inhibitor on hormone profiles in college-age man. Int J Sport Nutr Exe 2010;20:457–65.
- Daig I, Heinemann LAJ, Kim S, et al. The Aging Males Symptoms (AMS) scale: review of its methodological characteristics. Health Quality Life Outcomes 2003;1:77.
- Derogatis LR, Mellisaratos N. The DSFI: a multidimensional measure of sexual functioning. J Sex Marital Ther 1979;5:244–81.
- Sodergad R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–10.
- Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res 1989;28:193–213.
- Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract 1998;6:143–52.
- Craig CL, Marshall AL, Sjostrom A, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95.
- Heinemann LAJ, Zimmermann T, Vermeulen A, et al. A new ‘Aging Males’ symptoms’ rating scale. Aging Male 1999;2:105–14.
- Travison TG, Morley JE, Araujo AB, et al. The relationship between libido and testosterone levels in aging men. J Clin Endocrinol Metab 2006;91:2509–13.
- Morley JE, Perry HM, III Kevorkian RT, Patrick P. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas 2006;53:424–9.
- Clapauch R, Braga DJ, Marinheiro LP, et al. Risk of late-onset hypogonadism (andropause) in Brazilian men over 50 years of age with osteoporosis: usefulness of screening questionnaires. Arq Bras Endocrinol Metabol 2008;52:1439–47.
- Miwa Y, Kaneda T, Yokoyama O. Correlation between the Aging Males’ Symptoms Scale and sex steroids, gonadotropins, dehydroepiandrosterone sulfate, and growth hormone levels in ambulatory men. J Sex Med 2006;3:723–6.
- Kang J, II Ham BK, Oh MM, et al. Correlation between serum total testosterone and the AMS and IIEF questionnaires in patients with erectile dysfunction with testosterone deficiency syndrome. Korean J Urol 2011;52:416–20.
- Zengerling F, Schrader A, Cronauer M, et al. The Aging Males' Symptoms’ Scale (AMS): predictive value for lowered circulating androgens. Aging Male 2012;15:253–7.
- DeFina LF, Radford NB, Leonard D, et al. MON-0012: testosterone level in men is lower with increasing BMI and decreasing cardiorespiratory fitness but is not related to age. Endocrine Society's 96th Annual Meeting and Expo, 21–24 June 2014, Chicago.
- Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl 2014;16:192–202.
- Correa L, Rombaldi AJ, da Silva MC. Physical activity and aging male symptoms in a southern Brazilian population. Rev Bras Med Esporte 2011;17:228–31.
- Beutel ME, Wiltink J, Hauck EW, et al. Correlations between hormones, physical, and affective parameters in aging urologic outpatients. Eur Urol 2005;47:749–55.
- Pexman-Fieth C, Behre HM, Morales A, et al. A 6-month observational study of energy, sexual desire, and body proportions in hypogonadal men treated with a testosterone 1% gel. Aging Male 2014;17:1–11.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. The Aging Male 2015;18:5–15.