Abstract
We prospectively evaluated erectile function (EF) using the Sexual Health Inventory for Men (SHIM) and the erectile hardness score (EHS) as well as urinary statuses using the International Prostate Symptom Score (IPSS) and Overactive Bladder Symptom Score (OABSS) before and 3, 6, and 12 months after a daily treatment with 0.5 mg dutasteride (DUT). Significant improvements were observed in IPSS and OABSS in 98 patients with the DUT treatment, and the effects were similar between 28 patients with potency with baseline SHIM of 8 or greater and 70 severe erectile dysfunction (ED) patients at baseline. In the 28 patients with potency, significant decreases were observed in SHIM and EHS after 3, 6, and 12 months of the DUT treatment, with the severity of ED according to SHIM deteriorating in half of these patients after 12 months of the DUT treatment. Eighteen out of 28 patients (64.3%) with potency at baseline had awareness of the occurrence of ED before the DUT treatment, were younger, and had higher SHIM and EHS just before the DUT treatment than their counterparts. Regular assessments of EF may be needed, especially in younger patients and those with higher levels of EF before the administration of DUT.
Introduction
Lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) [Citation1] and decreases in sexual function [Citation2] both have negative impacts on the quality of life (QoL) of middle-aged and elderly men. Treatments with 5-alpha reductase inhibitors (5ARI) such as dutasteride (DUT) have been shown to reduce the size of the prostate, prevent the progression of BPH, and decrease the risk of urinary retention and BPH-related surgery in men with moderate to severe hyperplasia [Citation3,Citation4]. The American Urological Association and European Association of Urology guidelines recommend medical treatments with 5ARI for patients, especially those with severe BPH [Citation5,Citation6]. However, reduced erectile function (EF) due to treatments with DUT has been reported in clinical trials at an incidence rate of approximately 1.3–11% [Citation7–10].
Uygur et al. indicated that sexual adverse events (AE) increased with time in patients treated with 5ARI [Citation11]. On the other hand, Chi and Kim showed that EF had deteriorated after 1 month, but gradually improved by 12 months [Citation12]. However, sequential changes in the EF status with DUT treatments have not yet been elucidated in sufficient detail in a clinical setting. Furthermore, some reports indicated that the presence of erectile dysfunction (ED) is the important predictor for identifying severe LUTS [Citation13] and the presence of LUTS also affects the status of EF [Citation14], but few studies have evaluated differences in urinary improvements in patients with and without the AE of ED after DUT treatment.
Therefore, we herein investigated 1) sequential changes in EF statuses in more detail using the Sexual Health Inventory for Men (SHIM) and erection hardness score (EHS) as well as urinary statuses with the administration of DUT during a 12-month follow-up period, 2) differences in urinary improvements in patients with and without the deterioration of EF, and 3) whether patients were aware of the occurrence of ED at the beginning of the DUT treatment using a simple three-titer ad hoc questionnaire.
Methods
Ninety-eight patients who were followed-up for more than 12 months with a DUT treatment between 2009 and 2012 were included in our study population. They were treated with DUT for symptomatic BPH consisting of both an enlarged prostate size (total prostate size ≥20 mL on transrectal ultrasound) and moderate to severe LUTS (International Prostate Symptom Score (IPSS) ≥ 8). Any other kinds of medicine for urinary or sexual issues such as anti-muscarinic drugs and phosphodiesterase (PDE)-5 inhibitors were not administered after the DUT treatment. Patients with a previous history of prostate cancer, previous treatment with anti-androgens (chlormadinone acetate, allylestrenol), or 5ARI such as finasteride (FIN) or DUT, and those with spinal cord diseases and urinary tract infections, and in whom urethral catheters were inserted at the beginning of the DUT treatment were also excluded from our cohort.
In this prospective longitudinal survey, sexual status was evaluated by SHIM and EHS, and the urinary status was also assessed using IPSS and the Overactive Bladder Symptomatic Score (OABSS). SHIM was developed to diagnose the presence and severity of ED as a concise five-item version (range of scores, 1–25) of the 15-item International Index of Erectile Function [Citation15]. Severity was categorized into five groups based on a range of scores from 1 to 7; severe ED, 8–11; moderate ED, 12–16; mild to moderate ED, 17–21; mild ED, and 22–25; no ED. EHS consisted of five phases of scoring (range of scores, 0–4) self-reported by the patients in order to evaluate the erectile hardness of the penis [Citation16]. Furthermore, blood sampling, including measurements of total and free testosterone levels, was performed at baseline. The baseline survey was performed within 1 week before the DUT treatment and follow-up data were obtained after 3, 6, and 12 months of the DUT treatment. This study was approved by the Institutional Review Board at Keio University Hospital and written informed consent was obtained from all participants.
According to the EF status at baseline, 98 patients were classified into two groups: patients with potency (N = 28) and those with severe ED (N = 70). Patients with potency and those with severe ED were defined as having an SHIM score of 8 or greater and less than 8 before DUT, respectively. Furthermore, among the 28 patients with potency, we defined the deteriorated EF group as patients with one or more categories in the SHIM that had worsened after 12 months of the DUT treatment.
We also retrospectively evaluated how negative information regarding the possibility of decreased potency affected patients with potency at the beginning of the treatment after DUT-related AE information was routinely provided by the attending physician using an ad hoc questionnaire consisting of three phases: “very anxious”, “slight anxiety”, and “did not care”. Patients who answered “very anxious” and “slight anxiety” were categorized into the anxious about ED group.
Results were presented as the mean ± standard deviation (SD). Differences in IPSS, OABSS, SHIM, and EHS between the baseline and each point after the administration of DUT were analyzed using the Wilcoxon signed-rank test. Clinical parameters between the deteriorated EF group and non-deteriorated EF group were compared by the Chi-squared test or Mann–Whitney U test. A p value less than 0.05 was set as significant. These analyses were performed using a JMP version 10.0.2 statistical software package (SAS Institute, Cary, NC).
Results
Baseline patient characteristics before the DUT treatment
The mean ± SD age of the 98 patients was 71.5 ± 7.4 years and the mean ± SD prostate size was 51.3 ± 23.9 mL. The mean ± SD IPSS, QoL index, and OABSS before the DUT treatment were 16.6 ± 6.7, 4.5 ± 1.2, and 6.2 ± 2.9, respectively. The baseline mean ± SD total testosterone and free testosterone levels were 4.16 ± 1.25 (ng/mL) and 8.0 ± 2.7 (pg/mL), respectively. Twenty-nine out of 98 patients (29.6%) had hypertension, 13 (13.3%) had diabetes mellitus, and 28 (28.6%) had ischemic heart disease. The baseline mean ± SD SHIM and EHS were 6.5 ± 6.7 and 1.8 ± 1.4, respectively.
Changes in IPSS and OABSS after the DUT treatment in all patients, those with potency, and those with severe ED
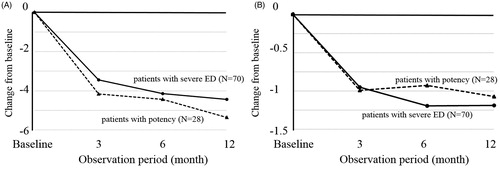
The mean total IPSS, QoL index, and OABSS in all patients after 3 and 12 months of the DUT treatment were 13.0, 3.9, and 5.2 and 12.0, 3.5, and 5.0, respectively, which were significantly lower than those at baseline (). The voiding subdomain score and storage subdomain score were also significantly reduced after 3, 6, and 12 months of the DUT treatment.
Table 1. Changes in IPSS, QoL, and OABSS with the DUT treatment in all patients.
Changes in the mean total IPSS after 3 and 12 months of the DUT treatment from baseline in 28 patients with potency were 4.2 and 5.4, respectively, which were not significantly different from those in 70 patients with severe ED (3.4 at 3 months and 4.4 at 12 months, ). No significant differences were observed in OABSS between patients with potency and those with severe ED (). Furthermore, the proportion of subjects with 25% IPSS improvements after 12 months was similar between patients with potency (64.3%) and those with severe ED (54.3%) (p = 0.366).
Changes in SHIM and EHS after the DUT treatment in 28 patients with potency at baseline
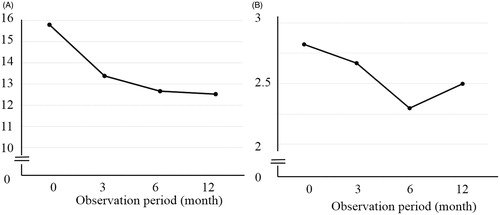
In the 28 patients with potency before the DUT treatment, the mean SHIM scores at baseline and after 3, 6, and 12 months of the DUT treatment were 15.8, 13.4, 12.7, and 12.5, respectively (). Although significant differences were observed between baseline and after 3, 6, and 12 months, a plateau was noted after 3 months. Furthermore, mean EHS at baseline and after 3, 6, and 12 months of the DUT treatment were 2.8, 2.7, 2.3, and 2.5, respectively (), with a significant difference being observed between baseline and after 6 and 12 months.
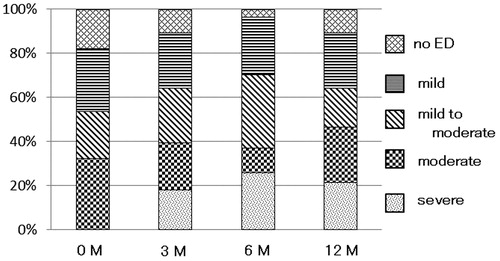
shows the distribution of ED severity in the 28 patients with potency over time. After 12 months of the DUT treatment, ED was severe in 6 (21.4%) out of 28 patients, moderate in 7 (25.0%), mild to moderate in 5 (17.9%), and mild in 7 (25.0%), whereas 3 patients (10.7%) had no ED. Of the 28 patients, the rates of deteriorated ED severity after 3, 6, and 12 months of the DUT treatment were 42.9% (12 of 28), 53.6% (15 of 28), and 50.0% (14 of 28) from the baseline.
Comparison of clinical characteristics between the deteriorated EF group and non-deteriorated EF group 12 months after the DUT treatment
Of the 28 patients with potency at baseline, the severity of ED according to SHIM deteriorated in 14 (50.0%) (deteriorated EF group), but remained unchanged in 14 (50.0%) (non-deteriorated EF group) after 12 months of the DUT treatment. In the deteriorated EF group, the mean ± SD IPSS and OABSS after 12 months of the DUT treatment were 14.6 ± 5.7 and 5.5 ± 2.3, respectively, which were significantly lower than those before the DUT treatment (21.1 ± 7.7, and 7.4 ± 1.8, respectively). In the non-deteriorated EF group, the mean ± SD IPSS after 12 months of the DUT treatment was 14.6 ± 5.7, which was also significantly lower than that before the DUT treatment (16.6 ± 7.4); however, the mean ± SD OABSS after 12 months of the DUT treatment was 5.6 ± 1.9, which was not significantly different from that before the DUT treatment (5.8 ± 2.5). No significant differences were observed in clinical parameters at baseline before the DUT treatment between the deteriorated EF group and non-deteriorated EF group (). However, in the deteriorated EF group, 11 out of 14 patients (78.6%) already reported that the severity of ED according to SHIM had deteriorated after 3 months. In contrast, in the non-deteriorated EF group, only 1 out of 14 patients (7.1%) reported that the severity of ED according to SHIM had deteriorated after 3 months. In the deteriorated EF group, the mean ± SD SHIM after 3 months of the DUT treatment was 11.1 ± 6.8, which was significantly lower than that before the DUT treatment (15.6 ± 4.8); however, in the non-deteriorated EF group, the mean ± SD SHIM after 3 months of the DUT treatment was 15.6 ± 5.4, which was not significantly different from that before the DUT treatment (15.9 ± 5.6).
Table 2. Comparison of clinical characteristics between the deteriorated EF group and non-deteriorated EF group after 12 months of the DUT treatment.
Awareness of AE related to ED due to the DUT treatment at baseline
We also retrospectively evaluated whether the 28 patients with potency at baseline were anxious about AE related to ED due to the DUT treatment using a simple three-titer ad hoc questionnaire. Ten patients (35.7%) did not care about sexual-related AE of ED, 11 had slight anxiety, whereas the reminder were very anxious at the beginning of the DUT treatment. Patients in the anxious about ED group were younger and had higher SHIM and EHS before the DUT treatment than those who did not care about ED due to the DUT treatment ().
Table 3. Baseline characteristics between patients anxious about ED and those who did not care about ED.
Discussion
This study indicated that 0.5 mg DUT administered once daily to men with an SHIM score of 8 or greater and symptomatic BPH resulted not only in significant improvements in the urinary status, but also the deterioration of sexual function evaluated by SHIM and EHS after 12 months of the DUT treatment. No significant differences were observed in baseline clinical features between patients in the deteriorated EF group and non-deteriorated EF group after 12 months of the DUT treatment. However, a significant difference was noted in SHIM deterioration after 3 months of the DUT treatment between the two groups. Furthermore, patients with potency at baseline, who were younger and had higher sexual function at the beginning of the DUT treatment, expressed more anxiety about the sexual-related AE of ED due to the DUT treatment.
Various sexual AE such as ED, gynecomastia, decreased libido, and ejaculation disorders have been associated with DUT treatments. According to one meta-analysis of 2411 patients treated with DUT and accumulated from three randomized control trials, side effects due to DUT were reported in 164 patients (6.8%) [Citation17]. In this study, EF severity deteriorated in 14 out of 98 patients (14.3%), and in 14 out of 28 patients (50%) with an SHIM score ≥8 at the beginning of DUT treatment. It is important to consider that the relatively high rate of deteriorated EF statuses after the DUT treatment in our study population may have been the “nocebo effect”, which means that information disclosure about potential AEs may have contributed to AE [Citation18], because we routinely and strictly provided information on the possibility of ED due to the DUT treatment by the attending physician before the treatment. A relationship between the disclosure of the possible side effects of sexual AEs and its occurrence has been reported previously [Citation19–21]. Mondaini et al. showed the rate of complaints of related ED was significantly higher in patients with awareness of drug-related AE than in those blinded to this information [Citation19]. Although approximately 70% of all subjects already had severe ED (SHIM <8) at the beginning of the DUT treatment in our population, in patients with potency at baseline, younger patients and those with higher EF had more awareness of the AE of ED due to the DUT treatment. Therefore, when DUT is administered, especially to these patients, physicians need to not only recognize this nocebo effect, but also explain the countermeasures available for ED due to the DUT treatment.
Casabe et al. recently reported that the co-administration of tadalafil (TAD) with FIN led to significantly greater improvements in the International Index of Erective Function (IIEF) score and IPSS in patients with BPH-LUTS than in the placebo/FIN group, and the incidence of sexual AE, even in the placebo/FIN group, was lower than that generally reported for 5ARI therapies [Citation22]. Recent studies indicated that the daily administration of 5 mg TAD effectively improved EF and increased patient satisfaction [Citation23–25]. Regardless of erectile ability at baseline, the co-administration of PDE5 inhibitors with DUT should be considered to reduce the psychological burden on patients regarding impairment of EF after administration.
The mechanisms by which decreases in EF may occur after DUT treatments have not yet been examined in sufficient detail, but may be explained by an androgenic role. In a castrated animal model, dihydrotestosterone treatment restored EF [Citation26] and improved erectile responses to stimuli [Citation27]. However, treatments with testosterone and 5ARI did not restore erectile responses in castrated models [Citation28]. These findings suggest that dihydrotestosterone plays an important role in maintaining EF. Moreover, histopathological evaluations revealed that the occurrence of ED after 5ARI treatments was associated with increases in the deposition of collagen in the penile smooth muscle and decreases in the expression of neuronal NOS [Citation29]. Further studies are warranted in order to elucidate the mechanisms underlying ED, especially hormonal effects and histopathological changes after DUT treatments in a clinical setting.
Homma et al. previously reported that the incidence of ejaculation disorders was 22.3% after the administration of the selective alpha-1A blocker, silodosin for BPH; however, patients with ejaculation disorders after being treated with silodosin achieved greater improvements in IPSS than those without ejaculation disorders [Citation30]. To date, few studies have investigated the relationship between the occurrence of sexual AE and improvements in LUTS in patients treated with DUT. In this study, no significant differences were observed in IPSS and OABSS improvements after 12 months of the DUT treatment between patients with potency and those with severe ED at baseline.
There were several limitations to this study. It was a retrospective study, and the number of patients with potency at baseline was not large. Moreover, the number of patients who were anxious about the onset of ED with the DUT treatment may have been small because our study population included older patients with a mean age of 71.5 years. Marumo et al. previously reported that the prevalence of men who had intercourse less than once a month was 68.8% in Japanese men who were 70 years or older [Citation31]. Furthermore, other sexual-related AE for orgasmic function and sexual desire were not evaluated in this study because SHIM only assessed the presence of and severity of ED. Treatments with 5ARI have also been reported to affect libido and ejaculatory function and, thus, may also have affected the SHIM score or EHS [Citation32]. Further studies are needed in order to evaluate sexual AE more completely using other questionnaires such as IIEF-15.
In conclusion, the daily administration of DUT not only resulted in an improved urinary status, but also deteriorated EF, especially in BPH patients with potency before the treatment. Regular assessments of the occurrence of ED due to DUT are needed, especially in younger patients and those having higher levels of EF before the administration of DUT.
Declaration of interest
All of authors have no conflict of interest. This study was conducted according to Helsinki Declaration and the ethic guideline about clinical studies.
References
- Abrams P, Chapple C, Khoury S, et al Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2009;181:1779–87
- Rosen RC, Fisher WA, Eardley I, et al The multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin 2004;20:607–17
- Roehrborn CG, Boyle P, Nickel JC, et al Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002;60:434–41
- Roehrborn CG, Nickel JC, Andriole GL, et al Dutasteride improves outcomes of benign prostatic hyperplasia when evaluated for prostate cancer risk reduction: secondary analysis of the REduction by DUtasteride of prostate Cancer Events (REDUCE) trial. Urology 2011;78:641–6
- McVary KT, Roehrborn CG, Avins AL, et al Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011;185:1793–803
- Oelke M, Bachmann A, Descazeaud A, et al EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013;64:118–40
- Debruyne F, Barkin J, van Erps P, et al Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol 2004;46:488–94. discussion 95
- Clark RV, Hermann DJ, Cunningham GR, et al Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004;89:2179–84
- Roehrborn CG, Marks LS, Fenter T, et al Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology 2004;63:709–15
- Tsukamoto T, Endo Y, Narita M. Efficacy and safety of dutasteride in Japanese men with benign prostatic hyperplasia. Int J Urol 2009;16:745–50
- Uygur MC, Arik AI, Altug U, Erol D. Effects of the 5 alpha-reductase inhibitor finasteride on serum levels of gonadal, adrenal, and hypophyseal hormones and its clinical significance: a prospective clinical study. Steroids 1998;63:208–13
- Chi BH, Kim SC. Changes in sexual function in benign prostatic hyperplasia patients taking dutasteride: 1-year follow-up results. Korean J Urol 2011;52:632–6
- Demir O, Akgul K, Akar Z, et al Association between severity of lower urinary tract symptoms, erectile dysfunction and metabolic syndrome. Aging Male 2009;12:29–34
- Tsai CC, Liu CC, Huang SP, et al The impact of irritative lower urinary tract symptoms on erectile dysfunction in aging Taiwanese males. Aging Male 2010;13:179–83
- Rosen RC, Cappelleri JC, Smith MD, et al Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–26
- Mulhall JP, Goldstein I, Bushmakin AG, et al Validation of the erection hardness score. J Sex Med 2007;4:1626–34
- Wu XJ, Zhi Y, Zheng J, et al Dutasteride on benign prostatic hyperplasia: a meta-analysis on randomized clinical trials in 6460 patients. Urology 2014;83:539–43
- Colloca L, Miller FG. The nocebo effect and its relevance for clinical practice. Psychosom Med 2011;73:598–603
- Mondaini N, Gontero P, Giubilei G, et al Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J Sex Med 2007;4:1708–12
- Silvestri A, Galetta P, Cerquetani E, et al Report of erectile dysfunction after therapy with beta-blockers is related to patient knowledge of side effects and is reversed by placebo. Eur Heart J 2003;24:1928–32
- Cocco G. Erectile dysfunction after therapy with metoprolol: the Hawthorne effect. Cardiology 2009;112:174–7
- Casabe A, Roehrborn CG, Da Pozzo LF, et al Efficacy and safety of the coadministration of tadalafil once daily with finasteride for 6 months in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia. J Urol 2014;191:727–33
- Burns PR, Rosen RC, Dunn M, et al Treatment satisfaction of men and partners following switch from on-demand phosphodiesterase type 5 inhibitor therapy to tadalafil 5 mg once daily. J Sex Med 2015;12:720–7
- Glina S, Roehrborn CG, Esen A, et al Sexual function in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia: results of a 6-month, randomized, double-blind, placebo-controlled study of tadalafil coadministered with finasteride. J Sex Med 2015;12:129–38
- Park MG, Yeo JK, Cho DY, et al The efficacy of combination treatment with injectable testosterone undecanoate and daily tadalafil for erectile dysfunction with testosterone deficiency syndrome. J Sex Med 2015;12:966–74
- Penson DF, Ng C, Rajfer J, Gonzalez-Cadavid NF. Adrenal control of erectile function and nitric oxide synthase in the rat penis. Endocrinology 1997;138:3925–32
- Garban H, Marquez D, Cai L, et al Restoration of normal adult penile erectile response in aged rats by long-term treatment with androgens. Biol Reprod 1995;53:1365–72
- Lugg JA, Rajfer J, Gonzalez-Cadavid NF. Dihydrotestosterone is the active androgen in the maintenance of nitric oxide-mediated penile erection in the rat. Endocrinology 1995;136:1495–501
- Pinsky MR, Gur S, Tracey AJ, et al The effects of chronic 5-alpha-reductase inhibitor (dutasteride) treatment on rat erectile function. J Sex Med 2011;8:3066–74
- Homma Y, Kawabe K, Takeda M, Yoshida M. Ejaculation disorder is associated with increased efficacy of silodosin for benign prostatic hyperplasia. Urology 2010;76:1446–50
- Marumo K, Nakashima J, Murai M. Age-related prevalence of erectile dysfunction in Japan: assessment by the International Index of Erectile Function. Int J Urol 2001;8:53–9
- Traish AM, Hassani J, Guay AT, et al Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med 2011;8:872–84