Abstract
The cell wall of the opportunistic human fungal pathogen, Candida albicans is a complex, layered network of rigid structural polysaccharides composed of β-glucans and chitin that is covered with a fibrillar matrix of highly glycosylated mannoproteins. Polymorphonuclear cells (PMNs, neutrophils) are the most prevalent circulating phagocytic leukocyte in peripheral blood and they are pivotal in the clearance of invading fungal cells from tissues. The importance of cell-wall mannans for the recognition and uptake of C. albicans by human PMNs was therefore investigated. N- and O-glycosylation-deficient mutants were attenuated in binding and phagocytosis by PMNs and this was associated with reduced killing of C. albicans yeast cells. No differences were found in the production of the respiratory burst enzyme myeloperoxidase (MPO) and the neutrophil chemokine IL-8 in PMNs exposed to control and glycosylation-deficient C. albicans strains. Thus, the significant decrease in killing of glycan-deficient C. albicans strains by PMNs is a consequence of a marked reduction in phagocytosis rather than changes in the release of inflammatory mediators by PMNs.
Introduction
C. albicans is ordinarily found as a commensal yeast colonizing the human gastrointestinal tract. It is, however, able to invade and cause deep-seated infections in individuals with compromised host defenses arising from trauma, the administration of immunosuppressive agents, a variety of disease and association with a number of genetic mutations that affect immune recognition [Citation1–3]. Invasive, disseminated candidiasis for a range of Candida species is associated with mortality rates of 30–60% with C. albicans being the most common and pathogenic of the species known to cause invasive disease [Citation4–8]. Many of the virulence traits of this fungus are related to the properties of highly glycosylated proteins in the outer wall and secretome of the organism.
Circulating polymorphonuclear (PMN) cells play a central role in the control mechanism preventing the spread of infection in the host. Fungal cells are recognized and engulfed by these leukocytes, thereby reducing the number of infectious cells in the body [Citation9,Citation10]. Contact between pathogen and PMN also leads to the further activation of the innate immune system via the production of cytokines and chemotactic factors [Citation11]. Cytokines activate PMNs and other cells in the immediate environment, while chemokine production generates a diffusible leukocyte attraction gradient, drawing increasing numbers of immune cells to the site of infection [Citation12]. Stimulated neutrophils activate a programmed antimicrobial response, known as the respiratory burst, leading to the release of significant quantities of toxic oxygen and nitrogen free radicals into the local environment, which can also cause inflammation and damage to the cellular integrity of local tissue [Citation10,Citation13]. The importance of PMNs in host defense is highlighted in studies showing that neutropenic patients [Citation14,Citation15] and those with peroxidase-deficiency are found to be highly susceptible to invasive C. albicans infections [Citation16,Citation17].
The primary point of interaction and recognition between the host immune system and C. albicans occurs at the interface of the outer cell wall of the fungus and the receptor proteins in the leukocyte membranes. Therefore the outer fungal cell wall has an important role to play in defining the outcomes of such exchanges. The composition and structure of fungal cell wall is highly regulated, but the major components in C. albicans consist of a rigid, inner skeletal layer of chitin and inter-linked β 1,3- and β 1,6- glucan to which a flexible mannoprotein matrix is attached covalently to β-glucans and chitin predominantly via glycosylphosphatidyinositol (GPI) anchors [Citation11,Citation18,Citation19]. Protein mannosylation occurs in two major forms, N- and O-linked, and many of the key glycosyl transferases have been highly studied in the model yeast Saccharomyces cerevisiae [Citation20–22] and in C. albicans [Citation23–26].
N-linked mannans are characterized by a conserved [mannose]8 [N-acetylglucosamine]2 core structure (numbers in subscript indicating repeating units), appended to more structurally diverse outer chains (). In C. albicans the outer chains consist of a α-(1,6)- mannose backbone to which are attached side chains consisting of α-(1,2)- β-(1,2)- and α-(1,3)- linked mannose and phosphomannose branches [Citation27]. C. albicans N-linked and O-linked mannans have been shown to be important for adhesion and other virulence traits and for the induction of the innate immune response in the host, including the production of pro-inflammatory cytokines and anti-mannan antibodies via the stimulation of a number of immune cell types [Citation28–30].
Fig 1. The structure of N-mannan (including phosphomannan) and O-linked mannan of C. albicans. The different glycosyl linkages are shown as different colored hexagons and the point of truncation of the various mutants used in this study is indicated by arrows. The extent of truncation of the N-mannan α-1,6 mannose backbone in the pmr1Δ mutant is not fixed, and does not remove all of the outer chains, as in the och1Δ mutant. See references [Citation23–26].
![Fig 1. The structure of N-mannan (including phosphomannan) and O-linked mannan of C. albicans. The different glycosyl linkages are shown as different colored hexagons and the point of truncation of the various mutants used in this study is indicated by arrows. The extent of truncation of the N-mannan α-1,6 mannose backbone in the pmr1Δ mutant is not fixed, and does not remove all of the outer chains, as in the och1Δ mutant. See references [Citation23–26].](/cms/asset/a85396d5-0791-4ef3-8c12-89181048ecd0/immy_a_551425_f0001_b.jpg)
C. albicans O-linked mannans are simpler structures, consisting of a linear (unbranched) chain of mannose residues, predominantly α-(1,2)-linked () [Citation26,Citation31]. O-linked mannans are attached to cell wall proteins via serine or threonine residues that are frequently clustered in domains that correspond to the rod-like stems of glycoproteins that present a globular N terminus towards the extracellular face of the wall [Citation22]. C. albicans strains deficient in O-mannan synthesis were found to be attenuated in virulence [Citation26,Citation32] and adhesion to epithelial cells [Citation33,Citation34], displayed altered cytokine stimulation profiles and were less sensitive to killing by neutrophils [Citation35].
To investigate the importance of C. albicans cell wall N- and O-linked glycosylation on the fungus-human PMN interaction, we used a series of C. albicans strains defective in different cell wall glycosylation components. The host-fungus interaction was characterized by quantifying the proportions of C. albicans bound or engulfed by the PMNs, the percentage of fungal cells killed following co-incubation, and PMN activation by measurement of supernatant myeloperoxidase and IL-8. Additionally, the sensitivity of C. albicans glycosylation-defective strains to PMN-derived anti-microbial compounds was investigated.
Materials and methods
Strains, media and culture conditions
All strains used in this study are listed in . Strains were maintained as frozen glycerol stocks and grown in Sabouraud broth (1% mycological peptone, 4% glucose) for 16 h at 30°C with shaking at 200 rpm. For the preparation of co-incubation inocula, above cultures were diluted 1:100 into 50 ml fresh sterile Sabouraud broth and incubated at 30°C with shaking at 200 rpm until the OD600 reached 0.6. The cells were then washed three times in sterile phosphate-buffered saline (PBS, pH 7.5), and resuspended to a final concentration of 1 × 108 cells/ml in PBS. Prior to use, an aliquot of the required number of cells was labeled with 0.1 mg/ml fluorescein 5-isothiocyanate (F2502, Sigma-Aldrich, UK) in 0.05 M carbonate-bicarbonate buffer (C3041, Sigma-Aldrich, UK), for 15 min at room temperature with continual agitation. Cells were then washed three times with sterile PBS and used immediately in experiments with C. albicans cells.
Table 1 Candida albicans strains used in this study.
Preparation of human PMNs
Human PMNs were isolated from freshly isolated, EDTA-anticoagulated peripheral venous blood of healthy human volunteers [Citation36]. Briefly, whole blood was diluted 1:2 in sterile PBS and 35 ml aliquots dispensed into 50 ml polypropylene tubes. Gently, 15 ml of Ficoll-Paque PLUS (17-1440-02, GE Healthcare, UK) was layered under the whole blood by sterile Pasteur pipette. Tubes were centrifuged at 400 g for 20 min at room temperature. The overlying plasma layer was carefully aspirated and centrifuged at 1500 g to generate platelet-poor plasma (PPP). The lymphocyte and Ficoll-Paque PLUS layers were aspirated and discarded. The pellet, containing erythrocytes and PMNs was lysed using RBC lysis buffer (420301, Biolegend, USA) according to the manufacturer's instructions. The resulting PMN pellet was washed twice in sterile PBS at room temperature, with centrifugation at 400 g and finally resuspended in pre-warmed RPMI-1640 medium supplemented with 10% donor's PPP. Neutrophils were examined by light microscopy and cell viability was established as >98% by Trypan Blue dye exclusion [Citation37]. All tests were completed within 5 h of PMN isolation.
As required aliquots of 1 × 106 PMNs were labeled with anti-CD13.PE-Cy5 (A07763, Beckman Coulter, USA) according to the manufacturer's instructions for 30 min at room temperature in the dark. After two washes in sterile PBS with centrifugation at 400 g the cells were ready for use.
Phagocytosis assay
A sample of 3 × 106 washed FITC-labeled C. albicans cells were co-incubated with 1 × 106 freshly isolated, anti-CD13.PE-Cy5 labeled human PMNs (effector: target ratio 3:1), and co-incubated in RPMI-1640 medium supplemented with 10% donor's own serum for 30 min at 37°C with continuous shaking. Cells were harvested by centrifugation at 500 g, resuspended and fixed in 500 μl ice-cold PBS with 40 mM EDTA and 1% (v/v) paraformaldehyde, and stored on ice.
Killing assay
Samples of 3 × 106 FITC-labeled C. albicans cells were co-incubated with 1 × 106 freshly isolated, anti-CD13.PE-Cy5 labeled human PMNs (effector: target ratio 3:1), and co-incubated in RPMI-1640 medium supplemented with 10% donor serum for 150 min at 37°C with continuous shaking. No hypha formation was observed inside the neutrophils by this time, thus no killing of neutrophils occurred due to hypha formation within the phagolysosome. Fungal cells were harvested by centrifugation at 1500 g, and the supernatant discarded. Hypotonic lysis of the PMNs (pure water, 1 min at room temperature) was followed by three washes in sterile PBS. Finally the released C. albicans cells were resuspended in 1% paraformaldehyde in PBS, containing 4 μg/ml propidium iodide (PI). The percentages of dead cells with PI-stained nuclei were quantified by fluorescence microscopy. A minimum of 3 groups of 100 cells were counted for each strain and the experiment repeated for a total of six independent biological replicates. Controls in the absence of PMNs showed 0% killing, therefore all killing was ascribed to the activities of the PMNs. For fluorescence images on PMNs with attached and phagocytosed C. albicans cells a Zeiss Axioplan 2 microscope (63 × magnification) with the Hamanatsu C4742-95 digital camera was used and images were analyzed with Openlab software V4.0.4.
Flow cytometry analysis of phagocytosis
Flow cytometry analysis of the samples was carried out using a LSRII flow cytometer (Becton Dickinson, Heidelberg, Germany), with computer-assisted evaluation of the generated data by FACS Diva software (Becton Dickinson, Heidelberg, Germany). The fungal cell wall dye Calcofluor White (CFW) was added to a final concentration of 10 μg/ml immediately prior to flow cytometry, allowing distinction between bound and engulfed C. albicans cells. In principle, internalized fungal cells are encapsulated by the intact cell membrane of the PMN, thereby preventing their staining by CFW. Free, suspended C. albicans cells or cells adhered to the outside of the neutrophil are stained by the fluorophore and were detected via the Pacific Blue channel.
Cytokine and chemokine assays for PMN activation
Supernatants collected following co-incubation of PMNs and C. albicans cells were assayed for markers of PMN activation. Simultaneous screening of 36 cytokines, chemokines and other secretory proteins was carried out using a human cytokine profiler kit (R&D Systems UK, Cat# ARY005) according to the manufacturer's instructions. The cytokine profiler data, in addition to conventional sandwich ELISA-based analysis confirmed evidence from other groups showing that activated neutrophils release the respiratory burst associated proteins myeloperoxidase and lactotransferrin (LTF) into the environment when stimulated [Citation10]. IL-8 secretion was found to be a consistent marker of PMN activation in this study. Quantification of IL-8 and MPO markers was carried out according to the manufacturer's instructions using commercially available sandwich ELISA-based assays (IL-8 – R&D Systems, Cat# DY208, MPO – Calbiochem, Cat# 475918).
Results
Flow cytometric analysis of attachment and phagocytosis of C. albicans by PMN cells
To characterize the effects of changing glycosylation status upon host:fungus interaction, we challenged purified human PMNs with a range of glycosylation-defective strains of C. albicans. Incubations were carried out for 30 min to evaluate glycosylation effects on adherence and phagocytosis. In the following description, we take ‘association’ to mean all Candida cells co-sorting with PMNs following the incubation period (including adhered and phagocytosed subsets), whilst ‘attached’ or ‘adhered’ cells refer to subsets of the associated cells bound to the PMN cell surface, undergoing phagocytosis. Various subsets of C. albicans yeast cells that were interacting with PMNs were distinguished in the analysis. An ‘association’ indicates C. albicans cells that co-sort with PMNs and includes adhered and phagocytosed yeast cells. Yeast cells that were ‘attached’ or ‘adhered’ refers to subsets of associated cells that were bound to the PMN cell surface but were not phagocytosed while ‘engulfed’ refers to C. albicans cells that had been phagocytosed. The strains used in this study are shown in and in . Briefly, the och1Δ strain lacks the branched, outer part of the N-glycan structure [Citation23], the pmr1Δ strain has a truncated O-glycan and N-glycan structure [Citation38], the mnt1Δ/mnt2Δ strain contains only a single O-linked mannose residue [Citation26] and the mnn4Δ strain lacks cell wall phosphomannan including the acid-labile β-1,2 mannan fraction [Citation24]. C. albicans serotype B strain A9 differs from the serotype A strains in being deficient in β-1,2 mannose in the acid stable component of the N-linked mannan [Citation39]. Serotype A strains include the SC5314 strain that is the genetic background for all the mutants used in this study.
Evaluation of FACS assay that measured the binding and uptake of C. albicans by neutrophils was also validated by fluorescence microscopy. Two sub-populations of C. albicans cells could be distinguished using FACS (). C. albicans cells association with PMNs was expressed as a function of FITC fluorescence versus CFW fluorescence (measured on the Pacific Blue channel). C. albicans cells in quadrant Q1-2 (FITC+, CFW-) represent engulfed cells whilst those in quadrant Q2-2 (FITC+, CFW +) represent cells attached to the PMN surface (). Engulfed and adhered (non-engulfed) cells from each of the sub-populations were also visualized by fluorescence microscopy () and corresponded to cells within the various FACS quadrants. Empty PMNs were excluded from the plot in , however they were visualized by fluorescence microscopy demonstrating positive α-CD13 antibody selection of the target immune cell type (). These data together demonstrate the robustness of the FACS technique in characterizing adherence and phagocytosis of C. albicans cells by human PMNs.
Fig 2. FACS assay for binding and phagocytosis and validation confocal fluorescence microscopy. Test co-incubation samples of C. albicans and PMNs (ratio 3:1) were analyzed by FACS (SI Materials and Methods – online only). (A) A scatter-graph of PMN associated-C. albicans cells plotted as a function of FITC versus CFW fluorescence intensities. Two sub-populations of fungal cells are resolved, corresponding to engulfed (quadrant Q1-2) and adhered (quadrant Q2-2) C. albicans cells. PMNs not associated with C. albicans cells were excluded from the analysis by FACS software. (B) Confocal fluorescence microscopic images of representative sorted cells from quadrants Q1-2 and Q2-2 validating the FACS analysis and gating strategy. Engulfed, FITC-labeled C. albicans cells are visible in the upper-left quadrant, whilst PMNs with adhered fungal cells stained with the cellwall specific dye CFW are shown in the upper-right quadrant. PMNs not associated with C. albicans cells were sorted and visualized (lower-left quadrant), thereby validating the selection of the PMN-specific antibody.
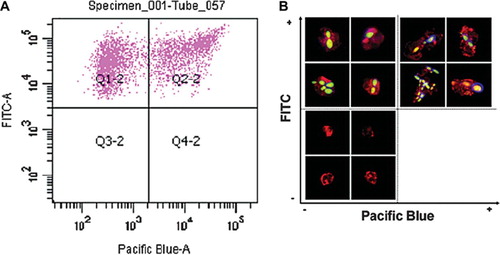
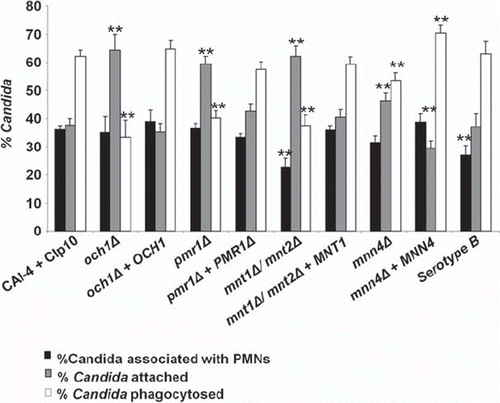
Flow cytometry was used to investigate the adherence and engulfment of C. albicans cells by human PMN following co-incubation for 30 min at 37°C. The gating strategy employed to analyze the flow cytometry data is described in the Online Supplementary Information (SI): Materials and Methods. After 30 min co-incubation, approximately 36 ± 2% (mean ± SE – and in all cases below) of the control C. albicans cells were co-sorted with PMNs (). The mnt1Δ/mnt2Δ and C. albicans Serotype B strains, had reduced association with PMN cells (23 ± 3 and 27 ± 3%, [P < 0.01 and P < 0.05] respectively) suggesting that O-mannan or β 1,2 linked mannan was implicated in PMN recognition and binding. PMN cells were attenuated in their ability to engulf N- and O-glycosylation-defective C. albicans strains (). Analysis of the flow cytometry data showed that 62 ± 2% of the cells of the CAI-4 + CIp10 control strain bound to PMNs were engulfed within 30 min co-incubation. In contrast, only 33 ± 6% of och1Δ, 40 ± 3% of pmr1Δ, and 38 ± 4% of mnt1Δ/mnt2Δ cells were engulfed (P < 0.01) (). Consequently, the reduced engulfment of mutant strains resulted in an accumulation of attached (adhered), non-engulfed C. albicans cells at the PMN surface. In turn the percentage of adhered C. albicans cells rose from 38 ± 2% in the control (CAI-4 + CIp10) strain to 64 ± 6% (och1Δ), 60 ± 3% (pmr1Δ), and 62 ± 4% (mnt1Δ/mnt2Δ) for the glycosylation-defective mutants respectively. Complementation of the deleted genes in all cases reverted the ratio of engulfed:adhered C. albicans to that resembling the control strain. The mnn4Δ strain lacking N-linked cell wall phosphomannan exhibited a relatively minor reduction in engulfment (53 ± 3%) in comparison to the other strains tested, although this was still significantly less than the control strain (). Likewise, the percentage of mnn4Δ cells adhered to the PMNs increased slightly but significantly with respect to the control strain (up to 46 ± 3%). Complementation of the mutant with the wild type gene restored the control profile of engulfed and adhered C. albicans cells to control levels although the mnn4Δ reintegrant strain, which contained multiple MNN4 alleles was more readily engulfed than the wild type (). The C. albicans Serotype B strain that lacked acid-stable β 1,2-linked mannose residues in the N-glycan outer chain had little difference in adherence and uptake into neutrophils (). Therefore, the cell wall glycosylation status (alterations in N- and O-linked glycosylation and phosphomannosylation) but not changes in the β 1,2 mannose content, reduced the engulfment of adherent fungal cells by human PMNs.
Fig 3. Analysis of binding and phagocytosis of C. albicans glycosylation-deficient mutant strains by FACS. Bars indicate the percentage of C. albicans cells associated with, bound to, or engulfed by human PMNs following 30 min of co-incubation at 37°C. The solid bars represent the percentage of C. albicans yeast cells (as a proportion of the total C. albicans cells counted) co-sorting with PMNs. This reflects differences in the recognition and binding of the various C. albicans strains by PMNs. Grey bars show the percentage of C. albicans yeast cells adhered to versus those engulfed by PMNs (subsets of the C. albicans yeast cells co-sorted with PMNs) and represent a measure of the relative ability of PMNs to phagocytose the C. albicans yeast cells. The open bars represent the proportion of associated cells that had been phagocytosed at this time point. Plotted values are the mean ± standard error of triplicate experi ments using PMNs from six independent donors. Statistical significance was carried out by a student's t-test (vs the value for the control strain in each case): **P < 0.05.
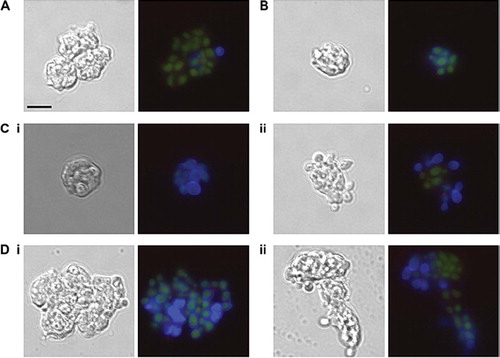
The effect of glycosylation status of C. albicans phagocytosis by purified anti CD13-PE-CY5-labeled human PMNs was validated independently by microscopy. PMNs were co-incubated with FITC-labeled C. albicans glycosylation mutants. After fixation, the C. albicans cell wall dye, Calcofluor White, was added immediately prior to microscopy to distinguish between C. albicans cells adhered to the PMN surface and those that had been internalized through phagocytosis. Prolonged exposure to the fixation solution (1% paraformaldehyde, 40 mM EDTA) resulted in non-specific staining of the PMN nuclei by Calcofluor White, presumably due to increased permeability of the cell membrane. Therefore, to ensure that any non-specific staining did not interfere with FACS analysis, samples were analyzed minutes after fixation when no non-specific CFW staining was observed. The majority of the CAI4-CIp10 (wild type control strain) cells associated with PMNs fluoresced only under the FITC filter, suggesting that they had been phagocytosed and were not exposed on the PMN surface. However, the C. albicans strains that were defective in cell wall glycosylation fluoresced under both the FITC and Pacific Blue channels, indicating that the majority of C. albicans cells had adhered to the PMN surface, but that the PMNs were unable to phagocytose the cells. Reintroduction of a single copy of the desired gene again restored the ability of the PMNs to phagocytose the C. albicans strain to almost wild type levels, confirming that the glycosylation status of C. albicans is important for phagocytosis ().
Fig 4. Glycosylation status of the C. albicans cell wall is important for phagocytosis by neutrophils. FITC-labeled C. albicans cells (3 × 106) were incubated with purified anti-CD13-PE-Cy5 labeled neutrophils (1 × 106) at 37°C for 30 minutes in RPMI medium supplemented with 10% donor's plasma. Cells were fixed with 1% paraformaldehyde and 40mM EDTA and 10 μg/ml Calcofluor White added 1 minute prior to microscopy. (A) CAI4 + CIp10, (B) Serotype B, (C) (i) Δmnn4 (ii) Δmnn4 (i) +MNN4, (D) (i) Δoch1 (ii) Δoch1 + OCH1. Note in (D) (i) that the FITC-stained yeast cells have a blue Calcofluor White-stained halo indicating they are on the PMN surface, while in (D) (ii) the FITC-stained cells are phagocytosed and do not stain with Calcofluor White. Scale bar represents 10 μm.
Comparison of killing of C. albicans glycosylation-defective mutant strains by PMN cells
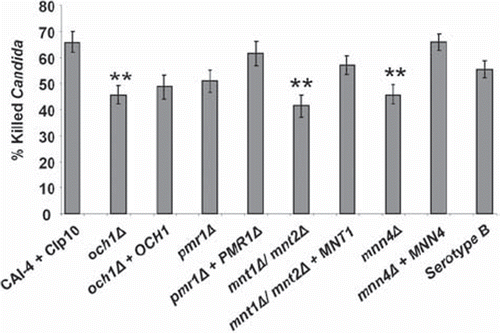
Next we assessed whether changes in the fungal cell-wall glycosylation status altered susceptibility to killing by PMNs. The sensitivity of C. albicans cell-wall glycosylation-deficient strains to PMN-mediated damage was investigated using phagocytosis-dependent and phagocytosis-independent killing assays. For the former, C. albicans cells were subjected to an extended co-incubation period of 150 min to assay the killing efficiency of PMNs with respect to fungal glycosylation status. Analysis of propidium iodide stained fungal cells showed that 66 ± 4% of the control fungal cells were killed in this period (). Glycosylation defects reduced the proportion of fungal cells killed by PMNs. Only 46 ± 4% of och1Δ, 51 ± 4% of pmr1Δ, 41 ± 4 % of mnt1Δ/mnt2Δ and 46 ± 4% of mnn4Δ cells were killed (P < 0.05 in all cases, except for pmr1Δ strain, where P = 0.15) (). Complementation of the deleted genes restored some measure of susceptibility to killing, although this was not always to control levels. A positive correlation existed between the percentage of dead C. albicans cells and the percentage of cells that had been engulfed (Pearson's correlation coefficient, [r] = +0.71).
Effects of glycosylation upon phagocytosis-independent killing of C. albicans by PMNs
PMNs can kill C. albicans by several mechanisms and reciprocally the ability of C. albicans to reduce PMN viability may be affected by the presence of other microorganisms and antigens. PMNs can ingest and digest pathogens but also release of large quantities of enzymes and toxic free-radicals into the environment, causing damage and death to non-ingested pathogens in the vicinity of the immune cells [Citation10]. Here we investigated the viability of PMNs in the presence and absence of bacterial cells or bacterial LPS and the effect of PMN supernatants on the viability of C. albicans.
First, we investigated the effect of 30 min co-incubation with LPS, two bacterial and one C. albicans glycosylation-deficient strain on PMN viability by Trypan Blue exclusion. Stimulation by LPS, heat-killed Pseudomonas aeruginosa and heat-killed Escherichia coli resulted in a higher proportion of dead PMNs (58 ± 10%, 40 ± 11% and 45 ± 5% respectively), as compared to pre-stimulation with any of the C. albicans strains CAI-4 (28 ± 3%), pmr1Δ (29 ± 1%) and pmr1Δ + PMR1 (37 ± 7%) respectively (SI Fig. S2 – online only). Therefore, more PMNs were killed following co-incubation with bacterial cells and LPS as compared to following co-incubation with the C. albicans strains tested.
Next, we investigated whether PMNs exposure to wildtype and C. albicans mutant strains would affect the composition and quantity of microbicidal compounds released into the cell-culture supernatant (Supplementary Materials and Methods: Supplementary method 2 – online only). The results in SI Table S1 indicate that following the 30 min pre-incubation period, fungal cells generally elicited higher levels (20–25 ng/ml) of MPO production by PMNs as compared to LPS (9 ± 1 ng/ml), P. aeruginosa (13 ± 2 ng/ml) and E. coli (12 ± 4 ng/ml). IL-8 induction was similar irrespective of the stimulus (∼16 ± 2 ng/ml) ). The efficacy of these cell-culture supernatants to kill C. albicans was characterized by co-incubation with fungal cells over 150 min.
Fig 5. C. albicans killing assay. Bars show the percentage of the tested C. albicans killed following 150 min co-incubation with PMNs at 37°C. Following incubation, PMNs were lysed by hypotonic shock. The remaining C. albicans were washed and stained with 4 μg/ml propidium iodide to stain exposed nucleic acid in dead and terminally damaged cells. A minimum of 100 cells of each strain were counted and the percentage of dead (PI+) cells calculated. Plotted values are the mean ± standard error of six experiments with independent donors, in which each combination was tested in triplicate. Statistical significance was carried out by a student's t-test (vs the value for the control strain in each case): **P < 0.05.
The percentage of dead C. albicans cells following 150 min incubation with cell culture supernatants from pre-stimulated PMNs showed no statistically significant difference, with respect to the pre-stimulant. All the C. albicans strains tested in this assay were equally sensitive to supernatant from pre-stimulated PMNs (42 ± 8% killed C. albicans cells) (SI Fig. S2 – online only). Supernatant concentrations of MPO and IL-8 did not change during co-incubation with fungal cells (SI Table S1 – online only). Yeast strains of the pmr1Δ mutant showed similar sensitivity to cell damaging agents released by activated neutrophils when compared to their wildtype control.
PMN stimulation by cytokine and chemokine analysis
Activated PMNs are able to secrete a variety of cytokines and chemokines into their immediate environment, resulting in autoinduction and the generation of chemoattractant gradients. The activation of PMNs in response to stimulation by glycosylation-defective C. albicans strains was characterized by quantifying supernatant levels of secreted markers of activation. Initial testing using the R&D Systems proteome profiler cytokine assay kit (R&D Systems UK, catalog # ARY005), which has been effective in the analysis of cytokine release from monocytes [Citation40] and T-cells [Citation41], identified IL-8, MPO and LTF as being upregulated. Supernatant levels of IL-8, IL13, macrophage migration inhibitory factor (MIF), macrophage inflammatory protein-1α (MIP1α), granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF) and complement component C5a, were found to be elevated with respect to the control, with C5a, IL-8 and MIF among the highest induced (not shown).
PMN cell activation was characterized by investigating the production of myeloperoxidase (MPO) and the cytokine IL-8 (CXCL8) in cell culture supernatant following 30 min co-incubation with C. albicans strains (LTF was not studied in detail as similar to MPO it is a marker of the respiratory burst). MPO catalyses the formation of hypochlorous acid and other toxic reactive oxidants following neutrophil activation, and has been shown to play a key role in the control of C. albicans infection by PMNs [Citation17,Citation42,Citation43]. IL-8 is produced by a variety of human immune cells including neutrophils, and is a potent stimulant of PMN cells [Citation44,Citation45]. There was a basal level of MPO production (8 ± 2 ng/ml) following PMN purification, as evidenced by the T = 0 sample ().
Fig 6. Myeloperoxidase and IL-8 quantification in C. albicans-PMN supernatants. The plot shows MPO and IL-8 production in cell culture supernatant following the co-incubation of PMNs (106 cells) with C. albicans strains (3 × 106 cells) for 30 min at 37°C. MPO and IL-8 concentrations were determined by ELISA assay kits. Controls were cell culture supernatant (RPMI only), pre-incubation supernatant (T = 0 control), and cell culture medium from samples lacking either PMNs (C. albicans only) or C. albicans cells (PMNs only). Plotted values are the mean ± standard error of six experiments with independent donors, in which each combination was tested in triplicate.
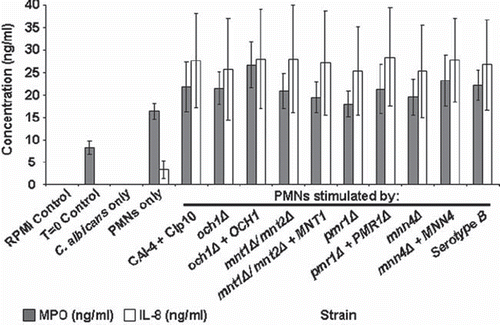
Analysis of the cell culture supernatant of PMNs that had not been exposed to C. albicans resulted in a basal level of MPO production (16 ± 2 ng/ml). Co-incubation with C. albicans resulted in increased levels of MPO with a mean of 22 ± 5 ng/ml. There was no significant difference in the induction of MPO production between the glycosylation-deficient and parent strains of C. albicans tested (). Following co-incubation of PMNs with C. albicans supernatants were assayed and found to contain higher levels of IL-8 (an average of 27 ± 11 ng/ml across the strains tested). However, no significant differences in IL-8 production were found between the mutant and wildtype strains that were tested. Thus PMNs upon exposure to C. albicans produced MPO and IL-8, but this was not dependent on the glycosylation status of the cell wall.
Discussion
C. albicans is the most common cause of invasive nosocomial disease [Citation46,Citation47]. Increased mortality rates and failure to clear infection in neutropenic patients [Citation48] and those with genetic abnormalities related to neutrophil function [Citation49] highlight the critical role played by PMNs in controlling the growth and spread of pathogens. In this study we examined the effects of C. albicans cell wall glycosylation on the binding and phagocytosis by PMNs. We show that O-glycosylation plays a role in the initial association between PMNs and Candida as O-glycan-deficiency resulted in significantly fewer cells associated with PMNs as compared to control and N-glycosylation mutant strains. In addition, loss of cell wall N- and O-glycans results in a reduced capacity of PMNs to phagocytose fungal cells. PMNs were able to phagocytose significantly fewer och1Δ, pmr1Δ, and mnt1Δ/mnt2Δ mutant cells as compared to the control strain. Binding of these mutants was not abolished however, implying that residual cell wall components such as β-glucans also play a role in uptake by PMNs. In such strains a corresponding increase in the percentage of adhered cells was found reflecting the disruption of the engulfment process leading to an accumulation of C. albicans on the PMN cell surface. The data suggest that recognition and uptake of fungal cells by PMNs is a two-step process and suggests that independent receptor-ligand interaction mediated by cell surface mannans may be required, for each stage to be successfully completed. This is in keeping with previous observations for the phagocytosis of apoptotic cells where a two step process for successful phagocytosis has been proposed [Citation36].
Previous work from our laboratory has shown that loss of cell wall phosphomannan had little impact upon C. albicans virulence in a murine model [Citation24]. This study advances the body of information relating to the role of phosphomannan at the fungal cell surface, showing that mutants lacking the epitope are slightly attenuated in their phagocytosis by PMNs. The decrease in phagocytosis was observed to be much greater in N- and O- glycan-deficient mutants as compared to the mnn4Δ strain indicating the relatively minor role of phosphomannan as compared to glycosylation, in the recognition, attachment and phagocytosis by neutrophils. Therefore non-phagocytosis-dependent interactions and adhesion to PMNs is unlikely to be due to charge interactions with PMN in the C. albicans cell wall. This highlights important differences in the phagocytosis of C. albicans between macrophages and PMNs as recent data shows that loss of phosphomannan content profoundly reduces the ability of macrophages to recognize and ingest C. albicans whereas loss of O-mannan enhances phagocytosis [Citation50]. Loss of mannan also results in reduced cytokine production by monocytes [Citation2,Citation51]. The percentage of cells of Candida albicans adhered to and phagocytosed by PMNs was not significantly different in the serotype A and serotype B strains. The serotype B strain bears cell wall mannans containing β1, 2 mannose residues, recently shown to be associated with both N and O mannans [Citation52]. This suggests that β1, 2 mannose residues and their putative ligand, galectin-3 do not appear to be required for PMN binding, phagocytosis or killing.
The dependence of the processes of recognition, phagocytosis and killing of C. albicans by PMN cells upon specific receptor-ligand combinations have been described previously. Lavigne et al. showed that fungal cell wall β-glucan is important for the recognition of adhered C. albicans cells, and is able to stimulate the antimicrobial respiratory burst in PMNs [Citation53]. The importance of complement binding to the C. albicans cell surface was described by Yamamura et al., who showed that complement factor C3 was required for C. albicans killing, but not phagocytosis [Citation54]. Work by Taylor et al. links these two studies by showing that murine dectin-1 (receptor for fungal β-glucans)-deficient neutrophils were found to be impaired in their ability to bind un-opsonized zymosan particles, resulting in a lowered respiratory burst [Citation55]. The use of opsonized zymosan particles or C. albicans cells restored binding, however the dectin-1 deficient PMNs remained attenuated in their ability to generate the respiratory burst. Dectin-1 deficiency resulted in a strong decrease in the ability of PMNs to kill unopsonized C. albicans cells, however, killing was restored with opsonized C. albicans [Citation54]. Purified soluble C. albicans cell wall mannan has also been shown to inhibit the interaction between C. albicans cells and human PMNs, demonstrating the requirement for glycans during attachment, and supports the data from our study [Citation56]. Studies in mice infected with C. albicans showed that TLR2 expression on neutrophils was required for efficient recognition of and chemotaxis towards fungal cells. Additionally, neutrophils from TLR2-/- mice were shown to be attenuated in their phagocytic activity towards C. albicans cells as well as expressing lower levels of nitric oxide and myeloperoxidase [Citation57].
There were no significant differences in the levels of MPO or IL-8 produced by PMNs in the first 30 min following contact with the C. albicans glycosylation mutants tested in our study. Our data confirmed that contact with fungal cells results in activation-dependent secretion of MPO and IL-8 into the culture medium [Citation16,Citation42,Citation58,Citation59]. Co-incubation of C. albicans cells with cell culture supernatant from pre-stimulated PMNs showed that: (i) there was no significant difference between the levels of MPO and IL-8 induced by the control strain and the pmr1Δ glycosylation-deficient mutant; and (ii) there was no significant difference in the percentage of C. albicans killed between the control and glycosylation-deficient strain. These data together suggest that PMNs at least in terms of their secretory profile do not respond specifically to the cell wall glycans of C. albicans cells, instead generating a broad non-specific cellular response to the fungus.
This study is the first to show that ubiquitous cell wall N- and O-linked glycans are involved in the recognition, binding and phagocytosis of C. albicans by human PMNs. Interestingly, studies using human peripheral blood mononuclear cells have found that N-linked glycans are predominantly recognized by the mannose receptor whilst O-glycans bound TLR4 and that these interactions are important for cytokine-stimulating activity by yeast cells [Citation60]. Here we show that the production of chemokines and respiratory burst enzymes by PMNs appears not to be dependent on cell wall mannosylation.
Tables 1 and 2
Download PDF (1.6 MB)Acknowledgements
We thank Héctor Mora-Montes, Gordon D. Brown, and Linda Duncan for helpful discussions and Kevin McKenzie and Raif Yucel for technical advice with imaging and FACS respectively and The Wellcome Trust (080088) for financial support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This paper was first published online on Early Online on 26 January 2011.
References
- Bodey GP, Mardani M, Hanna HA, . The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am J Med 2002; 112: 380–385.
- Odds FC. Candida and Candidosis, 2nd. London, England: Baillière Tindall, 1988.
- Pfaller MA, Messer SA, Houston A, . National epidemiology of mycoses survey: A multicenter study of strain variation and antifungal susceptibility among isolates of Candida species. Diagn Microbiol Infect Dis 1998; 31: 289–296.
- de Rosa FG, Garazzino S, Pasero D, di Perri G, Ranieri VM. Invasive candidiasis and candidemia: new guidelines. Minerva Anestesiol 2009; 75: 453–458.
- Gudlaugsson O, Gillespie S, Lee K, . Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 2003; 37: 1172–1177.
- Ostrosky-Zeichner L, Pappas PG. Invasive candidiasis in the intensive care unit. Crit Care Med 2006; 34: 857–863.
- Pfaller MA, Jones RN, Doern GV, Sader HS, The SENTRY Participant Group. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998. Antimicrob Agents Chemother 2000; 44: 747–751.
- Prado M, d. Silva MB, Laurenti R, Travassos LR, Taborda CP. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem I Oswaldo Cruz 2009; 104: 513–521.
- Lyman CA, Walsh TJ. Phagocytosis of medically important yeasts by polymorphonuclear leukocytes. Infect Immun 1994; 62: 1489–1493.
- Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest 2000; 80: 617–653.
- Netea MG, Brown GD, Kullberg BJ, Gow NAR. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 2008; 6: 67–78.
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature 2007; 449: 819–826.
- Salih HR, Husfeld L, Adam D. Simultaneous cytofluorometric measurement of phagocytosis, burst production and killing of human phagocytes using Candida albicans and Staphylococcus aureus as target organisms. Clin Microbiol Infect 2000; 6: 251–258.
- Maertens J, Vrebos M, Boogaerts M. Assessing risk factors for systemic fungal infections. Eur J Cancer Care 2001; 10: 56–62.
- Martino P, Girmenia C, Venditti M, . Candida colonization and systemic infection in neutropenic patients. A retrospective study. Cancer 1989; 64: 2030–2034.
- Hilal E-M, Fletcher J. Impaired neutrophil function and myeloperoxidase deficiency in myeloid metaplasia. Brit J Haematol 1977; 37: 323–329.
- Lehrer RI, Cline MJ. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest 1969; 48: 1478–1488.
- Klis FM, De Groot P, Hellingwerf K. Molecular organization of the cell wall of Candida albicans. Med Mycol 2001; 39(Suppl. 1): 1–8.
- Reid DM, Gow NAR, Brown GD. Pattern recognition: recent insights from dectin-1. Curr Opin Immunol 2009; 21: 30–37.
- Dean N. Asparagine-linked glycosylation in the yeast golgi. BBA-Gen Subjects 1999; 1426: 309–322.
- Lengeler K, Tielker D, Ernst J. Protein- O -mannosyltransferases in virulence and development. Cell Mol Life Sci 2008; 65: 528–544.
- Strahl-Bolsinger S, Gentzsch M, Tanner W. Protein O-mannosylation. BBA-Gen Subjects 1999; 1426: 297–307.
- Bates S, Hughes HB, Munro CA, . Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J Biol Chem 2006; 281: 90–98.
- Hobson RP, Munro CA, Bates S, . Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J Biol Chem 2004; 279: 39628–39635.
- Mora-Montes HM, Bates S, Netea MG, . Endoplasmic reticulum α-glycosidases of Candida albicans are required for N-glycosylation, cell wall integrity, and normal host-fungus interaction. Euk Cell 2007; 6: 2184–2193.
- Munro CA, Bates S, Buurman ET, . Mnt1p and Mnt2p of Candida albicans are partially redundant α-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J Biol Chem 2005; 280: 1051–1060.
- Cutler JE. N-glycosylation of yeast, with emphasis on Candida albicans. Med Mycol 2001; 39: 75–86.
- Fradin C, Poulain D, Jouault T. Beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun 2000; 68: 4391–4398.
- Han Y, Kanbe T, Cherniak R, Cutler J. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun 1997; 65: 4100–4107.
- Masuoka J. Surface glycans of Candida albicans and other pathogenic fungi: physiological roles, clinical uses, and experimental challenges. Clin Microbiol Rev 2004; 17: 281–310.
- Gow, NAR, Bates S, Brown AJP, . Candida cell wall mannosylation: importance in host-fungus interactions and potential as a target for the development of antifungal drugs. Biochem Soc Trans 1999; 27: 512–516.
- Rouabhia M, Schaller M, Corbucci C, . Virulence of the fungal pathogen Candida albicans requires the five isoforms of protein mannosyltransferases. Infect Immun 2005; 73: 4571–4580.
- Timpel C, Strahl-Bolsinger S, Ziegelbauer K, Ernst JF. Multiple functions of Pmt1p-mediated protein O-mannosylation in the fungal pathogen Candida albicans. J Biol Chem 1998; 273: 20837–20846.
- Timpel C, Zink S, Strahl-Bolsinger S, Schroppel K, Ernst J. Morphogenesis, adhesive properties, and antifungal resistance depend on the Pmt6 protein mannosyltransferase in the fungal pathogen Candida albicans. J Bacteriol 2000; 182: 3063–3071.
- Corbucci, C., E. Cenci, F. Skrzypek, . Immune response to Candida albicans is preserved despite defect in O-mannosylation of secretory proteins. Med Mycol 2007; 45: 709–719.
- McPhillips KA, Erwig LP. Assessment of apoptotic cell phagocytosis by macrophages. Methods Mol Biol 2009; 559: 2347–2356.
- Hanks JH, Wallace JH. Determination of cell viability. Proc Soc Exp Biol Med 1958; 98: 188–192.
- Bates S, MacCallum DM, Bertram G, . Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+ -ATPase, is required for glycosylation and virulence. J Biol Chem 2005; 280: 23408–23415.
- Hazen KC, Singleton DR, Masuoka J. Influence of outer region mannosylphosphorylation on N-glycan formation by Candida albicans: normal acid-stable N-glycan formation requires acid-labile mannosylphosphate addition. Glycobiology 2007; 17: 1052–1060.
- Feld M, Shpacovitch VM, Ehrhardt C, . Agonists of proteinase-activated receptor-2 enhance IFN-γ-inducible effects on human monocytes: role in influenza A infection. J Immunol 2008; 180: 6903–6910.
- Schade AE, Schieven GL, Townsend R, . Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 2008; 111: 1366–1377.
- Aratani Y, Koyama H, Nyui S, . Severe impairment in early host defence against Candida albicans in mice deficient in myeloperoxidase. Infect Immun 1999; 67: 1828–1836.
- Lehrer RI, Cline MJ. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol 1969; 98: 996–1004.
- Fradin C, Mavor AL, Weindl G, . The early transcriptional response of human granulocytes to infection with Candida albicans is not essential for killing but reflects cellular communications. Infect Immun 2007; 75: 1493–1501.
- Schaller M, Boeld U, Oberbauer S, . Polymorphonuclear leukocytes (PMNs) induce protective Th1-type cytokine epithelial responses in an in vitro model of oral candidosis. Microbiology 2004; 150: 2807–2813.
- Dimopoulos G, Ntziora F, Rachiotis G, Armaganidis A, Falagas ME. Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: differences in risk factors and outcome. Anesth Analg 2008; 106: 523–529.
- Leroy O, Gangneux J, Montravers P, . Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 2009; 37: 1612–1618.
- Betts R, Glasmacher A, Maertens J, . Efficacy of caspofungin against invasive Candida or invasive Aspergillus infections in neutropenic patients Cancer 2006; 106: 466–473.
- Cohen MS, Isturiz RE, Malech HL, . Fungal infection in chronic granulomatous disease: the importance of the phagocyte in defence against fungi. Am J Med 1981; 71: 59–66.
- McKenzie CGJ, Koser U, Bain JM, . Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun 2010; 78: 1650–1658.
- Gow NAR, Netea MG, Munro CA, . Recognition of Candida albicans β-glucan by dectin-1 induces cytokines and has non-redundant effects on the activation of innate immunity. J Infect Dis 2007; 196: 1565–1571.
- Fradin C, Slomianny MS, Mille C., . β-1,2 oligomannose adhesin epitopes are widely distributed over the different families of Candida albicans cell wall mannoproteins and are associated through both N- and O-glycosylation processes. Infect Immun 2008, 76: 4509–4517.
- Lavigne LM, Albina JE, Reichner JS. Beta-glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J Immunol 2006; 177: 8667–8675.
- Yamamura M, Valdimarsson H. Participation of C3 in intracellular killing of Candida albicans. Scand J Immunol 1977; 6: 591–594.
- Taylor PR, Tsoni VS, Willment JA, . Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol 2007; 8: 31–38.
- Diamond RD, Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro.J Clin Invest 1978; 61: 360–369.
- Tessarolli V, Gasparoto TH, Lima HR, . Absence of TLR2 influences survival of neutrophils after infection with Candida albicans. Med Mycol 2009; 25: 1–12
- Ali A, Rautemaa R, Hietanen J, . Expression of interleukin-8 and its receptor IL-8RA in chronic hyperplastic candidosis. Oral Microbiol Immunol 2006; 21: 223–230.
- Koziol-Montewka M, Magrys A, Paluch-Oles J, . MPO and cytokines in the serum of cancer patients in the context of Candida colonization and infection. Immunol Invest 2006; 35: 167–179.
- Netea MG, Gow NAR, Munro CA, . Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and toll-like receptors. J Clin Invest 2006; 116: 1642–1650.