Abstract
Objective A number of learned societies, including the International Menopause Society, have produced position statements pertaining to the use of postmenopausal hormone therapy. These documents are highly informative but are not designed for use by primary-care physicians and nurse practitioners during routine consultations. Our aim was to produce a toolkit for practitioners that could be used during office consultations to assist them in the assessment and management of the menopause.
Methods We used clinical experience in primary care, combined with published diagnostic algorithms, positions statements from learned medical societies and relevant peer-reviewed literature to develop assessment and management algorithms relevant to the primary care of women age 40 years and older.
Results The resultant ‘Practitioner’s Toolkit for Managing the Menopause’ comprises algorithms for the reasons why a woman might present, determination of menopausal status, key information that should be ascertained, issues that may influence treatment decision-making, hormonal and non-hormonal treatment options, symptom management and patient review, and a brief supporting document.
Conclusions We believe these algorithms and supporting document provide an accessible desktop tool for health-care practitioners caring for women at midlife. The toolkit has been endorsed by the International Menopause Society for global use.
Key words::
INTRODUCTION
Several position statements and consensus statements pertaining to postmenopausal hormone therapy and the management of the menopause have been publishedCitation1–5. These have been based on detailed literature reviews and expert opinion, and provide evidence-based clinical practice guidance. However, a simple assessment and decision-making tool for use by primary health-care practitioners is lacking. Therefore our aim was to develop such a tool that started at the point a woman aged 40 years or more walked in the door.
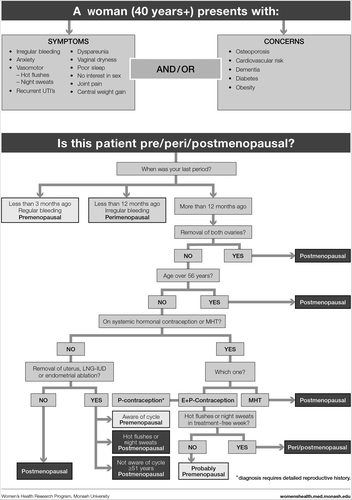
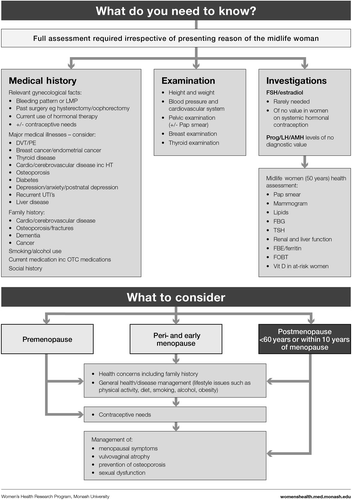
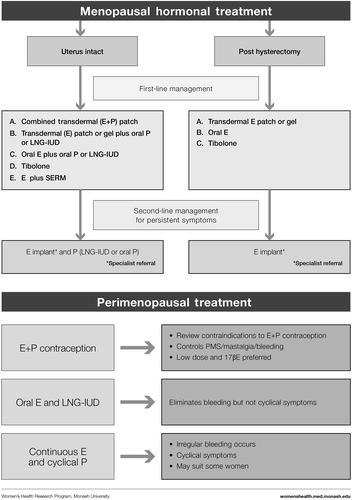
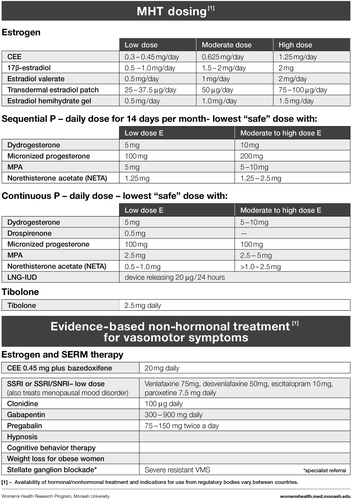
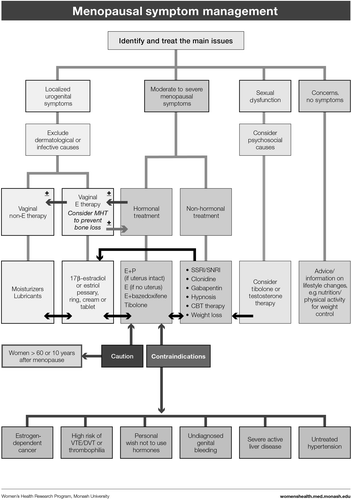
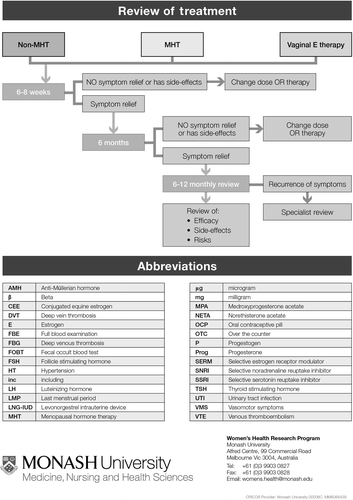
Based on clinical practice experience, our first step was to address the reasons why a woman might present, that is, her symptoms or concerns (). We then followed with the menopause staging decision tool (), a pragmatic algorithm developed to accommodate women who may have amenorrhea due to hysterectomy, endometrial ablation or hormonal contraception, and settings in which hormonal levels cannot be measured (due to cost or remoteness)Citation6. The assessment (), treatment options ( and ) and symptom management algorithms ( and ) are derived from the published literature. The included therapies are comprehensive to enable global application, with the caveat that the availability of the included hormonal and non-hormonal treatments, and indications for their use by regulatory bodies, vary between countries.
This manuscript includes the assessment and management algorithms that can be assembled into a folded desktop reference and the brief supporting text. To our knowledge, this is the first clinical practice tool for the management of the menopause in primary care that has international application.
A PRACTITIONER’S TOOLKIT FOR MANAGING THE MENOPAUSE
Supporting notes
The menopause is a normal biological event that affects all women. Each woman experiences and views the menopause transition and their postmenopausal years differently. The experience will be influenced by the age at which menopause occurs, why it occurs (surgical versus natural), the health and well-being of the individual, and their ethnicity, environment and culture.
Treatment will be determined by symptom severity, weighing of benefits and risks, and each woman's personal expectations.
DEFINITIONS
Menopause is the permanent cessation of menstruation in a non-hysterectomized woman. As a woman with an intact uterus may have stopped menstruating for a range of reasons, such as having had an endometrial ablation or a hormonal intrauterine device (IUD) inserted, a more pragmatic approach is to define menopause as the permanent cessation of ovarian function.
The average age of natural menopause has been reported as being at 51.5 years in developed countriesCitation7. In general, it occurs between the ages of 45 and 55 years.
The perimenopause is the time from the onset of cycle irregularity through until 12 months after the menstrual period.
Surgical menopause is the removal of both ovaries.
Primary ovarian insufficiency (POI) is cessation of ovarian function before the age of 40 years.
Factors associated with earlier menopause include hysterectomy, smoking, lower level of education, living at an altitude above 2000 m and being single. Factors that have been associated with a later menopause include parity, higher body mass index (BMI) and oral contraceptive useCitation7–9.
BASIC PHYSIOLOGY
Menopause occurs because the ovaries run out of eggs. The basic reproductive unit of the ovary is the ovarian follicle. Each ovarian follicle contains a single oocyte. A female infant at birth has approximately 300 000 ovarian follicles. By approximately 37 years of age, this number is depleted to about 25 000, and at menopause few/none remain.
Loss of ovarian follicles is associated with diminished estradiol (E2) and ovarian inhibin production, and increased production of pituitary follicle stimulating hormone (FSH). Loss of follicles also results in a fall in the production of anti-Müllerian hormone (AMH). AMH is produced by developing ovarian follicles. When follicle numbers decline, AMH levels fall. Hence, AMH levels decline with age. Measurement of AMH is useful in predicting ovarian response to ovulation induction (low level predicts a poor response).
Changes in FSH, E2, inhibin B and AMH may precede or coincide with the development of menstrual irregularity or symptoms.
The stages of menopause have been classified by the Stages of Reproductive Aging Workshop (STRAW), most recently updated as STRAW + 10Citation10. Using this guide, the following phases are characterized as follows.
Late reproductive phase
Changes in menstrual cycle flow/length;
FSH, E2 variable;
AMH, inhibin B low;
Some women develop intermittent symptoms.
Menopause transition (perimenopause)
Increased cycle variability;
FSH increased;
E2 variable;
AMH, inhibin B low;
Symptoms more likely.
Postmenopause
Cessation of menstruation;
FSH elevated;
E2, AMH, inhibin B low, progesterone continually low;
Symptoms much more likely.
Androgens and the menopause
Androgens are produced by the adrenal cortex and the ovaries. Circulating blood levels of total and free testosterone, dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS) and androstenedione decline with age, with the decline commencing in the late reproductive yearsCitation11,Citation12.
There is no acute change in androgens across the natural menopauseCitation12,Citation13.
Surgical menopause is associated with a significant reduction in testosteroneCitation12,Citation14 and lower androgens have been reported in women with POICitation15,Citation16.
HOW TO DIAGNOSE MENOPAUSE
The diagnosis of menopause is mostly straightforward if a woman is over the age of 45 years and reports cessation of menstruation for over 12 months, with or without symptoms ().
If a woman has had a hysterectomy and has classic menopausal symptoms, treatment can be instituted without a firm diagnosis, as menstrual bleeding is not an issue if estrogen is to be prescribed.
Challenging situations, in terms of diagnosis, include women who have had an endometrial ablation, have a progestin-releasing IUD, are using systemic hormonal contraception (estrogen plus progestin oral, transdermal or vaginal ring or implanted/depot progestin) or have symptoms and are less than 45 years old.
Measurement of hormones, when and why
Most women do not need to have any hormonal tests done to diagnose menopause ().
Progestogen IUD in situ – can usually be treated with estrogen if symptomatic without any diagnostic blood tests needed.
Endometrial ablation – still need to prescribe progestogen protection for the endometrium. It is appropriate to institute treatment if the woman is symptomatic without any hormonal tests.
For women using systemic hormonal contraception – hormonal tests are completely uninformative as ovarian function is suppressed. The only way to ascertain a woman's menopausal status while using systemic hormonal contraception is to cease usage.
Hormone measurement may be useful for:
Women with subtle/fluctuating symptoms, for example predominant mood change and few vasomotor symptoms. Of note, a single observation of normal FSH and estradiol does not exclude perimenopause as hormone levels may fluctuate at this time.
Women younger than 45 years old.
Hormone measurements are required:
To diagnose POI – diagnosis requires FSH to be elevated and E2 to be low on at least two occasions at least 4–6 weeks apart. Other investigations are usually indicated once POI is diagnosed.
Other biochemical investigations based on clinical assessment
Exclude other causes of amenorrhea in younger women
– pregnancy
– hyperprolactinemia
– thyroid disease
– hypothalamic amenorrhea (anorexia nervosa, etc.)
Exclude other common causes of fatigue, mood change, hotness
– thyroid disease – thyroid stimulating hormone (TSH)
– iron deficiency – hemoglobin/iron stores
– type 2 diabetes – fasting blood glucose
Consider whether fasting lipids, vitamin D measurement required.
PERIMENOPAUSAL SYMPTOMS
Because hormone levels fluctuate during the perimenopausal years, women might present with symptoms of relative estrogen excess, estrogen deficiency or both. Typical estrogen excess symptoms include breast tenderness, menorrhagia, migraine, nausea, shorter cycle length and a shorter follicular phaseCitation17.
THE SYMPTOMS OF THE MENOPAUSE
There is substantial variability between women in the symptoms that occur due to the hormonal changes at menopause. The symptoms listed below are primarily due to systemic estrogen deficiency and most are alleviated by estrogen therapy. Common symptoms are:
Vasomotor symptoms (VMS)
– hot flushes
– night sweats
Psychological
– depressive symptoms
– anxiety/irritability
– sleep disturbance
– overall diminished well-being
– lessened memory
– lessened concentration
General physical
– sleep disturbance
– fatigue
– headaches
– muscle/joint pains
– crawling sensations on skin (formication)
Urogenital and sexual
– vaginal itching, burning
– dryness and dyspareunia
– urinary frequency, urgency.
Symptom prevalence
It is well established that menopausal symptoms may range from none at all through to debilitating.
VMS are most commonly reported in the late menopause transition and after menopause. VMS are more common in obese women.
Within the US population, symptoms are more common in African-American women than Caucasian women, and less common in Chinese and Japanese womenCitation18.
A UK study reported that:
| – | 1 in 4 women experience severe VMS, | ||||
| – | 1 in 3 experience severe psychological symptoms (depression, anxiety), | ||||
| – | 1 in 2 women report moderate to severe symptoms of sleep disturbance, joint pain or headache) and, | ||||
| – | at least 1 in 4 women has sexual problemsCitation19,Citation20. | ||||
High rates of sleeping difficulty, VMS, joint and muscular discomfort and of vaginal dryness have been reported for women living in urban and remote regions across the globe, dispelling the myth that menopausal symptoms are phenomena of the developed worldCitation21–24.
Symptom duration
There is no age limit at which menopausal symptoms cease. Women who experience severe symptoms, either from early in the menopause transition or from their final menstrual period, continue to experience severe symptoms for several yearsCitation19.
At least 10% of women have bothersome VMS 10 years after their menopause has occurred, with as many as 16% of 85-year-olds continuing to experience VMSCitation25. Urogenital atrophy due to estrogen deficiency persists unless treated, so effectively all untreated postmenopausal women are affected.
OTHER HEALTH CONSEQUENCES OF THE CHANGE IN HORMONES AT MENOPAUSE
The fall in E2 at menopause has a number of adverse metabolic effects and health effects:
Metabolic
Central abdominal fat deposition (even in slim women),
Insulin resistance and increased risk of type 2 diabetes.
Cardiovascular
Impaired endothelial function (impaired vascular integrity),
Increased cholesterol (total cholesterol and low density lipoprotein cholesterol).
Skeletal
Accelerated bone loss,
Increased fracture risk.
Neurological
Persistent controversy as to whether the fall in estrogen at menopause and decline in androgens with age adversely affect cognitive performance.
Urogenital
Atrophic vaginitis,
Urinary tract – frequency, cystitis, urge incontinence, dysuria.
MANAGEMENT ()
Considerations for ALL women at menopause
The importance of improving lifestyle factors such as good nutrition, being physically active, cessation of smoking and limiting alcohol should be highlighted, as these can confer benefits to all women. All women should be reviewed in terms of:
Cardiovascular disease risk (blood pressure and lipids),
Diabetes (fasting blood glucose),
Urogenital health (consider local hormonal/non-hormonal therapy),
Cancer screening – breast check, PAP smear, mammogram (recommended frequency varies between countries).
General advice for symptom management
Dressing in layers, using a small fan, etc. may help women manage VMS, but will not reduce VMS or alleviate other symptoms.
Weight reduction may result in reduced VMS in overweight womenCitation26.
The effects of exercise on VMS are mixedCitation27; however, increased physical activity may improve sleep and general well-being.
Menopausal hormone therapy
Since publication of the first of the Women's Health Initiative (WHI) hormone studies, the use of menopausal hormone therapy (MHT) has raised concerns. In 2013, several international medical societies formulated a ‘Global Consensus Statement on Menopausal Hormone Therapy’Citation28 which can be found at http://informahealthcare.com/doi/pdf/10.3109/13697137.2013.771520.
This statement was endorsed by the American Society for Reproductive Medicine, the Asia Pacific Menopause Federation, the Endocrine Society, the European Menopause and Andropause Society, the International Menopause Society, the International Osteoporosis Foundation and the North American Menopause Society. Several core recommendations are listed in this document, with the first being:
‘MHT is the most effective treatment for vasomotor symptoms associated with menopause at any age, but benefits are more likely to outweigh risks for symptomatic women before the age of 60 years or within 10 years after menopause.‘
Other documents summarizing the benefits and risk of MHT in detail are accessibleCitation2,Citation3,Citation29. There is general consensus that:
Endometrial protection with a progestogen is essential in non-hysterectomized women, whereas hysterectomized women should be prescribed estrogen alone.
Women with premature ovarian insufficiency should be treated with MHT at least until the age of natural menopause.
Oral estrogen is associated with an increased risk of venous thromboembolic disease (VTE), although the absolute risk is small for women < 60 years old. The risk appears to be lower/not at all with transdermal estrogen. Therefore transdermal estrogen is preferred for women at increased risk of VTE, i.e. smokers, obese women.
Breast cancer is a contraindication to the use of MHT.
The prescription of individually formulated and compounded hormone preparations is not recommended.
MHT prevents bone loss and fractures in women aged up to 60 years, or within 10 years of menopause.
Testosterone therapy, given in a dose appropriate for a woman, may improve sexual desire and arousal.
Oral DHEA is not effective for the treatment of menopausal VMS, mood changes or sexual dysfunction.
Perimenopausal women
Treatment goals are cycle control, contraception and symptom relief.
Oral contraceptive pill
For the perimenopausal woman needing contraception, the combined oral contraceptive pill (OCP) provides contraception, menstrual cycle control, and relief from VMS and other symptoms. It also prevents bone loss and treats acne that can occur at this time. Each woman's risks must be assessed including smoking status, blood pressure, lipid profile, migraine aura history, thrombosis and cardiovascular risk, and family history.
Low-dose ethinylestradiol OCP (20 μg) or estradiol-containing OCP may be preferred. A 30 μg OCP can probably be used with equal safety (UK Medical Eligibility Criteria for Contraceptive use, http://www.fsrh.org/pdfs/UKMEC2009.pdf). However, some women have VMS when using ethinylestradiol that are alleviated when they are switched to an estradiol-containing OCP.
VMS in the pill-free week can be managed by eliminating the placebo tablets or adding a low dose of supplemental estrogen.
In some countries, weekly transdermal, monthly injectable, or monthly intravaginal contraceptive options will provide the same benefits with cyclical bleeding.
Women can transition from contraceptive hormone therapy to MHT when contraception is no longer required.
Progestogen-only regimens
The levonorgestrel-releasing intrauterine device (LNG-IUD) provides contraception and suppresses the endometrium and is an excellent option for the management of heavy bleeding. Although initial breakthrough bleeding may occur, 80% of women are amenorrheic at 1 yearCitation30. The LNG-IUD can be combined with oral/transdermal estrogen and can be left in situ for 5 years.
High-dose oral progestogen-only regimens (medroxyprogesterone acetate or micronized progesterone) may alleviate hot flushes and treat endometrial hyperplasia. However side-effects (weight gain, mastalgia, fluid retention, vaginal discharge, and dry mouth) can be a problem at these doses. Lower doses can be tried but may be less effective. Short-term use may be applicable in women who do not want to take estrogen. They can be used cyclically for the first 12–14 days of the cycle and produce predictable bleeding in the majority of women.
Cyclical MHT
This can be instituted during the perimenopause, with the progestin dose timed to the first 14 days of a woman's own cycle. However, as the dose of estrogen is not sufficient to suppress ovulation, women often experience symptoms of estrogen excess including mastalgia and erratic bleeding.
Menopausal hormone therapy after menopause
The primary use of MHT (estrogen ± progestogen therapy) is to alleviate symptoms of the menopause, namely hot flushes, night sweats, sleep disturbance, arthralgia and vaginal dryness and therefore improve the quality of life of women who, without MHT, find these symptoms intolerable.
For women with an intact uterus, progestogen therapy is taken with estrogen to protect the lining of the uterus from over-stimulation by estrogen.
This can be continuous estrogen with cyclic progestogen for 14 days out of a monthly cycle, or as continuous combined MHT where both the estrogen and progestogen are taken every day.
Cyclic MHT results in scheduled menstrual bleeding after the progestogen is ceased.
Continuous combined MHT results in no bleeding in 90% of women after 12 months. Breakthrough bleeding is not uncommon in the first few months of this type of regimen. If breakthrough bleeding is persistent or prolonged, then investigation of the endometrium is required.
For women who have undergone a hysterectomy, the administration of estrogen therapy alone is appropriate.
MHT FORMULATIONS ()
Estrogen
Estrogen can be used systemically as oral conjugated equine estrogen, estradiol valerate, estrone sulfate or micronized estradiol; transdermal estradiol (patches, gels, spray); a vaginal estradiol ring; and as implanted estradiol pellets. Intranasal estradiol, which is also highly effective, is no longer available. Vaginal estrogen is used exclusively for the treatment of urogenital atrophy.
Oral estrogen preparations
Advantages include:
Convenience, and
Reliable absorption for most users.
Disadvantages include:
Increased risk of VTE disease,
Increased risk of cholelithiasis,
Increased sex hormone binding globulin (SHBG), and therefore decreased free testosterone,
Increased thyroid binding globulin (TBG) – may need to adjust thyroxine dose,
Administration of higher total dose.
Transdermal estradiol preparations
Advantages include:
Avoidance of gut and first-pass hepatic metabolism: no change in SHBG or TBG, null effect on hepatic coagulation proteins,
Little/no increase in VTE disease,
Lower total dose,
Convenience for some women (e.g. once- or twice-a-week patch).
Disadvantages include:
Patches may cause skin irritation and rarely general allergic reaction,
Gel can be ‘sticky’ and inconvenient,
Occasionally poorly absorbed,
Women may forget to change twice-weekly patch.
Progestogen
Progestogen therapy is required for all women who have not had a hysterectomy. Progestogens include micronized progesterone and the synthetic progestins. There is evidence that supports micronized progesterone as being safer than synthetic progestin therapy in terms of breast cancer and cardiovascular disease riskCitation31,Citation32.
Micronized progesterone is taken either orally or the same capsule can be used as an intravaginal pessary. Synthetic progestins are mostly taken orally, separately or combined with oral estrogen, or in a combined estradiol–progestin patch.
The LNG-IUD is available in some countries and in appropriate circumstances is an excellent option for progestin effects to be achieved in the endometrium with minimization of systemic side-effects.
Non-prescription progesterone creams are widely available and are being used by many women who believe this treatment will preserve bone, act as an alternative to MHT, may be substituted for synthetic progestins in MHT regimes and will alleviate menstrual and premenstrual symptoms. SomeCitation33,Citation34, but not all studiesCitation35 indicate that, if a sufficient amount of transdermal progesterone can be administered, it may alleviate vasomotor symptoms and afford endometrial protection in the short term, but long-term benefit and safety need to be established.
Managing clinical side-effects of MHT therapy
Common adverse effects of estrogen include nausea, headache and breast tenderness. Combined estrogen–progestogen therapy can result in irregular, and occasionally heavy, bleeding.
Progestin therapy may cause lowered mood or irritability. When this occurs, either the dose needs to be reduced or the patient needs to be switched to another progestin. Micronized progesterone may result in less adverse mood effects.
Initiating treatment with low-dose estrogen will minimize the likelihood of adverse effects. Transdermal estrogen is less likely than oral estrogen to cause nausea. Changing from one estrogen regimen to another in many cases can alleviate certain adverse effects.
All women using systemic MHT require medical review every 6 months. Review should include updating medical history and a routine general health and breast check (). Investigations should be individually assessed with at least biannual mammography.
Bone densitometry measurement (DXA) should be performed where indicatedCitation36.
All unexpected vaginal bleeding warrants investigation and, when appropriate, specialist referral. Transvaginal ultrasound and/or endometrial biopsy is required if there is any excessive or prolonged bleeding 3–6 months after commencing MHT.
The need for ongoing MHT, the formulation and dose requirement should be regularly reviewed ().
Effectiveness
Estrogen therapy alleviates VMS and the other commonly reported symptoms of the menopause in 96% of womenCitation5. Low-dose therapy can be highly effective.
Treatment tips
If symptoms persist on high-dose oral therapy, there is no point increasing the dose if SHBG is high, as the administered estrogen will just be bound by the SHBG. Switch to non-oral.
If symptoms persist on high-dose, non-oral therapy, check serum estradiol to be sure the patient is actually absorbing the administered dose.
Although there is substantial controversy about whether menopause causes depression or major mood disturbance, many women report alleviation of anxiety and improved well-being with the use of estrogen therapy. There is also evidence for improved sleep quality.
OTHER THERAPIES WITH HORMONE-LIKE ACTIONS ()
Tibolone
Tibolone is a unique chemical compound that provides an alternative to estrogen–progestin therapy. Tibolone itself has very weak actions. It is metabolized in the gut and target tissues to metabolites that exhibit estrogenic, progestogenic and androgenic actions. Therefore it alleviates VMS, has favorable mood effects, treats urogenital atrophy and does not activate the endometrium – and therefore does not cause vaginal bleeding. Tibolone can also be converted to an active form which has weak androgen action. As a result, women may experience an improvement in sexual interest and responsiveness when they use tiboloneCitation37. It is uncommon for women using tibolone to experience breast tenderness, and tibolone does not increase mammographic density (unlike standard oral estrogen–progestin therapy). Tibolone prevents bone loss and has been shown to reduce fractures in older womenCitation38.
Tibolone should not be prescribed with other hormone therapy and is contraindicated in women with breast cancer. Tibolone has not been shown to increase the risk of VTE, cardiovascular disease or endometrial cancer.
Occasionally, women report fluid retention and mild weight gain with tibolone. Vaginal bleeding or spotting may occur in women just after commencing tibolone; however, this is uncommon.
Tibolone 1.25 mg/day has been associated with a small increase in the risk of ischemic stroke in older womenCitation38. For women aged 60–69 years, the risk of stroke was 2.8/1000 person-years for tibolone and 1.0/1000 person-years for placebo. In the same study, the risks for both breast and colon cancer were significantly reduced with tibolone compared with placebo.
Estrogen + selective estrogen receptor modulator
The combination of a selective estrogen receptor modulator (SERM) with estrogen has been described as a tissue-selective estrogen complex (TSEC) therapy. The first of these has now been approved in the USA: the oral combination of conjugated equine estrogen (CEE) 0.45 mg/day with 20 mg of bazedoxifene (BZE). This therapy alleviates VMS, alleviates urogenital atrophy, preserves bone and does not stimulate the endometrium such that vaginal bleeding is minimalCitation39,Citation40. Mastalgia is uncommon with this therapy. The incidence of VTE with CEE/BZE needs further clarification. An increase in VTE has been reported in studies of BZE aloneCitation41, but not in studies of combined CEE/BZECitation42.
Oral therapy for urogenital atrophy
Ospemifene is a SERM that has been approved in the USA for the treatment of vulvovaginal atrophy in postmenopausal women. The recommended dose is 60 mg/day. Ospemifene has an estrogen-like effect in the vagina (increases superficial cells and decreases parabasal cells and lowers vaginal pH). It results in a small, but statistically significant reduction in dyspareuniaCitation43. The most common adverse effect of ospemifene is VMS, which has been reported to occur in 10% of treated womenCitation44.
NON-HORMONAL OPTIONS WITH EVIDENCE TO SUPPORT EFFICACY ()
An array of non-hormonal therapies are recommended for the treatment of VMS, few of which have evidence to support efficacy.
Selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are effective in some, but not all women with VMS, and have the added benefit of improving mood and well-being. Recommended doses are listed in the Toolkit ().
A recent systematic review and meta-analysis concluded: ‘SSRI use is associated with modest improvement in the severity and frequency of hot flushes but can also be associated with the typical profile of SSRI adverse effects’Citation45.
Clonidine
Clonidine is sometimes prescribed for the management of VMS for women who cannot take estrogen. Studies of clonidine for VMS have been small and most show no benefit over placeboCitation46,Citation47. A dose of 100–150 μg/day may be effective in some women, although the effect is modestCitation48,Citation49. The most common side-effect, dry mouth, is dose-related.
Gabapentin
Gabapentin is an anti-epileptic that also is indicated for the treatment of neuropathic pain. It has been found in several studies to reduce VMS in postmenopausal women in doses of 300–900 mg/dayCitation50,Citation51. The anxiolytic effects of gabapentin may also be beneficial for some women. Side-effects include headache, dizziness and somnolence and are dose-related. Preliminary data also support the off-label use of pregabalin, in a dose of 75–150 mg twice daily, for the treatment of VMSCitation52.
Hypnosis
Hypnosis has been shown to diminish VMS in a study of postmenopausal womenCitation53. Although this needs further verification, it can be considered as a treatment option for women who are unable to take hormone therapy and who have not achieved a satisfactory outcome with other non-hormonal interventions.
Cognitive behavior therapy
Cognitive behavior therapy (CBT) employs psychotherapeutic behaviour modification to help women deal with VMS. In expert hands, CBT has been shown to significantly reduce VMSCitation54,Citation55.
Stellate ganglion blockade
Blockade of the stellate ganglion at the anterolateral aspect of the C6 vertebra on the right side under fluoroscopy has been shown to alleviate severe VMS for up to 12 weeksCitation56. This option could be considered for women with severe, debilitating VMS when other treatments are contraindicated or ineffective and where expertise in this procedure is available.
ANDROGEN THERAPY
Testosterone
There is no level of testosterone below which a woman can be said to be androgen-deficient, and a ‘testosterone deficiency syndrome’ has never been biochemically definedCitation57. However, several randomized, clinical trials have demonstrated efficacy of testosterone, in doses appropriate for women, for the treatment of low sexual desire/arousal disorder. This has been shown for naturally and surgically menopausal women, and for MHT users and non-usersCitation58–60.
Although a transdermal patch, releasing 300 μg of testosterone per day, was approved for surgically menopausal women in Europe, this treatment was not approved outside EU countries. Presently, it is not available in most countries, including EU countries. Testosterone pellets have been used for the past three decades in Australia and the UK for testosterone therapy for women (recommended dose being one half of a 100 mg testosterone pellet every 6 months or more), but these pellets are no longer available. A transdermal testosterone cream (AndroFeme 1%) can be prescribed in Australia, although it has not been approved by the Australian Therapeutics Goods Administration. The recommended starting dose is 0.5 ml/day, applied to the lower torso/upper thigh. It is essential that testosterone levels are monitored shortly after commencement of treatment and regularly during treatment (6-monthly) as absorption is variable. Blood levels of calculated free testosterone should be kept below the upper limit of the range for premenopausal women.
Women should be advised that, when testosterone is administered in a dose that results in levels within the normal female range, the effects of treatment usually do not emerge for 6– 8 weeks. Therefore treatment needs to be continued at least this long as a therapeutic trial. If no benefit is seen by 6 months, treatment should be discontinued.
Side-effects of testosterone therapy are rare when treatment is prescribed for appropriately selected women and given in the appropriate dose. Side-effects from excessive dosage can include masculinization with acne and excess body hair, fluid retention and cliteromegaly. These side-effects are rare if the appropriate dose of testosterone is administered.
Women with severe acne or severe excess body hair should not use testosterone. Similarly, women who are pregnant, lactating or who have a suspected cancer should not use testosterone as a standard precaution.
DHEA
DHEA was popularized in the 1990s as a treatment to improve well-being, sexual function and possibly reduce menopausal symptoms. Subsequent randomized, placebo-controlled trials have not shown oral DHEA to be effective as a treatment for estrogen deficiency symptoms in postmenopausal women. It has been shown to be no more effective than placebo for low sexual desire, diminished well-being and cognitive functionCitation61. Data to support the routine prescription of DHEA to postmenopausal women with adrenal insufficiency are also lackingCitation62.
Conflict of interest S.R.D. is presently an investigator for Trimel Pharmaceuticals Canada and has received unrestricted research grant support from Lawley Pharmaceuticals and Besins Healthcare.
Source of funding S.R.D. is an NHMRC Principal Research Fellow (Grant number 1041853).
References
- Position statement: Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause 2010;17:25–54
- de Villiers TJ, Pines A, Panay N, et al. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 2013;16:316–37
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab 2010;95(7 Suppl 1):S1–66
- Wierman ME, Basson R, Davis SR, et al. Androgen therapy in women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2006;91:3697–710
- National Institutes of Health State-of-the-Science Conference Statement: management of menopause-related symptoms. Ann Intern Med 2005;142:1003–13
- Bell RJ, Lijovic M, Fradkin P, Davis SR. A pragmatic approach to the classification of menopausal status for community-based research. Menopause 2008;15:978–83
- Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013;178:70–83
- Qiu C, Chen H, Wen J, et al. Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab 2013;98:1612–21
- Castelo-Branco C, Blumel JE, Chedraui P, et al. Age at menopause in Latin America. Menopause 2006;13:706–12
- Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012;97:1159–68
- Zumoff B, Strain GW, Miller LK, Rosner W. Twenty-four hour mean plasma testosterone concentration declines with age in normal premenopausal women. 1995;80:1429–30
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005;90:3847–53
- Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab 2000;85:2832–8
- Judd HL, Lucas WE, Yen SS. Effect of oophorectomy on circulating testosterone and androstenedione levels in patients with endometrial cancer. Am J Obstet Gynecol 1974;118: 793–8
- van der Stege JG, Groen H, van Zadelhoff SJ, et al. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause 2008;15:23–31
- Kalantaridou SN, Calis KA, Vanderhoof VH, et al. Testosterone deficiency in young women with 46,XX spontaneous premature ovarian failure. Fertil Steril 2006;86:1475–82
- Van Look PF, Lothian H, Hunter WM, Michie EA, Baird DT. Hypothalamic-pituitary-ovarian function in perimenopausal women. Clin Endocrinol 1977;7:13–31
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation. Am J Public Health 2006;96:1226–35
- Mishra GD, Kuh D. Health symptoms during midlife in relation to menopausal transition: British prospective cohort study. BMJ 2012;344:e402
- Avis NE, Brockwell S, Randolph JF Jr, et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause 2009;16:442–52
- Chuni N, Sreeramareddy CT. Frequency of symptoms, determinants of severe symptoms, validity of and cut-off score for Menopause Rating Scale (MRS) as a screening tool: a cross-sectional survey among midlife Nepalese women. BMC Women’s Health 2011;11:30
- Olaolorun FM, Lawoyin TO. Experience of menopausal symptoms by women in an urban community in Ibadan, Nigeria. Menopause 2009;16:822–30
- Waidyasekera H, Wijewardena K, Lindmark G, Naessen T. Menopausal symptoms and quality of life during the menopausal transition in Sri Lankan women. Menopause 2009;16:164–70
- Blumel JE, Chedraui P, Baron G, et al. A large multinational study of vasomotor symptom prevalence, duration, and impact on quality of life in middle-aged women. Menopause 2011;18: 778–85
- Vikstrom J, Spetz Holm AC, Sydsjo G, Marcusson J, Wressle E, Hammar M. Hot flushes still occur in a population of 85-year-old Swedish women. Climacteric 2013;16:453–9
- Kroenke CH, Caan BJ, Stefanick ML, et al. Effects of a dietary intervention and weight change on vasomotor symptoms in the Women’s Health Initiative. Menopause 2012;19:980–8
- Elavsky S, Gonzales JU, Proctor DN, Williams N, Henderson VW. Effects of physical activity on vasomotor symptoms: examination using objective and subjective measures. Menopause 2012;19:1095–103
- de Villiers TJ, Gass ML, Haines CJ, et al. Global Consensus Statement on menopausal hormone therapy. Climacteric 2013;16: 203–4
- Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of The North American Menopause Society. Menopause 2007;14:168–82
- Chrisman C, Ribeiro P, Dalton VK. The levonorgestrel- releasing intrauterine system: an updated review of the contraceptive and noncontraceptive uses. Clin Obstet Gynecol 2007;50:886–97
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause 2014;21:769–83
- Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol 2008;26:1260–8
- Leonetti H, Longo S, Anasti J. Transdermal progesterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol 1999;94:225–8
- Leonetti HB, Wilson KJ, Anasti JN. Topical progesterone cream has an antiproliferative effect on estrogen stimulated endometrium. Fertil Steril 2003;79:221–2
- Cooper A, Spencer MI, Whitehead M, Ross D, Barnard GJR, Collins WP. Systemic absorption of progesterone from Progest cream in postmenopausal women. Lancet 1998;351:1255
- Birkhauser MH, Panay N, Archer DF, et al. Updated practical recommendations for hormone replacement therapy in the peri- and postmenopause. Climacteric 2008;11:108–23
- Nijland EA, Weijmar Schultz WC, Nathorst-Boos J, et al. Tibolone and transdermal E2/NETA for the treatment of female sexual dysfunction in naturally menopausal women: results of a randomized active-controlled trial. J Sex Med 2008;5:646–56
- Cummings SR, Ettinger B, Delmas PD, et al. The effects of tibolone in older postmenopausal women. N Engl J Med 2008;359:697–708
- Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective Estrogens, Menopause and Response to Therapy (SMART) Trials. J Womens Health (Larchmt) 2014;23:18–28
- Mirkin S, Komm BS, Pan K, Chines AA. Effects of bazedoxifene/conjugated estrogens on endometrial safety and bone in postmenopausal women. Climacteric 2013;16:338–46
- de Villiers TJ, Chines AA, Palacios S, et al. Safety and tolerability of bazedoxifene in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled phase 3 trial. Osteoporos Int 2011;22:567–76
- Pinkerton JV, Komm BS, Mirkin S. Tissue selective estrogen complex combinations with bazedoxifene/conjugated estrogens as a model. Climacteric 2013;16:618–28
- Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013;20:623–30
- Simon J, Portman D, Mabey RG Jr. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas 2014;77:274–81
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med 2014;29:204–13
- Salmi T, Punnonen R. Clonidine in the treatment of menopausal symptoms. Int J Gynaecol Obstet 1979;16:422–6
- Lindsay R, Hart DM. Failure of response of menopausal vasomotor symptoms to clonidine. Maturitas 1978;1:21–5
- Bolli P, Simpson FO. Clonidine in menopausal flushing: a double-blind trial. N Z Med J 1975;82:196–7
- Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med 2000;132:788–93
- Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat 2012;136:479–86
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet 2005;366:818–24
- Nguyen ML. The use of pregabalin in the treatment of hot flashes. Can Pharm J 2013;146:193–6
- Elkins GR, Fisher WI, Johnson AK, Carpenter JS, Keith TZ. Clinical hypnosis in the treatment of postmenopausal hot flashes: a randomized controlled trial. Menopause 2013;20:291–8
- Mann E, Smith MJ, Hellier J, et al. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol 2012;13:309–18
- Ayers B, Hunter MS. Health-related quality of life of women with menopausal hot flushes and night sweats. Climacteric 2013;16:235–9
- Lipov EG, Joshi JR, Sanders S, et al. Effects of stellate-ganglion block on hot flushes and night awakenings in survivors of breast cancer: a pilot study. Lancet Oncol 2008;9:523–32
- Davis SR, Davison SL, Donath S, Bell R. Relationships between circulating androgen levels and self-reported sexual function in women. JAMA 2005;294:91–6
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab 2005;90:5226–33
- Davis SR, Moreau M, Kroll R, et al. Testosterone for low libido in menopausal women not taking estrogen therapy. N Engl J Med 2008;359:2005–17
- Panay N, Al-Azzawi F, Bouchard C, et al. Testosterone treatment of HSDD in naturally menopausal women: the ADORE study. Climacteric 2010;13:121–31
- Davis SR, Panjari M, Stanczyk FZ. DHEA replacement for postmenopausal women. J Clin Endocrinol Metab 2011;96:1642–53
- Alkatib AA, Cosma M, Elamin MB, et al. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab 2009;94:3676–81