Abstract
Objective We investigated the effect of a combination of vaginal ultra-low-dose estriol with lactobacilli on the sexual functioning domain of quality of life during the treatment of breast cancer survivors on an aromatase inhibitor with vaginal atrophy.
Subjects and methods This was an open-label, bicentric, exploratory, clinical study in 16 postmenopausal breast cancer survivors on aromatase inhibitors suffering from vaginal atrophy-induced sexual disorders. Atrophy symptoms were assessed by scoring with an 11-point estimation scale (0 = not at all, 10 = worst imaginable feeling). Sexuality parameters of quality of life and medication adherence were recorded in a patient's diary and in the Female Somatic Sexual Experience Instrument (FSSEI) questionnaire. Patients underwent an initial treatment for 4 weeks (one vaginal tablet of Gynoflor® containing 0.03 mg estriol daily), followed by maintenance therapy (three vaginal Gynoflor® tablets weekly) for 8 weeks.
Results Vaginal dryness continuously improved from a median score of 8 at entry to a score of 4 at the end of initial therapy, and a median score of 2 at the end of maintenance therapy. Normal sexual activity before breast cancer diagnosis was reported by 14 women (88%). At study entry, only three women (19%) were sexually active. At the end of the Gynoflor® regimen, ten women (63%) reported sexual activity, of which seven (44%) reported sexual intercourse. The FSSEI demonstrated a non-significant trend of improvement of parameters related to sexuality.
Conclusions Local vaginal therapy with Gynoflor® in breast cancer survivors on aromatase inhibitors reporting atrophic vaginitis could be considered as a useful treatment for the quality of sexual life.
INTRODUCTION
The use of an aromatase inhibitor (AI) in hormone receptor-positive breast cancer patients often induces or enhances symptoms of vaginal atrophyCitation1. This side-effect dramatically impacts quality of life, thereby hampering adherence and, as a consequence, affecting long-term survival ratesCitation2. Furthermore, atrophic vulvovaginitis interferes with other aspects of life, such as relationships, rehabilitation, and enjoyment of the remaining life span of these womenCitation3.
Quality of life is a multidimensional construct that encompasses various areas of functioning. Four primary domains of quality of life have been suggested: mental, physical, social, and sexualCitation4. Sexual quality of life generally refers to body image distress, changes in sexual desire, and perceived sexual functioning. From a medical viewpoint, sexuality encompasses more than genital functioningCitation5. Women surviving breast cancer and treated with AIs may feel that their altered sexuality is not a medically legitimate complaint, and their treating physicians often emphasize disease-free survival more than such side-effects, which are often seen as insignificant and minor. However, sexual dysfunction is well known severely to interfere with quality of life and with the strong need for intimacy during cancer diagnosis and treatment.
Various models have been used to describe the female sexual responseCitation6. In 1966, Masters and Johnson proposed a model consisting of four successive phases: excitement, plateau, orgasmic, and resolution phases. In 1979, Kaplan proposed the three-phase model, consisting of desire, arousal, and orgasm, with desire being the factor inciting the overall response cycle. Recently, it has been suggested that sexual function should be considered as a circuit, with four main domains: libido, arousal, orgasm, and satisfactionCitation7,Citation8. Each aspect may overlap and negatively or positively feedback on the nextCitation6. Anyhow, normal sexuality includes adequate sexual desire, arousal with orgasm which leads to relaxation and induces a feeling of sexual pleasure, fulfilment, and satisfaction.
The best treatment for vaginal atrophy is local estrogen therapyCitation9,Citation10; however, evidence to support the use of conventional local estrogen therapy among breast cancer patients is conflictingCitation11. Interestingly, some scientific dataCitation12 suggest that estrogen therapy (including application of local vaginal estrogen) also improves local genital sensitivity and sexual response, thereby potentially improving a woman's sexuality, although some authors disagree with thisCitation13.
The present assessment of sexual life improvement is a part of a broader exploratory, pharmacokinetic, clinical studyCitation14 (n = 16) wherein we investigated the pharmacokinetics, safety, and efficacy of a ultra-low-dose vaginal estriol–lactobacilli combination therapy in breast cancer survivors on an AI suffering from vaginal atrophy. This product, Gynoflor®, contains 10Citation8 cfu viable lyophilized Lactobacillus acidophilus KS400 bacteria and 0.03 mg estriol (E3). The latter is not only given at ultra-low dose (0.03 mg), but is also a much less potent estrogen than estradiol (E2). Its dose is 16–32 times lower than used in conventional E3 vaginal preparations (0.5–1 mg). All women during the mentioned study applied a daily vaginal tablet for 4 weeks followed by a maintenance therapy of three tablets weekly for 8 weeks. Primary outcomes were serum concentrations and pharmacokinetics of E3, E2 and estrone (E1) using highly sensitive gas chromatography–mass spectrometry (GC/MS). Compared with baseline, serum E1 and E2 did not increase in any of the women at any time following vaginal application. Serum E3 transiently increased after the first application in 15 of 16 women, with a maximum 2–3 h post-insertion of 168 pg/ml; after 4 weeks, serum E3 was slightly increased in eight women with a maximum of 44 pg/ml, i.e. application of the low-dose 0.03 mg E3 and Lactobacillus acidophilus vaginal tablets in postmenopausal breast cancer patients during AI treatment suffering from vaginal atrophy leads only to small and transient increases in serum E3, but not E1 or E2.
Gynoflor® has been proven to be safe and efficacious in the restoration of disturbed vaginal floraCitation15,Citation16 and in treatment of postmenopausal atrophic vaginitisCitation17–19. The current clinical study demonstrated that an initial daily dose of a vaginal tablet for 4 weeks followed by one tablet every second day as maintenance therapy led only to small and transient increases in serum E3, but not E1 or E2 in women with breast cancer on AIs with severe atrophyCitation14. Therefore, ultra-low-dose 0.03 mg E3 and Lactobacillus acidophilus vaginal tablets can be considered safe and efficacious for treatment of atrophic vaginitis in breast cancer patients taking AIs.
This article describes the study results with regard to the sexual domain of quality of life during treatment of breast cancer survivors on AIs suffering from symptomatic vaginal atrophy and dyspareunia with the ultra-low-dose estriol– lactobacilli combination (Gynoflor®).
METHODS
From April 2011 to July 2012, 16 postmenopausal breast cancer survivors on AIs suffering from sexual dysfunction related to vaginal atrophy were included in an open-label, clinical trial conducted at two centers in Belgium and in Germany. The study was approved by both Ethical Committees and the national authorities as appropriate (EudraCT No: 2010–022007-22) and all patients signed informed consent before any study action was taken, according to GCP guidelines and the Declaration of Helsinki.
This small (n = 16) open study with subjective evaluation of both signs and symptoms of vaginal atrophy assessed also sexual quality of life of during the pharmacokinetic clinical study as reported elswhereCitation14. For the pharmacokinetic study, 16 women where statistically sufficient to detect the systemic effects of locally applied E3. Due to the fact that most of the breast cancer patients under endocrine therapy suffer from significant side-effects with a major impact on daily life, the study protocol also included an extended work-up concerning sexual quality of life issues. Of course, for this question, the number of patients was clearly insufficient to demonstrate clear statistical changes but, since this topic is of high clinical relevance, the generated sexual quality of life data trends are published here as a separate publication.
Included women were postmenopausal as defined as either aged 52 years or older or ≥ 46 years after bilateral oophorectomy with cessation of menses for at least 12 months. Furthermore, in women with intact ovaries following hysterectomy, follicle stimulating hormone levels had to be above 30 IU/l. All women had started AIs for adjuvant treatment of breast cancer at least 6 months ago. Additional criteria were presence of clinical symptoms of vaginal atrophy, and a Karnofsky score ≥ 80%. Women had the right to withdraw from the study at any time.
Main exclusion criteria were use of any other sex hormones or use of any other vaginal medication 6 months before or during the study, use of steroidal AIs, any severe genital diseases or conditions. Women with a body mass index (BMI) lower than 18.5 kg/mCitation2 or higher than 30 kg/mCitation2 were also excluded.
Gynoflor® vaginal tablets (100 million viable Lactobacillus acidophilus KS400 and 0.03 mg E3) were supplied by Medinova AG, Switzerland. Recruited women underwent an initial treatment for 4 weeks by inserting one tablet deeply into the vagina while in the recumbent position before going to sleep, followed by maintenance therapy (three vaginal tablets weekly) for 8 weeks.
Clinical examinations were performed at screening (S = week [– 1]), at entry (E = Day 0) and at days 14 (C1 = week 2), 28 (C2 = week 4), 56 (C3 = week 8) and 84 (C4 = week 12) to assess hormone levels, efficacy, and safety ()Citation14.

Clinical vaginal atrophy symptoms were assessed by a visual analog scale with an 11-point estimation scale (0 = not at all, 10 = worst imaginable feeling). Additionally, symptoms, sexuality parameters of quality of life, and medication adherence were recorded in a patient's diary and in the Female Somatic Sexual Experience Instrument (FSSEI), version ‘breast cancer’, sexual questionnaire (at E and C4). The FSSEI is a validated sexual questionnaire used by the University Hospitals Leuven, BelgiumCitation20; it consists of 30 questions (qualitative and quantitative types, in the native patient's language) covering the main topics of women's sexual domain of quality of life: sexual history, desire, arousal/lubrication, orgasm and sexual satisfaction ().
Table 1 Female Somatic Sexual Experience Instrument (FSSEI), version breast cancer
At each control visit, the global efficacy and tolerability were assessed by both investigator and patient. Treatment adherence was assessed by asking women about their medication, checking the medication, and by reviewing the diaries.
The individual subject values were tabulated with descriptive statistics. Values between visits were compared using the Wilcoxon signed rank sum test, the McNemar test or the sign test. All continuous parameters were summarized using standard summary statistics as appropriate.
RESULTS
From 19 screened women, 16 were included in this study, eight from each center. One protocol violation was noted: a woman, who was treated with the steroidal AI exemestane before she switched to a non-steroidal AI, was recruited as the investigator was not aware of that.
All 16 patients were Caucasian, with a mean age of 57.0 (range 52.0–63.0) years and a BMI of 23.5 ± 3.0 kg/mCitation2. The diagnosis of breast cancer had been a median of 2.6 years ago with one subject diagnosed with the disease 28.2 years ago; the median duration of AI therapy was 2.1 years with one subject having it for 7.7 years. The daily AI dose was either 1 mg letrozole (n = 11) or 2.5 mg anastrozole (n = 5). The mean Karnofsky score was 98.1 ± 5.4%. The most frequently used concomitant medications were taken for gastrointestinal conditions (56%) and for improving the function of the musculoskeletal system, such as antiphlogistics (44%).
Treatment adherence was very good during both initial (range 92.9–100.0%) and maintenance therapy (range 95.8–100.0%).
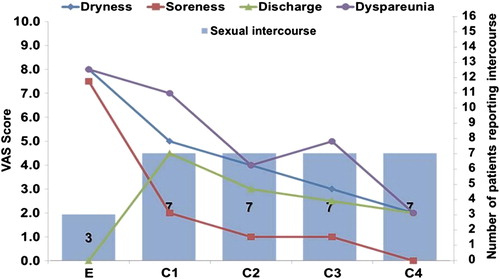
During treatment, the subjective improvement of all clinical signs and symptoms of vaginal atrophy were statistically not significant due to the small number of patients. Vaginal dryness improved continuously from a median score of 8 at entry to a score of 4 at the end of initial therapy, and a score of 2 at the end of maintenance therapy. Dryness and soreness improved from entry to control visits (p < 0.001), while statistical evaluation of the improvement in dyspareunia and other symptoms was hampered by low subject numbers ().
Table 2 Signs and symptoms
In total, 16 breast cancer patients completed the FSSEI (sexual questionnaire) and were included in the evaluation and analysis. In addition to a clinical examination at baseline, patients were asked whether they were sexually active before and during breast cancer diagnosis as a question in the FSSEI. Before breast cancer diagnosis, 14 women (88%) reported having sexual activity. At entry, only three women (19%) were sexually active, indicating the severity of the side-effects of AIs and the impact of the breast cancer diagnosis. At visit C4, ten women (63%) reported sexual activity (FSSEI analysis), of whom seven (43%) reported normal, regular penetrative vaginal sexual intercourse. A trend to correlation between improvement of vaginal atrophy symptoms and improvement of sexual functioning could be considered ().
The FSSEI analysis (, ) demonstrated a trend for improvement of all main domains of sexual quality of life (desire, arousal, orgasm, and satisfaction), being statistically significant for ‘body image’ (p = 0.0009) and ‘sexual desire’ (p = 0.0042), but not for the others (due to the small number of subjects).
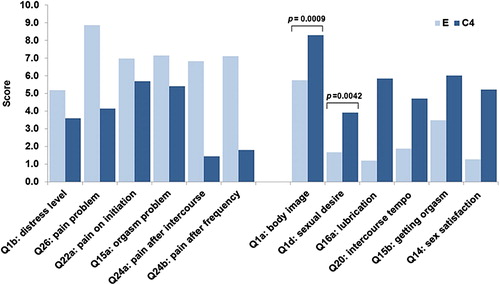
Table 3 Results of Female Somatic Sexual Experience Instrument (FSSEI)
Symptom dynamics based on patient diary data showed a median time of 11 days (range 4–24 days) until improvement of vaginal dryness, and 27 days (range 4–89 days) until complete disappearance of this symptom. So the 28-day initial therapy was, on average, sufficient to treat the symptomatic vaginal atrophy in the investigated subjects. It should be noted that increased discharge was observed immediately after initiation of estriol–lactobacilli combination therapy. However, it was unclear whether this indicated some remnants of the tablets, enhanced lubrication (physiological discharge), or pathological discharge.
DISCUSSION
The impact of estrogen deprivation on vaginal health during treatment with AIs is broadly recognized; however, the indirect effect on sexuality and quality of life is still underestimatedCitation21. Estrogen deficiency disrupts many of the physiological responses which characterize sexual arousal, including smooth muscle relaxation, vasocongestion and lubricationCitation13,Citation22. Vaginal dryness, dyspareunia and loss of sexual desire are the main factors destroying the sexual life of affected women. Therefore, support and information are needed not only by younger but also by older, postmenopausal women taking these drugsCitation3.
Estrogen receptors are heavily concentrated in the vulvovaginal area, making this body region extremely sensitive to estrogen deprivationCitation23. Low estrogen levels result in a reduction of squamous epithelial cell layers of the vaginal epithelium, leading to a predominance of parabasal and basal cells, while levels of collagen, glycogen, mucopolysaccharides, and hyaluronic acid significantly declineCitation23,Citation24. Concomitantly, a severe reduction of the vaginal lactobacilli is seen, which in turn leads to an increased vaginal pH and further contributes to the greater risk for urogenital tract infectionsCitation25. The resulting vaginal atrophy is a main contributor to sexual dysfunction: lubrication and tissue elasticity are reduced, and the vagina becomes shorter and narrower, leading to dyspareunia. Furthermore, the diminished sensory response reduces the orgasmic intensity, decreases libido, and leads to loss of sexual satisfactionCitation22,Citation26.
The ‘gold standard’ treatment for these problems in healthy postmenopausal women is application of vaginal estrogen preparations, but in breast cancer survivors such treatment is in general contraindicated due to safety concerns. Earlier reported results of the current studyCitation14 have demonstrated that therapy with ultra-low-dose 0.03 mg E3 and Lactobacillus acidophilus vaginal tablets (Gynoflor®) can be considered as a safe and efficacious treatment of atrophic vaginitis in breast cancer patients taking AIs. Only a few studies have evaluated the effects of vaginal estrogen for improvement in sexual function of postmenopausal women. Gast and colleaguesCitation27 demonstrated that local estrogen therapy was also associated with improvement in sexual desire, frequency of orgasm, and sexual satisfaction; however, there was no effect on coital frequency. Cayan and colleaguesCitation28 evaluated, with a 19-item Sexual Function Index questionnaire, sexual desire, arousal, lubrication, orgasm, satisfaction, and pain during various estrogen application regimens. In this study, the vaginal therapy-only group did not have a favorable response in desire, arousal, orgasm or satisfaction compared to the oral group. It could be stated that the effect of local vaginal estrogen application on the sexuality of women still needs more in-depth investigationsCitation13. Nonetheless, it seems that the restoration of vaginal epithelial health with local estrogen application results in increased vaginal compliance, decreased vaginal pH, increased vaginal blood flow, and lubricationCitation12,Citation29. Women subsequently report decreased vaginal irritation, soreness, dryness, and pain during intercourse, resulting in increased sexual desire, arousal, i.e. subjective improvement in the sexual domain of quality of lifeCitation12,Citation29,Citation30.
Our study demonstrated similar findings for postmenopausal breast cancer patients taking AIs. We have seen that, even in the absence of a systemic increase in circulating estrogen levels (as reported earlierCitation14), symptoms of vaginal atrophy and sexual functioning both improved and approached the level of functioning that was present before diagnosis (75% vs. 88%). The FSSEI demonstrated a trend for improvement of all main domains of sexual quality of life (desire, arousal, orgasm, satisfaction); however, statistics failed to show significance due to small subject numbers (only seven of 16 patients reported normal sexual intercourse).
The results of our study also support the positive role of local vaginal E3 and probably other estrogens in achieving better results in sexualityCitation27,Citation28 as E3 really succeeds in transforming the pelvic tissues into better shape for intercourseCitation12,Citation29,Citation30, and it seems that this is especially relevant in the AI-treated breast cancer women suffering from vaginal atrophy with dyspareunia. It still remains unclear and could be further investigated whether this concept could be adapted for other, especially postmenopausal, women with dyspareunia-induced sexual problems. The strengths of the present study were the precise study design and comprehensive evaluation of various relevant parameters in the investigated patient population. The weaknesses of the study were the small numbers of subjects for the testing of some parameters of sexuality, but this will probably be the only study looking at the quality of life in women with an endocrine treatment of breast cancer. Hence, for consolidation of these exploratory findings, larger studies including more women are needed.
In conclusion, it was demonstrated that local Gynoflor® therapy of postmenopausal breast cancer survivors treated with a non-steroidal AI reporting atrophic vaginitis could be considered as a useful treatment opportunity having probably a positive impact on the sexual domain of quality of life of affected women.
Conflict of interest Dr Gilbert Donders is a member of the Global Advisory Board of Medinova AG, Switzerland. Dr Philipp Grob and Dr Valdas Prasauskas are employees of Medinova AG, Switzerland. The authors alone are responsible for the content and writing of the paper.
Source of funding This study and online free access of the publication were sponsored by Medinova AG, Switzerland.
References
- Crandall C, Petersen L, Ganz PA, Greendale GA. Association of breast cancer and its therapy with menopause-related symptoms. Menopause 2004;11:519–30
- Hickey M, Saunders C, Partridge A, Santoro N, Joffe H, Stearns V. Practical clinical guidelines for assessing and managing menopausal symptoms after breast cancer. Ann Oncol 2008;19:1669–80
- Pumo V, Milone G, Iacono M, et al. Psychological and sexual disorders in long-term breast cancer survivors. Cancer Manag Res 2012;4:61–5
- Yanez B, Thompson EH, Stanton AL. Quality of life among Latina breast cancer patients: a systematic review of the literature. J Cancer Surviv 2011;5:191–207
- Derzko C, Elliott S, Lam W. Management of sexual dysfunction in postmenopausal breast cancer patients taking adjuvant aromatase inhibitor therapy. Curr Oncol 2007;14(Suppl 1):S20–40
- Berman JR. Physiology of female sexual function and dysfunction. Int J Impot Res 2005;17(Suppl 1):S44–51
- Basson R, Berman J, Burnett A, et al. Report of the International Consensus Development Conference on Female Sexual Dysfunction: definitions and classifications. J Urol 2000;163:888–93
- Basson R. The female sexual response: a different model. J Sex Marital Ther 2000;26:51–65
- MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010;85:87–94
- Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008;111:67–76
- Kendall A, Dowsett M, Folkerd E, Smith I. Caution: Vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol 2006; 17:584–7
- Sarrel PM. Effects of hormone replacement therapy on sexual psychophysiology and behavior in postmenopause. J Womens Health Gend Based Med 2000;9(Suppl 1):S25–32
- Krause M, Wheeler TL, Snyder TE, Richter HE. Local effects of vaginally administered estrogen therapy: a review. J Pelvic Med Surg 2009;15:105–14
- Donders G, Neven P, Moegele M, et al. Ultra-low-dose estriol and Lactobacillus acidophilus vaginal tablets (Gynoflor®) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res Treat 2014;145:371–9
- Donders GG, Van Bulck B, Van de Walle P, et al. Effect of Lyophilized lactobacilli and 0.03 mg estriol (Gynoflor®) on vaginitis and vaginosis with disrupted vaginal microflora: a multicenter, randomized, single-blind, active-controlled pilot study. Gynecol Obstet Invest 2010;70:264–72
- Ozkinay E, Terek MC, Yayci M, Kaiser R, Grob P, Tuncay G. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG 2005;112:234–40
- Jaisamrarn U, Triratanachat S, Chaikittisilpa S, Grob P, Prasauskas V, Taechakraichana N. Ultra-low-dose estriol and lactobacilli in the local treatment of postmenopausal vaginal atrophy: a double-blind randomized trial followed by open-label maintenance therapy. Climacteric 2013;16:347–55
- Kanne B, Patz B, Wackerle L. [Local treatment of vaginal infections with Doederlein bacteria and estriol in climacterium and senium]. Frauenarzt 1986;3:35–40
- Kanne B, Jenny J. [Local administration of low-dosed estriol and viable Lactobacillus acidophilus in the post-menopausal period]. Gynäkol Rundsch 1991;31:7–13
- Morales L, Neven P, Timmerman D, et al. Acute effects of tamoxifen and third-generation aromatase inhibitors on menopausal symptoms of breast cancer patients. Anticancer Drugs 2004;15:753–60
- Carter J, Goldfrank D, Schover LR. Simple strategies for vaginal health promotion in cancer survivors. J Sex Med 2011;8: 549–59
- Goldstein I, Alexander JL. Practical aspects in the management of vaginal atrophy and sexual dysfunction in perimenopausal and postmenopausal women. J Sex Med 2005;2(Suppl 3): 154–65
- Trinkaus M, Chin S, Wolfman W, Simmons C, Clemons M. Should urogenital atrophy in breast cancer survivors be treated with topical estrogens? Oncologist 2008;13:222–31
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Julia MD. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas 2005;52(Suppl 1):S46–52
- Bruno D, Feeney KJ. Management of postmenopausal symptoms in breast cancer survivors. Semin Oncol 2006;33:696–707
- Wylie KR. Sexuality and the menopause. J Br Menopause Soc 2006;12:149–52
- Gast MJ, Freedman MA, Vieweg AJ, De Melo NR, Girao MJ, Zinaman MJ. A randomized study of low-dose conjugated estrogens on sexual function and quality of life in postmenopausal women. Menopause 2009;16:247–56
- Cayan F, Dilek U, Pata O, Dilek S. Comparison of the effects of hormone therapy regimens, oral and vaginal estradiol, estradiol + drospirenone and tibolone, on sexual function in healthy postmenopausal women. J Sex Med 2008;5:132–8
- Bachmann GA, Leiblum SR. The impact of hormones on menopausal sexuality: a literature review. Menopause 2004;11: 120–30
- Semmens JP, Tsai CC, Semmens EC, Loadholt CB. Effects of estrogen therapy on vaginal physiology during menopause. Obstet Gynecol 1985;66:15–18

