Abstract
Some pharmacological activities of Cochlospermum planchonii Kunth (Cochlospermaceae) root extract were studied. The extract yielded 3.4% w/w dry matter. The extract was well tolerated in all doses (250- 3000 mg/kg bw, per os) used in the experiment. Brine shrimps lethality test gave LC50 of 4.42 ppm at 95% confidence interval. The extract significantly (p <0.05) increased pentobarbital-induced sleeping time at all doses. The extract (250, 500, and 1000 mg/kgbw, p.o.) significantly (p <0.05) decreased the paw licking time in the second (late) phase of the formalin test. Also, it significantly (p <0.05) decreased the number of acetic acid-induced writhings in all doses used. The anti-inflammatory study showed that the extract caused a biphasic inhibition of carrageenan-induced paw edema, similar to indomethacin (20 mg/kgbw). Despite the potent analgesic effect of the extract, it did not protect mice from leptazole-induced convulsions. The extract (250, 500, 1000 mg/kgbw p.o.) significantly (p <0.05) decreased blood glucose levels in alloxan-induced hyperglycemic mice in a dose- and time-dependent manner. The phytochemical tests showed the presence of saponins, tannins, glycosides and carbohydrates. In conclusion, C. planchonii root extract contains very potent bioactive compounds, which showed CNS depressant, analgesic, anti-inflammatory, and antihyperglycemic effects with minimal toxicity. Therefore, it is endowed with a potential for pharmacological control of pain, inflammation, and diabetes mellitus.
Introduction
Cochlospermum planchonii Kunth Bixaceae (Cochlospermaceae) (CitationMabberley, 1981) has been an important economic medicinal plant in some parts of Africa. It is a low shrubby plant and a common weed of cultivation in both Guinea and Sudan savannah zones (CitationBurkill, 1985). It is widespread from Senegal to East and West Cameroon up to Nigeria (CitationAkobundu & Agyakwa, 1987). In Benue State of Nigeria, where the plant is found in abundance in the wild, it is used traditionally in the management of some ailments such as jaundice, pre-menstrual pain, infertility, diabetes mellitus, gonorrhea and enteric fever (CitationBurkill, 1985; CitationIgoli et al., 2002). The decoction of the root bark of the plant is used for treating hepatic fever, hepatobiliary affections (black toilet fever), and hemolytic anemia in Burkina Faso (CitationAliyu et al., 1995). Economically, the stem bark of C. planchonii is used in northern Sierra Leone and Nigeria for rope and string production. The root material is a very good source of reddish yellow dyes used by the traditional textile mills in the Nupe tribe and as culinary colorant in Lagos, Nigeria (CitationBurkill, 1985).
The leaf oil of C. planchonii showed a superior anti-plasmodial effect in vitro when compared to chloroquine, with LC50 of 22-35 μg/mL against Plasmodium falciparum (CitationBenoit-Vical et al., 1999). Also, CitationAtawodi (2005) showed that the petroleum extract of the stem bark (4 mg/mL) has trypanocidal properties in vitro. Phytochemical analysis of the root extract showed the presence of egallic acid, zinc salt, and manganese (CitationAliyu et al., 1995). The zinc salt showed hepatoprotective effect because it inhibited cytochrome P450 enzyme in the liver.
The present study further investigated the pharmacological activities of the methanol root extract of C. planchonii in vivo and in vitro to confirm the analgesic and antidiabetic uses.
Materials and methods
The experimental protocols used in this study were approved by the Ethics Committee of the University of Nigeria, Nsukka, in accordance with the guide to the care and use of laboratory animals in research and teaching in the university. Freshly prepared solutions of drugs and physiological solutions were used in all experiments.
Plant collection and extraction
The root material of C. planchonii was collected in November, 2005 from the premises of the University of Agriculture, Makurdi, Benue State, Nigeria. The plant was identified by Patrick Ekwuno of the Department of Wildlife and Forestry, University of Agriculture, Makurdi, Benue State, Nigeria. A voucher specimen (voucher no. DWF-H1105-97) was deposited in the departmental herbarium of the same university.
Dried and pulverized root material of C. planchonii (270 g) was extracted by cold maceration for 48 h at room temperature (27°C) using 70% aqueous methanol. The methanol solution was evaporated under reduced pressure and dried to give a crude residue (9.1 g). The extract was stored at 4°C throughout the duration of this study.
Experimental animals
Albino Wistar mice (190) of either sex (20-35 g) and 25 albino Wistar rats (65-100 g) of either sex were procured from the Animal Unit of the Faculty of Veterinary Medicine, University of Nigeria, Nsukka. They were kept in stainless steel cages and were fed ad libitum with standard laboratory animal feed (Guinea Feed®) and with access to tap water, except in situations where fasting was required. They were maintained in accordance with the recommendation in the Guide for the Care and Use of Laboratory Animals (DHHS, 1985). They were allowed two weeks to acclimatize before the commencement of the experiments.
Brine shrimps lethality test (BSLT)
The method of McLaughlin and coworkers (1991) was used to study the toxicity of C. planchonii extract. Briefly, Artemia salina eggs obtained from a pet shop in Davis, California, were incubated in natural sea water (from Bar Beach, Lagos, Nigeria) in a dam-well under room conditions. About ten 48 h - shrimp nauplii in 1 mL of autoclaved sea water were put into each Bijou bottle using a Pasteur pipette under a stereo-microscope with a light source. They were separated into 4 groups of three.
Increasing concentrations (10, 100, 1000 ppm) of the methanol root extract of C. planchonii were added into each of the groups, and distilled water was added into the control group. The nauplii were incubated at room temperature (28°C) for 24 h, after which the survivors in each well were counted. The results were analyzed using Finney Probit Analysis (MS-DOS computer-program) to determine the LC50 at 95% confidence interval. Weak nauplii were noted as an indication of central nervous system depression.
Acute toxicity test
Thirty albino Wistar mice (21-39 g) of either sex were used for the study. They were randomly divided into six groups of five mice each. They were kept in stainless steel cages and were provided feed and water ad libitum. The mice in the different groups were orally dosed with increasing doses (50,100, 200, 400, and 1000 mg/kgbw) of the C. planchonii extract, while the control group received distilled water (10 mL/kg bw). The mice were observed for mortality and toxic signs for 48 h (CitationAnaga et al., 2006).
Effect of the extract in pentobarbital-induced sleeping time
The method of CitationShetty and Anika (1982) was used for the study. Twenty-five albino Wistar mice (22-31 g) of either sex were used for the study. The mice were randomly divided into five groups of five mice each. They were treated with increasing doses (250, 500, 750, and 1000 mg/kg bw of the extract by the oral route, while the control group received distilled water (10 mL/kg bw). After 30 min, all mice were given sodium pentobarbital (35 mg/kg bw) intraperitoneally. The time of injection, time of sleep (loss of righting reflex) and the time of awakening (regain of righting reflex) were recorded.
Anti-nociceptive (analgesic) effects of C. planchonii extract
The method of CitationMarchioro et al. (2005) was used for the acetic acid-induced writhing test. Five groups of mice consisting of five mice each were fasted for 12 h, but given free access to drinking water. Mice in group A received distilled water (10 mL/kgbw), which served as the negative control, while mice in group B received 400 mg/kgbw acetyl salicylic acid (ASA), which served as the positive control. Mice in groups C-E were treated with 250, 500 and 1000 mg/kg bw of the extract by oral administration respectively. After 45 min, the mice were treated with acetic acid (0.7%, 10 mL/kg bw) through intraperitoneal administration. The number of writhings or abdominal stretches produced in each mouse was counted for 30 min. Antinociception was calculated by the method described by CitationDambisya and Lee (1995) as percentage inhibition of abdominal constrictions using the formula:
The formalin test was studied according to the method of CitationMarchioro et al. (2005). Thirty mice (21-32 g) of either sex were randomly divided into five groups (A-E) of six mice each. Mice in group A received distilled water (10 mg/kgbw) orally, which served as the negative control, while those in group B were treated with ASA (400 mg/kgbw p.o.), which served as the positive control. Groups C-E received graded doses (250, 500, 1000 mg/kgbw) of C. planchonii extract by oral administration, respectively.
After 30 min of the drugs and extract administration, the mice were injected with 50 μL of 1% formalin into the sub-plantar area of the hind limb. The paw licking time (PLT) was recorded using a stop watch after the administration of formalin. The duration the mice continued licking or biting the paws during the first phase (0-5 min) and second phase (20-25 min) of the reaction was recorded. Antinociception was calculated by the method described by CitationDambisya and Lee (1995) as percentage inhibition of abdominal constrictions using the formula:
Anti-inflammatory effect of C. planchonii extract
The anti-inflammatory effect of the extract of C. planchonii was studied using carrageenan-induced paw edema in rats (CitationWinter et al., 1962). Twenty-five albino Wistar rats (149-199 g) of either sex were randomly divided into five groups (A-E) of five rats each. Rats in group A were treated with indomethacin (20 mg/kgbw, orally) suspended in 1% carbonated buffer solution, which served as the positive control. Rats in groups (B-D) were treated with graded doses (250, 500, 1000 mg/kgbw) of the extract by oral administration, while mice in group E received distilled water (10 mL/kgbw), which served as the negative control. Before the treatment, the volume displacement by the normal paw (Vo) was measured for each rat. After 45 min of the extract and indomethacin administration, 50 μL of carrageenan (1%) in normal saline was injected into the sub-plantar area of the hind paw. The change in volume due to carrageenan-induced paw swelling (Vt) of the paw was measured at 1, 2, 3, 4, and 5 h after treatment, using an improved method of CitationGarcia et al. (2004). The percentage inhibition was calculated using the modified formula (CitationDambisya & Lee, 1999; CitationOjewole. 2004) below:
where, Vt = volume displacement by carrageenan-induced paw edema in treated animal, Vo = volume displacement by normal paw in untreated rats. The result was presented as percentage edema inhibition of the mean of each group of rats.
Anticonvulsant effect of C. planchonii extract
Briefly, 15 mice (22-29 g) of either sex were divided into three groups of five mice each. Mice in group A were treated with sodium pentobarbital (35 mg/kg bw, i.p.) and leptazole (90 mg/kg bw, i.p.) simultaneously, while group B mice were pre-treated with the extract (1000 mg/ kg bw, p.o.) and after 45 min with leptazole (90 mg/kg, i.p.). Mice in group C received leptazole (90 mg/kg bw) only by intraperitoneal route, which served as the negative control. The duration of clonic convulsions and mortality were recorded and percentage protection was recorded (CitationHossein & Fatemeh, 2005).
Effect of C. planchonii extract in normoglycaemic mice
Twenty-five male albino Wistar mice (19-24 g) were used for the study. They were fasted for 16 h and fasting blood glucose level was determined. They were divided into five groups of five mice each. The mice were treated as follows: mice in group I received distilled water (10 mL/kg bw), which served as the negative control, while group II received glibenclamide (2.5 mg/kg bw, p.o.), which served as the positive control. Mice in groups (III-V) were treated with graded doses (250, 500, and 1000 mg/kg bw, p.o.) of the extract, respectively. Blood glucose levels were determined at 60, 120, 180, and 360 min after administration of the extract and drug.
Blood glucose levels were determined using blood glucose meter (Roche Diagnostics, London, East Sussex, UK) with ACCU-CHEK (Advantage II) strips using the protocol in the Quick Reference Guide (CitationAtkins et al., 1991). Briefly, a snip was made on the tip of the tail of each mouse to produce a drop of blood and then placed on the strip already inserted into the blood glucose meter, to read off the blood glucose level. The result was presented in mg/100 mL blood.
Effect of C. planchonii extract in alloxan-induced hyperglycemia in mice
Thirty male albino Wistar mice (24-27 g) were used. They were fasted for 16 h and the fasting blood glucose levels were determined. Hyperglycemia was induced by a single intraperitoneal injection of 150 mg/kg of alloxan monohydrate (AM) in 10 mM citrate buffer (pH 4.5) (CitationAnaga et al., 2004). The blood glucose levels were determined 7 days post-administration of AM. Mice having high blood glucose (> 400 mg/100 mL of blood) were considered hyperglycemic.
The hyperglycemic mice were randomly divided into five groups of six mice each and were treated as follows: Group I, distilled water (10 mL/kg bw, negative control); Group II, glibenclamide (2.5 mg/kg bw, positive control); Groups (III-V), graded doses (250, 500 and 1000 mg/kgbw) of the extract respectively. The extract and the drug were given orally. The blood glucose levels were determined 60, 120, 180, and 360 min after administration of the drug and the extract. The blood glucose level was determined following the procedure described above.
Phytochemical spot test
The methanol extract of C. planchonii roots was tested for the presence of alkaloids, flavonoids, tannins, glycoside, saponins, proteins, and carbohydrate using the standard procedures (CitationTrease & Evans, 2002).
Data analysis
The results were presented as mean ± SEM and subjected to one-way analysis of variance (ANOVA) followed by post-hoc multiple-comparison Dunnett’s test to determine the level of significance between “test” and “control” group data means. Values of p <0.05 were considered statistically significant.
Results
The yield of the extract of C. planchonii roots after the removal of the methanol in vacuo was 3.4% w/w dry matter. The extract was dark brown in color with a syrupy consistency and pleasant odor.
No mortality was recorded in the acute toxicity test. The clinical signs observed in the treated mice include drowsiness, depression, reduced activity and clumping together. The effect of the methanol extract of C. planchonii roots on brine shrimp nauplii showed that the LC50 was 4.45 ppm at 95% confidence interval. The surviving nauplii were very sluggish in movement.
The effect of C. planchonii extract on pentobarbital-induced sleeping time is presented in . The extract significantly (p <0.05) increased the sleeping time at the doses (500, 750, and 1000 mg/kg bw) when compared with the control. The result of the effect of the C. planchonii extract on acetic acid-induced abdominal constrictions in mice is presented in . The extract significantly (p <0.05) decreased the number of abdominal writhings in all the doses of the extract and the drugs used in the study. Also, the extract at 500 mg/kg bw (p.o.) gave the highest activity (74%) when compared with the control (). The extract (500 mg/kg bw, p.o.) significantly (p <0.05) decreased the number of abdominal writhings when compared with ASA (400 mg/kg bw, p.o.), the reference drug. The result of the effect of the extract on formalin-induced nociception is presented in . The extract (250, 500, 1000 mg/kg bw, p.o.) and ASA (400 mg/kg bw, p.o.) significantly (p <0.05) decreased the number of paw licking by the mice in both early (0-5 min) and late phases (25-20 min) of formalin-induced nociception, when compared with the control group. The percentage inhibition of pain showed that the extract was more potent in late phase (70-88%) than early phase (44-77%) of nociception (). On comparing the extract-treated groups with the group that received ASA, reference drug, extract in all doses was more effective than the reference drug. The extract had no protection against leptazole-induced convulsion in mice ().
Figure 1. Effect of the methanol root extract of C. planchonii on pentobarbital-induced sleeping time. *Significant at p <0.05 compared with the control group.
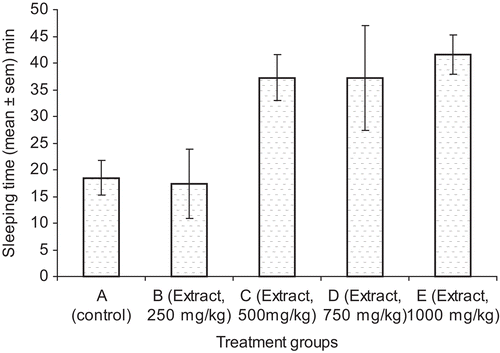
Table 1. Effect of the methanol root extract of C. planchonii on acetic acid-induced writhing (analgesic test).
Table 2. Effect of the methanol root extract of C. planchonii on formalin (analgesic) test.
Table 3. Effect of the methanol root extract of C. planchonii on leptazole-induced convulsions.
The anti-inflammatory effect of the methanol extract of C. planchonii roots on carrageenan-induced paw edema is presented in . The extract at the dose of 250 mg/kg bw (p.o.) and the reference drug, indomethacin (20 mg/kg bw, p.o.) induced biphasic inhibition of paw edema development. The first phase of edema inhibition occurred in the first 2 h and the second phase occurred between 4-6 h post-administration of the drug and the extract. The extract (250 mg/kg bw, p.o.) caused about 40% inhibition of carrageenan-induced paw edema at 1 h, which declined to zero at 3 h post-administration of the extract in the first phase observation. The second phase of paw edema inhibition by the extract commenced immediately, gradually increased, and peaked (50%) at 6 h after administration of the extract (). Indomethacin (20 mg/kg bw, p.o.) caused about 45% edema inhibition at 2 h (first phase) and about 60% activity (second phase) at about 4 h after administration of the drug ().
Figure 2. Effect of the methanol root extract of C. planchonii in carrageenan-induced paw edema in rats (anti-inflammatory test). CP, C. planchonii extract, p.o., per os.
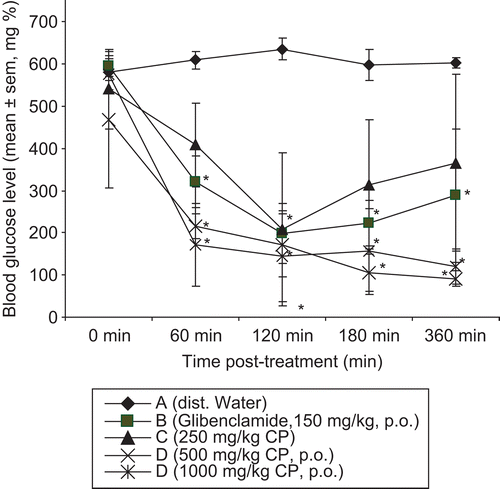
The extract had no significant effect on blood glucose level in normoglycemic rats (). The effect of the methanol root extract of the plant on AM-induced hyperglycemia is presented in . The extract (250, 500, 1000 mg/kg bw, p.o.) and glibenclamide (2.5 mg/kg bw, p.o.) significantly (p <0.05) decreased the blood glucose level throughout the duration of the study. The antihyperglycemic effect of the plant extract was very prominent at 60 min post-administration, which continued throughout the period of study. At 360 min after administration, the extract (1, 000 mg/kg bw, p.o.) reduced the blood glucose level slightly below the normoglycemic level (). Weight loss and decrease in feed intake were observed during the period of the study, but improved in recovered mice (data not provided).
Figure 3. Antihyperglycemic effect of the methanol root extract of C. planchonii in alloxan-induce hyperglycemic mice. Significant at p < 0.05 with the control group; CP, C. planchonii extract; p.o., per os.
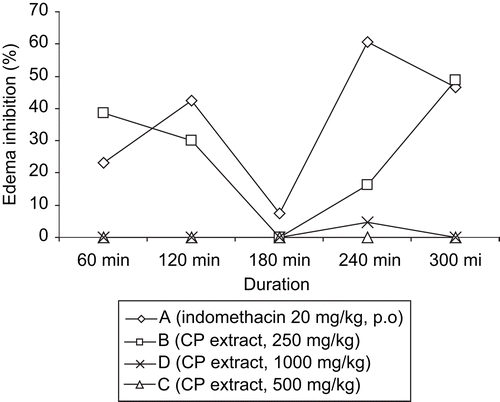
Table 4. Effect of the methanol root extract of C. planchonii on blood glucose level in normoglycemic rats.
The phytochemical spot test result showed the presence of saponins, tannins, glycosides, and carbohydrates.
Discussion
The present investigation was studied to scientifically confirm the ethno-medicinal uses of C. planchonii in vivo and in vitro. The oral administration of the methanol root extract of C. planchonii was well tolerated in all doses (250- 3000 mg/kg bw, p.o.), as no mortality was recorded over 48 h duration. The animals exhibited clinical signs such as drowsiness, depression, reduced activity and clumping together, which may be due to the involvement of the nervous system. Full recovery was 48 h post-administration of the extract. The result of BSLT established that the extract contains very potent bioactive compounds. The bioactivity was rated very high due to the very low LC50 (4.42 ppm) of the extract. The ED50 value for general bioactivity is approximately one tenth of the value of the LC50 in BSLT (CitationMcLaughlin et al., 1991). Therefore, the ED50 of C. planchonii extract was approximated to 0.44 ppm (440 μg/mL). The surviving nauplii were dull and inactive, which is a sign of CNS depression (CitationAllurin et al., 2005; CitationMcLaughlin et al., 1991).
The extract caused CNS depression in mice because it significantly increased the pentobarbital-induced sleeping time by more than 200% at the highest dose (1000 mg/kg bw, p.o.). Though the extract of C. planchonii depressed the CNS, it was expected that it would protect mice from leptazole-induced convulsions and mortality, but the result of the anticonvulsant study showed that the extract did not protect the mice from leptazole toxicity (). The CNS depressant effect of the extract corroborates with some of the clinical observations in the acute toxicity study and BSLT.
Two anti-nociceptive models were used to determine whether the analgesic effect was central or peripheral in origin, inflammatory or non-inflammatory. The extract showed a promising anti-nociceptive effect because it significantly decreased the number of acetic acid-induced abdominal constrictions in all the doses (250, 500, and 1000 mg/kg bw, p.o.) of the extract. The percentage pain inhibition showed that the extract (250 and 500 mg/kg bw, p.o.) was more effective than ASA (400 mg/kg bw, p.o.) (). Further increase in the dose (1000 mg/kg, p.o.) led to decrease in activity as observed in the study. Since the extract increased pentobarbital-induced sleeping time and weakened the movement of surviving nauplii in BSLT, it is presumed that the extract has central nervous system depressant effect, as central depressants and antihistamines are known to reduce the number of abdominal writhings (CitationOnasanwo & Elegbe, 2006).
In an effort to further classify the type of analgesia – inflammatory or non-inflammatory, the formalin model was used. Conversely, the extract (500 and 1000 mg/kg) potentiated the direct effect of formalin by more than 250% increase in the PLT in the early (0-5 min) phase reaction. The methanol extract of C. planchonii roots at all doses (250, 500, 1000 mg/kg bw) significantly decreased the PLT in the late phase (20-25 min) when compared with the positive and negative controls (). This effect observed in the late phase showed the involvement of inflammatory nociception (CitationHunskaar & Hole, 1997; CitationOlajide et al., 2000). Also, the percentage pain inhibition showed that the extract was similar to ASA (400 mg/kg bw, p.o.) in decreasing formalin-induced pain. Therefore, the analgesic effect of C. planchonii extract is presumed to be mediated by inflammatory mechanism.
To confirm the inflammatory involvement of the extract in relieving pain, the effect of the extract on carrageenan-induced paw edema (anti-inflammatory effect) was studied. The methanol root extract of C. planchonii at the dose of 250 mg/kg bw (p.o.) produced a typical biphasic inhibition of paw edema induced by carrageenan in rats (). C. planchonii extract induced about 40% reduction in paw edema at 60 min post- administration (early phase). The early phase begins after the injection of carrageenan and lasts for 60 min, characterized by the release of histamine and serotonin, which are blocked by antihistamine and antiserotonin agents (CitationCrunkhon & Meacock, 1971). Therefore, extract is presumed to be mediated via antihistamine and antiserotonin mechanisms as observed in the early phase. The effect of the plant extract was similar in time and pattern with indomethacin (20 mg/kg bw). Hence, the effect was indomethacin-like in action, by reducing carrageenan-edema in the delayed phase, probably by inhibiting tissue prostaglandins and plasma kinins (CitationVinegar et al. 1969; CitationFerreira et al., 1991; CitationMahgoub, 2002). Therefore, the results of the present study suggest that the methanol root extract of C. planchonii induced analgesic and anti-inflammatory effects, probably by inhibiting the release, synthesis and/or production of inflammatory mediators such as prostaglandins, histamine and other polypeptide kinins (CitationGamache et al., 1986, CitationHunskaar & Hole, 1997; CitationOlajide et al., 2000).
Acute treatment of the control group of mice with distilled water alone did not produce any change in blood glucose level throughout the duration of the study in AM-diabetic mice (). However, the methanol root extract of C. planchonii, like glibenclamide produced significant reductions in the blood glucose levels of the fasted AM-treated diabetic mice in all the doses used and over the duration of the study. The extract at 1000 mg/kg bw (p.o.) showed the highest activity by decreasing the blood glucose level by more than 500%. The effect was superior to that of glibenclamide that was used as a standard. The antihyperglycemic effect of C. planchonii confirmed its use in Benue State, Nigeria, in traditional management of diabetes mellitus (CitationIgoli et al., 2002). Unlike some oral sulfonylureas, the extract did not cause any significant effect in blood glucose level in normoglycemic rats, which is an advantage in preventing hypoglycemic coma in normal patients (CitationJohnson & Bressler, 1981). Therefore, the antihyperglycemic effect of the extract would appear to be mediated via a mechanism that is similar to glibenclamide, an oral hypoglycemic sulfonylurea (CitationOjewole, 2004).
Finally, the extract of C. planchonii contains saponins, tannins, glycosides and carbohydrates. The presence of alkaloids and flavonoids was questionable. The extract contains very high concentration of saponins, which have been associated with some of the CNS effects. CitationAliyu et al. (1995) have isolated zinc and manganese from C. planchonii, and these minerals have been associated with antidiabetic effect in experimental animals (CitationMarles & Farnsworth, 1995).
In conclusion, the methanol root extract of C. planchonii contains very potent biologically active compounds, which have moderate sedative property, a potent analgesic effect mediated by neurogenic and inflammatory mechanisms, a good anti-inflammatory activity, potent antihyperglycemic effect in hyperglycemic rats, and low toxicity. Further studies to systematically isolate the active metabolites from the root of C. planchonii will be needed. The extract or its isolated chemical components could present a new resource for the development of new plant-based therapy useful in the control of pain, inflammation, and diabetes mellitus.
Acknowledgements
We are grateful to P. Ekwuno of the Department of Wildlife and Forestry, University of Agriculture, Makurdi, Nigeria, for the collection and identification of the plant material. We acknowledge Professor I.U. Asuzu of the Department of Veterinary Physiology and Pharmacology, University of Nigeria for providing carrageenan used in this study and proof reading the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akobundu JO, Agyakwa CN (1987): A Handbook of West African Weeds. Ibadan, Nigeria, IITA Publications, pp. 232–233.
- Aliyu S, Okoye ZSC, Thomas SW (1995): The hepatoprotective cytochrome P450 enzyme inhibitor isolated from Nigerian medicinal plant: C. planchonii is a zinc salt. J Ethnopharmacol 48: 89–97.
- Alluri UK, Tayi VN, Roa-Dodda S, Mulabagal V, Hsin-Sheng T, Guttumukkala VS (2005): Assessment of bioactivity of Indian medicinal plants using brine shrimps (Artemia salina) lethality assay. Int J Appl Sci and Eng 3: 125–134.
- Anaga AO, Njoku CJ, Ekejiuba ES, Esiaka MN, Asuzu, IU (2004): Investigation of the methanolic leaf extract of Costus afer Ker for pharmacological activities in vitro and in vivo. Phytomedicine 11: 242–248.
- Anaga AO, Shoyinka SVO, Asuzu IU (2006): Toxic effects of Dennettia tripetala root extract. Pharm Biol 44: 451–461.
- Atawodi SE (2005): Comparative in vitro trypanocidal activities of petroleum ether, chloroform, methanol and aqueous extracts of some Nigeria Savannah plants. Afr J Biotech 4: 177–182.
- Atkins SH, Dasmahapatra A, Jaker MA, Chorost MI, Reddy S (1991): Fingerstick glucose determination in shock. Ann Int Med 114: 1020–1024.
- Benoit-Vical F, Valentin A, Mallie M, Bastide SM, Bessiere JM (1999): In vitro antimalarial activity and cytotoxicity of C. tinctorium and C. planchonii leaf extracts and essential oils. Planta Med 65: 378–381.
- Burkill HM (1985): The Useful Plants of West Tropical Africa, Vol. 1. Kew, Royal Botanic Gardens, pp. 386–387.
- Crunkhon P, Meacock SER (1971): Mediators of the inflammation induced in the rat paw by carrageenan. Br J Pharmacol 42: 392–402.
- Dambisya YM, Lee TL (1995): Effects of l-NAME, l-NMMA, and l- arginine on the analgesic effects of morphine in mice. Methods Fund Exp Clin Pharmacol 17: 577–582.
- Dambisya YM, Lee SB (1999): Influence of temperature, pH and naloxone on antinociceptive activity of Channa striatus (Haruan) extract in mice. J Ethnopharmacol 66: 181–186.
- Ferreira SH, Duarte IDG, Lorenzetti BB (1991): Molecular mechanism of action of peripheral morphine analgesia: Stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol 201: 121–122.
- Gamache DA, Poysishock J, Ellis EF (1986): Anti-inflammatory and antinociceptive properties of dantrolene sodium in rats and mice. Pharmacol Res 45: 456–460.
- Garcia MD, Fernendez MA, Alvarez A, Saenz MT (2004): Anti-nociceptive and anti-inflammatory effects of the aqueous extracts from the leaves of Pimenta racemosa var. ozua. J Ethnopharmacol 91: 67–73.
- Hossein H, Fatemeh T (2005): Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia 76: 722–724.
- Hunskaar S, Hole K (1997): The formalin test in mice: dissociation between inflammatory and non-inflammatory and pain. Pain 30: 103–114.
- Igoli T, Anyim TA, Usman AA, Oluma HOA, Igoli, NP (2002): Folk medicines of the lower Benue valley of Nigeria, in: Sigh VK, Govil JN, Hashmi S, Singh G, eds, Recent Progress in Medicinal Plants, Vol. 7. Ethnomedicine and Pharmacognosy II. Science Technology Publication, Chicago, Illinois, pp. 327–388.
- Johnson JE, Bressler R (1981). Clinical pharmacology of suphonylureas hypoglycaemic agent. Part 1. Drugs 22: 211–245.
- Mabberley, DJ (1981): The Plant Book: A Portable Dictionary of Higher Plants. Cambridge, Cambridge University Press, p. 424.
- Marchioro M, Blank MFA, Mourão RHV, Antoniolli AR (2005): Antinociceptive activity of the aqueous extract of Erythrina velutina leaves. Fitoterapia 75: 637–642.
- Mahgoub AA (2002): Grapefruit juice potentiates the anti-inflammatory effect of diclofenac on carrrageenan-induced rat’s paw edema. Pharmacol Res 45: 1–5.
- Marles RJ, Farnsworth NR (1995): Antidiabetic plants and their constituents. Phytomedicine 2: 137–189.
- McDonald LC (1988): Hormones influencing metabolism, in: Booth NH, McDonald LE, eds., Veterinary Pharmacology and Therapeutics, 6th edn. Iowa State University Press, Ames, pp. 616–656.
- McLaughlin JL, Chang CJ, Smith D (1991): Bench-top bioassays for the discovery of bioactive natural products: An update, in: Atta-ur-Rahman, ed., Studies in Natural Product Chemistry, Vol. 9. Amsterdam, Elsevier, pp. 383–408.
- Ojewole JAO (2004): Evaluation of the analgesic, anti-inflammatory and antidiabetic properties of Sclerocarya birrea (A. Rich.) Hosch stem-bark aqueous extract in mice and rats. Phytother Res 18: 601–608.
- Olajide OA, Awe SO, Makinde SM, Ekhelar AI, Olusola A, Morebise O, Okpako DT (2000): Studies on anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. J Ethnopharmacol 71: 79–89.
- Onasanwo SA, Elegbe RA (2006): Anti-nociceptive and anti-inflammatory properties of the leaf extracts of Hedranthera barteri in rats and mice. Afr J Biomed Res 9: 109–118.
- Shetty SN, Anika SM (1982): Laboratory Manual of Pharmacology and Toxicology. Enugu, Nigeria, Fourth Dimension, pp. 43–47.
- Trease GE, Evans WC (2002): Pharmacognosy 15th edition. London, W.B. Saunders, pp. 58–302.
- Vinegar R, Schreiber W, Hugo R (1969): Biphasic development of carrageenan edema in rats. J Pharmacol Exp Therap 166: 96–103.
- Winter C A, Risley EA, Nuss CW (1962): Carrageenan-induced edema in the hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soci Expl Biol and Med 111: 544–547.
