Abstract
A methanol extract from the stem bark of Antiaris africana Engler (Moraceae), as well as compounds isolated and identified as betulinic acid (1), 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene (2), ursolic acid (3), oleanolic acid (4), strophanthidol (5), periplogenin (6), convallatoxin (7), strophanthidinic acid (8), methyl strophanthinate (9), and 3,3′-dimethoxy-4′-O-β-d-xylopyronosylellagic acid (10), were tested for their antioxidant and anticancer activities. The DPPH radical scavenging assay was used for the antioxidant test while the potato disk tumor induction and XTT assays were used to detect antitumor activities. The antioxidant test showed that the methanol extract and compounds 1, 9, and 10, as well as vitamin C, used as reference antioxidant drug, were able to interact with more than 50% DPPH. The results of the potato disk tumor induction assay also indicated a pronounced tumor reducing activity of the methanol extract (83.12%) and compound 10 (96.64%). Samples showing more than 20% inhibition of Crown gall tumors were then tested against human DU-145 and hepatocarcinoma Hep G2 cells. The results showed inhibitory activities of 64.12% and 73.62%, respectively, on DU-145 and Hep G2 cells for the methanol extract. Compound 10 showed the highest inhibition potency on both cell lines with more than 70% inhibition at 50 μg/mL. The methanol extract showed an IC50 value lower than this at 30 μg/mL, a threshold value for potential antineoplastic extracts. The results of the present study provide supportive data for the traditional anticancer use of A. africana and indicate that the methanol extract as well as compound 10 represent a potential source of medicine for the treatment of cancer, having also interesting antioxidant properties.
Introduction
Antiaris africana Engler (Moraceae) is a tree usually about 15–20 m high, but it can grow sometimes up to 40 m, and has white latex and alternate dissymmetric leaves (CitationBerg et al., 1985; CitationBerhaut, 1979). The wood is used in some regions of Africa as timber (CitationBerhaut, 1979; CitationOkogun et al., 1976). The bark extract is used in traditional medicine for the treatment of chest pains (CitationOkogun et al., 1976). Leaf decoctions are applied in the treatment of syphilis (CitationBerhaut, 1979), and the latex is a purgative agent. It is also used as a cure for sore throat and leprosy. This plant is also used in Cameroon folk medicine in the treatment of cancer (personal communication; by Victor Kuete as collected from traditional healers). Cancer is the largest single cause of death in both men and women. Recently, resistance to anticancer drugs has been observed. Therefore, the research and development of more effective and less toxic drugs by the pharmaceutical industry has become necessary. Many substances derived from medicinal plants are known to be effective and versatile chemopreventive and antitumoral agents in a number of experimental models of carcinogenesis. Crown gall tumor antiproliferative screening models in vitro provide important preliminary data to help select plant extracts with potential antineoplastic properties for future study. The inhibition of A. tumefaciens-induced tumor (or Crown gall) in potato disk tissue is an assay based on antimitotic activity, and can detect a broad range of known and novel antitumor effects (CitationMcLaughlin & Rogers, 1998). Crown gall is a neoplastic plant disease caused by A. tumefaciens (CitationKahl & Schell, 1982; CitationLippincott & Lippincott, 1975). The validity of this bioassay is predicted on the observation that certain tumorigenic mechanisms are similar in plants and animals (CitationBecker, 1975; CitationBraun, 1972; CitationKarpas, 1982). The present study was therefore aimed to assess the antioxidant and anticancer activity of the methanol extract and compounds from the stem bark of A. africana.
Materials and methods
Plant material and extraction
The stem bark of Antiaris africana was collected from Mont Eloundem (Yaoundé) in the Central Province of Cameroon, in May 2006. Mr. Victor Nana of the National Herbarium of Cameroon identified the plant. A voucher specimen (No. 5925 SRF/Cam) is deposited at the National Herbarium, Yaoundé, Cameroon.
Extraction and compound isolation
The air-dried and powdered stem bark (5 kg) of Antiaris africana was macerated with 15 L MeOH at room temperature for 72 h. Removal of the solvent from the extract under reduced pressure yielded a dark brown residue (100 g).
A part (80 g) of the methanol extract was adsorbed on 100 g of silica gel 60 (0.05–02 mm (230–400 mesh)) with 0.1 L methanol as solvent, and dried under reduced pressure. The brown powder obtained was submitted to gradient flash chromatography on a 600 g silica gel 60 column (open, 15 × 15 cm) (eluent: n-hexane, n-hexane–EtOAc, EtOAc, as the mobile phase and collecting 500 mL fractions). This yielded 75 fractions pooled into eight major components by combining the eluates on the basis of thin layer chromatography (TLC). Further separation of these fractions was done by repeated column chromatography using silica gel 60 (0.05–0.2 mm, 230–400 mesh) and Sephadex LH-20. Fraction 3 (5.1 g) (eluted with n-hexane–EtOAc 8:2) was further separated by silica gel column chromatography (30 × 350 mm), eluting with n-hexane–ethyl acetate in order of increasing polarity from 15 to 35% to give 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene C32H52O4 (2; 3 mg; white powder; m/z: 500; m.p.: 246–248°C) (CitationGonzalez et al., 1987), and a mixture which was further purified on Sephadex LH-20 (20 × 750 mm), eluting with CH2Cl2–MeOH 3:2 to give ursolic acid C30H48O3 (3; 8 mg; white powder; m/z: 456; m.p.: 283–284°C) (CitationSeebacher et al., 2003), and oleanolic acid C30H48O3 (4; 4 mg; white amorphous powder; m/z: 456) (CitationSeebacher et al., 2003). Further purification of fraction 4 (2.5 g), eluted with n-hexane–EtOAc (6.5:3.5), afforded betulinic acid C30H40O3 (1; 8.6 mg; white grains; m/z: 456; m.p.: 282–283°C) (CitationMahato & Kundu, 1994). Fraction 6 (5.0 g) (eluted with n-hexane–EtOAc 1:1) gave periplogenin C23H34O5 (6; 35 mg; white crystals; m/z: 390; m.p.: 138–139°C) (CitationJunior & Wichtl, 1980) when fixed on silica gel and subjected to silica gel column (25 × 500 mm) eluting with n-hexane–EtOAc 1:1 in order of increasing polarity up to n-hexane–EtOAc 25:75. Further purification of fraction 7 (eluted with n-hexane–EtOAc 4:6) on a 25 × 300 mm silica gel column with n-hexane–EtOAc 1:1 in order of increasing polarity up to n-hexane–EtOAc 2:8 gave strophanthidol C23H34O6 (5; 13 mg; white crystals; m/z: 406; m.p.: 263–265°C) (CitationSharipov et al., 1969). Fraction 8 (eluted with n-hexane–EtOAc 2.5:7.5) afforded a white precipitate identified as convallatoxin C29h42o10 (7; 5 mg; white amorphous powder; m/z: 550) (CitationSchenk et al., 1980), and a filtrate. The filtrate was chromatographed on a 15 × 250 mm silica gel column with n-hexane–EtOAc 25:75 to yield strophanthidinic acid C23H32O7 (8; 9 mg; white grains; m/z: 420; m.p.: 163–164°C) (CitationRobien et al., 1987), and 3,3′-dimethoxy-4′-O-β-d-xylopyronosylellagic acid C21H18O12 (10; 3 mg; yellow amorphous powder; m/z: 462) (CitationLi et al., 1999) and methyl strophanthinate C24H34O7 (9; 15 mg; yellow amorphous powder; m/z: 434) (CitationRobien et al., 1987). The chemical structures of the isolated compounds are illustrated in .
General procedure
Aluminum sheet pre-coated with silica gel 60 F254nm (Merck) was used for thin layer chromatography, eluted with mixtures of solvents such as: hexane/CH2Cl2 (9:1); CH2Cl2/MeOH (19:1); CH2Cl2/MeOH (9:1); and the spots were visualized using both ultraviolet light (254 and 366 nm) and 50% H2SO4 as spray reagent. The structure of an isolated compound was determined using a Bruker AC 400 spectrometer and a Varian Gemini 400 MHz instrument for one-dimensional (1D) nuclear magnetic resonance (NMR). Multi-impulsional 2D NMR experiments [1H-1H COSY (correlation spectroscopy], 1H-1H NOESY (nuclear Overhauser effect spectroscopy), 13C-1H HSQC (heteronuclear single quantum correlation), 13C-1H HMBC (heteronuclear multiple bond connectivity) were performed using standard Bruker or Varian Gemini micro-programs. The melting points were determined using a Kofler microhot stage apparatus. Mass spectra were recorded with ZQ 2000 Waters and Q-Tof1 Micromass spectrometers using electrospray ionization (ESI-MS: Uc = 30 V), a Nermag R10-10C spectrometer, and an HP-5973 mass selective detector.
Antioxidant investigation: DPPH assay method
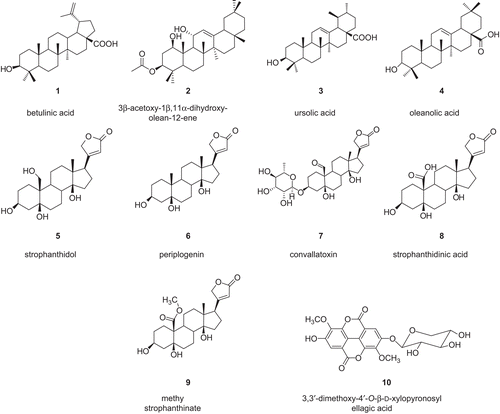
The free radical scavenging activity of the methanol extract and compounds was evaluated as described by CitationMensor et al. (2001). Briefly, the test samples, prior dissolved in DMSO (dimethylsulfoxide; Sigma), were mixed with a 0.3 mM 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) ethanol solution, to give final concentrations of 5, 10, 50, 100, and 500 μg/mL of sample per microliter of DPPH solution. After 30 min at room temperature, the absorbance values were measured at 517 nm and converted into percentage antioxidant activity. Ascorbic acid was used as a standard control. Each assay was repeated three times and the results, recorded as a mean of the triplicated experiments, were graphically illustrated (). The inhibition ratio (%) was calculated as follows: % inhibition = [(absorbance of control – absorbance of test sample)/absorbance of control] × 100.
Figure 2. Free radical scavenging activity of the methanol extract, compounds from Antiaris africana, and vitamin C: values with the same letter are not significantly different (ANOVA, p < 0.05). 1, betulinic acid; 2, 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene; 3, ursolic acid; 4, oleanolic acid; 5, strophanthidol; 6, periplogenin; 7, convallatoxin; 8, strophanthidinic acid; 9, methylstrophanthinate; 10, 3,3′-dimethoxy-4′-O-β-d-xylopyronosylellagic acid.
Preliminary antitumor test: potato disk tumor induction assay
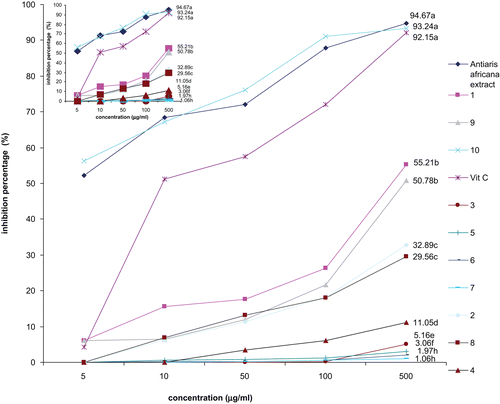
The antitumor assay was carried out as described by CitationCoker et al. (2003) as follows. Agrobacterium tumefaciens LMG 184 (provided by the Laboratory of Microbiology, University of Gent, Belgium) was grown on yeast extract medium (YEM) for 48 h at 28°C. Red potatoes [Solanum tuberosum (Solanaceae)] obtained from the local market (Yaoundé, Cameroon) were washed under running water and disinfected in 10% bleach for 20 min. Potato sections (0.5 cm thick) were made and placed in bleach (20%) for 15 min, then aseptically transferred in sterile distilled water. These disks were placed in a 24-well culture plate containing 15% water agar. Suspensions of A. tumefaciens in phosphate-buffered saline (PBS) were standardized to 1 × 109 colony forming units (CFU), prepared following a McFarland turbidity standard. The methanol extract and compounds were dissolved in DMSO at a concentration of 100 μg/mL. Controls included DMSO with PBS; DMSO without the bacterium; and DMSO with the bacterium. The test solutions consisted of 400 μL of each solution, 100 μL water, and 400 μL of the standardized bacterium suspension. Each disk in the 24-well culture plate was overlaid with 50 μL of the appropriate extract/water/bacterium mixture and incubated at room temperature for 12 days. On day 12, the disks were stained with Lugol’s reagent. The stained potato disks were viewed under a dissecting microscope and the tumors were counted. Twelve replicates were analyzed for each sample, and the final results were graphically () reported as the mean of 12 experiments. Bacterial viability was determined by incubating each sample solution with 1 × 109 CFU of bacterial suspension. At 10, 20, 30, and 60 min after inoculation, 10 μL of each solution was removed and placed on YEM plates, and incubated for 24 h. Bacterial growth was evidenced by growth across the plates.
Antitumor assay on human DU-145 prostate carcinoma and hepatoma Hep G2 cells
The anticancer activity of samples showing more than 20% inhibition of Crown gall tumors on human DU-145 (androgen-insensitive prostate cancer cells) and hepatocarcinoma Hep G2 cells was evaluated following XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxyanilide inner salt) assay (CitationGerlier & Thomasset, 1986; CitationItharat et al., 2004; CitationZee-Cheng, 1997). Briefly, cells were cultured in Dulbecco’s minimum essential medium (DMEM) supplemented with 5% fetal calf serum (FCS), gentamicin sulfate (0.004%), glucose (0.57%), and NaHCO3 (0.12%). Cells were seeded into 96-well flat-bottomed plates at a concentration of 3.0 × 105 cells per mL. After 24 h, cells were treated with samples, which were diluted with culture medium to a final concentration of 25 μg/mL (CitationItharat et al., 2004; CitationZee-Cheng, 1997). XTT labeling reagent (50:1) was added and the absorbance (560 nm) read after 72 h (CitationGerlier & Thomasset, 1986). Experiments were carried out three times, in triplicate. Active samples (with less than 50% survival) after an exposure time of 72 h were serially diluted in a concentration range of 3.12–50 μg/mL and tested. The concentration of the sample that inhibited 50% cell proliferation (IC50) was determined graphically. Doxorubicin, a known antitumor agent, was used as positive control. The cell survival percentage was determined using the formula: % cell survival = (ODT/ODC) × 100; ODT and ODC being the absorbance of the test sample-treated group and the control group (0.1% DMSO), respectively.
Statistics
One-way analysis of variance (ANOVA) at the 95% confidence level was used for all statistical analysis.
Results and discussion
The structures of the isolated compounds were established using spectroscopic analysis, especially NMR spectra in conjunction with 2D experiments, COSY, HMQC, HSQC, and direct comparison with published information and with authentic specimens obtained by our research group for some cases. The compounds isolated from the stem bark of A. africana () were four pentacyclic triterpenes identified as betulinic acid (1), 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene (2), ursolic acid (3), oleanolic acid (4), cardenolide, known as strophanthidol (5), periplogenin (6), convallatoxin (7), strophanthidinic acid (8), methylstrophanthinate (9), and a known ellagic acid derivative, 3,3′-dimethoxy-4′-O-β-d-xylopyronosylellagic acid (10). In this study, we evaluated the DPPH scavenging activity and anticancer activities of these compounds together with those of the methanol extract, and the results are reported in – and .
Figure 3. Antitumor activity of the methanol extract and compounds from Antiaris africana as obtained in Crown gall assay: values with the same letter are not significantly different (ANOVA, p < 0.05). 1, betulinic acid; 2, 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene; 3, ursolic acid; 4, oleanolic acid; 5, strophanthidol; 6, periplogenin; 7, convallatoxin; 8, strophanthidinic acid; 9, methylstrophanthinate; 10, 3,3′-dimethoxy-4′-O-β-d-xylopyronosylellagic acid.
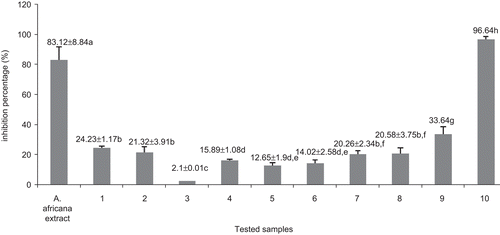
Figure 4. Effects of the methanol extract and compounds from Antiaris africana on (A) DU-145 and (B) Hep G2 cell survivals: values with the same letter are not significantly different (ANOVA, p < 0.05). 1, betulinic acid; 2, 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene; 7, convallatoxin; 8, strophanthidinic acid; 9, methylstrophanthinate; 10, 3,3′-dimethoxy-4′-O-β-d-xylopyronosylellagic acid.
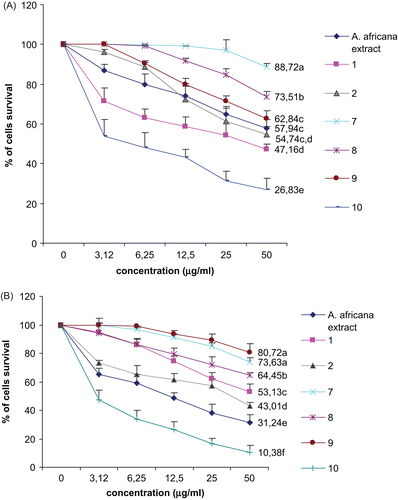
Table 1. Activity of the crude extract from A. africana, selected compounds, and doxorubicin on human cancer cell lines DU-145 and Hep G2.
The DPPH scavenging activity of the tested samples is summarized in . It appeared that all tested compounds showed dose-dependent inhibitory potency. At the concentration of 500 μg/mL, only the methanol extract, compounds 1, 9, and 10, and vitamin C, used as the reference antioxidant drug, showed more than 50% inhibition (). Compound 10 and the methanol extract exhibited very high activity (93.24 and 94.67%, respectively), not significantly different (p < 0.05) from that of vitamin C (92.15%). The high scavenging potency of the tested ellagic acid derivative (10) could also explain the activity of the methanol extract. However, the antioxidant activity of such derivatives is well known (CitationLiu et al., 2007).
The results of the potato disk tumor induction assay are illustrated in . It was found from the results of the bacterial viability test that the concentration of the tested sample did not alter A. tumefaciens growth at 10, 20, 30 min, and 1 h of treatment. The three controls used in this assay included DMSO with PBS; DMSO without bacterium; and DMSO with bacterium. The two first controls did not induce tumor, showing that neither DMSO nor PBS interfere with the activity of A. tumefaciens or induce tumor themselves. DMSO with A. tumefaciens induced an average of 32 tumors. Pronounced tumor reducing activity was observed with the methanol extract (83.12%) and compound 10 (96.64%). This experiment supports the traditional use of this plant as an anticancer drug, and indicates that compound 10 could be the most implicated antineoplastic principle. These data allowed selection of compounds for more specific human tumor cell line testing. It has been demonstrated that samples with more than 20% inhibitory potency of Crown gall tumors can show interesting activity on many human cancer cells (CitationCoker et al., 2003; CitationMcLaughlin & Rogers, 1998). In this study, more than 20% inhibition activity was obtained with the methanol extract, and compounds 1, 2, 7, 8, 9, and 10. They were therefore selected for their activity against human DU-145 and hepatocarcinoma Hep G2 cells. The results are illustrated in both and .
The highest DU-145 and Hep G2 cell survival was noted after treatment with compounds 7, 8, and 9 (). At 50 μg/mL, compound 7 induced 73.63 and 88.72% survival of Hep G2 and DU-145 cancer cell lines, respectively, while compound 8 allowed 73.51% DU-145 and 64.65% survival of the Hep G2 cell line. Compound 9 induced growth of 62.84% DU-145 and 80.72% Hep G2 cells (). The inhibitory activity recorded with the methanol extract was 64.12 and 73.62% on DU-145 and Hep G2 cells, respectively. Compound 10 showed the highest inhibition potency on both cell lines, with more than 70% inhibition at 50 μg/mL. The American National Cancer Institute guidelines set the limit of activity for crude extracts at a 50% inhibition (IC50) of proliferation of less than 30 μg/mL after an exposure time of 72 h (CitationSuffness & Pezzuto, 1990).
The methanol extract and compound 10 showed IC50 values lower than this threshold value, suggesting that they are potential antineoplastic agents. The results of this experiment validate the use of the disk tumor induction assay as a preliminary step for selecting anticancer samples, as the good activity observed for the methanol extract and the ellagic acid derivative (compound 10) was confirmed on human carcinoma DU-145 and Hep G2 cells. However, the anticancer activity of ellagic acid-like compounds is well demonstrated (CitationPettit et al., 1987).
The overall results of this study are very interesting, taking into account the incidence of prostate cancer and hepatocarcinoma. Prostate carcinoma appears as the most common tumor in men (CitationThompson et al., 1997). Human liver tumors, particularly hepatocellular carcinoma, are among the most common malignancies worldwide (CitationLotze et al., 1993), with an estimated annual incidence of 1 million cases, mainly in Africa and Asia (CitationCarr et al., 1997).
The results of the present study provide supportive data for the traditional anticancer use of A. africana. Further studies on the effects of this extract as well as compound 10 on normal human hepatocytes will help to determine whether the decoction has selective cytotoxic effects on liver cancer cells. This will be done in conjunction with the study of the mechanism of action of these samples.
Acknowledgements
The authors report no conflict of interest. The authors acknowledge the technical support of the National Herbarium of Cameroon, and Mr. Victor Nana for sample collection.
Declaration of interest: The authors alone are responsible for the content and writing of this paper.
References
- Becker FF (1975): Etiology: Viral carcinogenesis. In: Cancer. A Comprehensive Treatise, Vol. 2. New York, Plenum Press, pp. 323–341.
- Berg CC, Hijman MEE, Weerdenburg E (1985): Cameroon Flora. Paris, Satabié, pp. 104–110.
- Berhaut J (1979): Flore Illustrée du Sénégal. Paris, Satabié, pp. 402–405.
- Braun AC (1972): The relevance of plant tumor systems to an understanding of the basic cellular mechanisms underlying tumorigenesis. Prog Exp Tumor Res 15: 165–187.
- Carr BI, Flickinger JC, Lotze MT (1997): Hepatobiliary cancers. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds., Cancer: Principles and Practice of Oncology, 5th ed. Philadelphia, Lippincott, pp. 1087–1114.
- Coker PS, Radecke J, Guy C, Camper ND (2003): Potato disc tumor induction assay: a multiple mode of drug action assay. Phytomedicine 10: 133–138.
- Gerlier D, Thomasset N (1986): Use of MTT colorimetric assay to measure cell activation. J Immunol Methods 94: 57–63.
- Gonzalez AG, Ferro EE, Ravelo AG (1987): Triterpenes from Maytenus horrida. Phytochemistry 26: 2785–2788.
- Itharat A, Houghton PJ, Eno-Amooquaye E, Burke PJ, Sampson JH, Raman A (2004): In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J Ethnopharmacol 90: 33–38.
- Junior P, Wichtl M (1980): 3-epi-Periplogenin: a new cardenolid from Adonis vernalis. Phytochemistry 19: 2193–2197.
- Kahl G, Schell JS (1982): Molecular Biology of Plant Tumors. New York, Academic Press.
- Karpas A (1982): Viruses and leukemias. Am Sci 70: 277–285.
- Li XC, Elsohly HN, Hufford CD, Clark AM (1999): NMR Assignments of ellagic acid derivatives. Magn Reson Chem 37: 856–859.
- Lippincott JA, Lippincott BB (1975): The genus Agrobacterium and plant tumorigenesis. Ann Rev Microbiol 29: 377–405.
- Liu X, Ardo S, Bunning M, Parry J, Zhou K, Stushnoff C, Stoniker F, Yu L, Kendall P (2007): Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT - Food Sci Technol 40: 552–557.
- Lotze MT, Flickinger JC, Carr BI (1993): Hepatobiliary neoplasms. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds., Cancer: Principles and Practice of Oncology, 4th ed. Philadelphia, Lippincott, pp. 883–914.
- Mahato SB, Kundu AP (1994): 13C NMR spectra of pentacyclic triterpenoids – a compilation and some salient features. Phytochemistry 37: 1517–1575.
- McLaughlin JL, Rogers LL (1998): The use of biological assays to evaluate botanicals. Drug Inf J 32: 513–524.
- Mensor LL, Menezes FS, Leitao GG, Reis AS, Dos Santos TC, Coube CS, Leitão SG (2001): Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15: 127–130.
- Okogun IJ, Spiff AI, Ekong DEU (1976): Triterpenoids and betaines from the latex and bark of Antiaris africana. Phytochemistry 15: 826–827.
- Pettit GR, Cragg GM, Singh SB (1987): Antineoplastic agents constituents of Combretum caffrum. J Nat Prod 50: 386–391.
- Robien W, Kopp B, Schabl D, Schwarz H (1987): Carbon-13 NMR spectroscopy of cardenolides and bufadienolides. Prog NMR Spectrosc 19: 131–181.
- Schenk B, Junior P, Wichtl M (1980): Cannogenol-3-O-α-l-rhamnoside and cannogenol-3-O-β-d-allomethyloside, two new cardiac glycosides from Convallaria majalis. Planta Med 40: 1–11.
- Seebacher W, Simic N, Weis R, Saf R, Kunert O (2003): Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn Reson Chem 41: 636–638.
- Sharipov AK, Gorovits MB, Makarichev GK, Yagudaev MR, Abubakirov NK (1969): NMR spectra of cardenolides with an oxygen-containing function at C-10. Khim Prirod Soed 5: 270–273.
- Suffness M, Pezzuto JM (1990): Assays related to cancer drug discovery. In: Hostettmann K, ed., Methods in Plant Biochemistry: Assays for Bioactivity, Vol. 6. London, Academic Press, pp. 71–133.
- Thompson IM, Coltman JCA, Crowley J (1997): Chemoprevention of prostate cancer: The prostate cancer prevention trial. Prostate 33: 217–221.
- Zee-Cheng R (1997): Anticancer research on Loranthaceaea plants. Drugs Future 22: 519–530.