Abstract
The aqueous extract of Emblica officinalis Gaertn. (syn: Phyllanthus emblica L.) (Euphorbiaceae) seeds was investigated for its anti-diabetic activity in animal models. Streptozotocin (STZ)-induced type 2 diabetes models were used for the study. The standardized doses of 100, 200, 300, and 400 mg kg−1 body weight of the extract were administered orally to normal and diabetic rats in order to define its glycemic potential. The maximum fall of 27.3% (p < 0.001) in the blood glucose level of normal rats was observed at 6 h during fasting blood glucose studies, with the dose of 300 mg kg−1 identified as the most effective dose. The same dose produced a fall of 25.3% (p < 0.001) in the same models during the glucose tolerance test (GTT) at 3 h after glucose administration. However, the dose of 300 mg kg−1 of aqueous seed extract in sub- and mild-diabetic animals produced a maximum fall of 34.1 and 41.6% (p < 0.01), respectively, during the GTT at 3 h after glucose administration. This evidence clearly indicates that the aqueous extract of E. officinalis seeds has definite hypoglycemic potential as well as anti-diabetic activity.
Introduction
The disease diabetes is well known in each and every part of the world. Type 2 diabetes mellitus (T2DM) is a major and growing health problem throughout the world. T2DM results from both peripheral insulin resistance and impaired insulin secretion. Insulin resistance arises as a consequence of obesity, a sedentary life, and aging, resulting in hyperglycemia and diabetes. Blood pressure elevation and dyslipidemia are collectively called “metabolic syndrome X” (CitationArulmozhi & Portha, 2006). As diabetes is a global burden, its management should be explored systematically and scientifically. This is the main reason for the persistent interest all over the world to explore remedies from the so-called “alternative system of medicine.” Since the natural remedies are of low toxicity with minimal or no side effects (CitationRai et al., 2007; CitationSingh et al., 2007), therefore medicinal plants are of significant interest to scientists. It is important to justify the various uses of medicinal plants enunciated in traditional systems of medicine, scientifically.
Emblica officinalis Gaertn. (syn: Phyllanthus emblica L.) (Euphorbiaceae) grows in tropical and subtropical parts of China, India, and the Malay Peninsula. The fruit is commonly known as amla or emblic myronalan, and is of high value in traditional Indian medicine. Several constituents of E. officinalis have been identified: the hydrolyzable tannins and its derivatives are the key ingredients (CitationChaudhuri, 2004). The two derivatives emblicanin A and B have been proposed to be the active constituents, with significant in vitro antioxidant activity (CitationGhosal et al., 1996). The fruits of E. officinalis have been reported to have potent antimicrobial (CitationAhmad et al., 1998), antioxidant (CitationBhattacharya et al., 1999; CitationBabu et al., 2004; CitationRao et al., 2005), adaptogenic (CitationRage et al., 1999), hepatoprotective (CitationJeena et al., 1999), antitumor (CitationJose et al., 2001), and antiulcerogenic (CitationSairam et al., 2002; CitationKumar et al., 2004) activities. Its antioxidant activity is related to the presence of gallic and ellagic acids that inhibit the degradation of vitamin C (CitationDamodaran & Nair, 1936). Since the seeds of E. officinalis have not been explored scientifically for their role in diabetes management, these were our choice for the present study. This report on the anti-diabetic effect of the aqueous extract of E. officinalis seeds is the first from our research group using experimental models.
Materials and methods
Plant material and extraction procedure
The fruits of E. officinalis (15 kg) were purchased during the month of November from the local market of Allahabad, India and authenticated by Professor Satya Narayan, Taxonomist Department of Botany, University of Allahabad, India. A voucher specimen has been submitted to the University herbarium. The fruits were boiled at 70°C and pulps of fresh fruits were separated to take out the hard seeds. The shade-dried seeds (2.5 kg) were mechanically crushed and extracted with distilled water (65°C) using Soxhlet apparatus for up to 72 h. The extract was filtered and concentrated in a rotary evaporator at 45–50°C under reduced pressure to obtain semisolid material, which was then lyophilized to obtain a powder (yield 12.3%, w/w) (CitationRai et al., 2007, Citation2008).
Chemicals
Streptozotocin was purchased from Sigma-Aldrich Co., USA. Blood glucose level (BGL) for fasting blood glucose (FBG) and glucose tolerance test (GTT) studies was assayed using kits from Bayer Diagnostics, India and a one touch Accu-Chek sensor from Roche Diagnostics, Germany. The solvents were from E. Merck.
Preliminary phytochemical screening
Preliminary phytochemical screening was carried out for phenols and flavonoids (CitationKokate, 1994; CitationHarborne, 1998). Phenolic extraction of the dried powder sample was performed using 70% ethanol, and the total phenolic content was analyzed using Folin–Ciocalteu reagent (CitationMcDonald et al., 2001).
Experimental animals
More than 100 male albino Wistar rats of the same age group with body weight 150–200 g were selected for all experiments. The animals, obtained from the National Institute of Communicable Disease (NICD), New Delhi, India, were housed in polypropylene cages at an ambient temperature of 25–30°C and 45–55% relative humidity, with a 12 h each dark/light cycle. Animals were fed a pellet diet (Pashu Ahar Kendra, Varanasi) and water ad libitum. The study was approved by the Institutional Ethics Committee.
Induction of diabetes
Diabetes was induced by a single intraperitoneal injection of freshly prepared streptozotocin (STZ) 50 mg kg−1 (Sigma-Aldrich, Seelze, Germany) in 0.1 M citrate buffer (pH = 4.5) (CitationEl-Fiky et al., 1996) to rats deprived of food overnight. After 3 days of STZ administration, rats with marked hyperglycemia were selected for the study (CitationRai et al., 2008). The rats with stable hyperglycemia were divided into two groups of 36 rats each:
sub-diabetic animals with normal FBG (80– 120 mg dL−1) and abnormal postprandial glucose (PPG; > 210 mg dL−1) levels, also known as streptozotocin recovered (SR);
mild-diabetic animals with FBG 150– 200 mg dL−1 and PPG > 250 mg dL−1.
Blood glucose estimation
BGL was estimated by the glucose oxidase method (CitationBarham & Trinder, 1972) using a standard kit from Bayer Diagnostics India Ltd.
Experimental design
Initial screening of the aqueous extract for hypoglycemic activity was done with a range of variable doses in normal healthy rats by conducting FBG and GTT studies. The anti-diabetic effect was assessed in sub- as well as mild-diabetic models (CitationShukla et al., 1994; CitationRai et al., 2008) with the same range of doses based on similar studies of FBG and GTT (CitationRai et al., 2008).
Assessment of hypoglycemic activity by FBG studies in normal healthy rats
Five groups of six rats each, fasted overnight, were used in the experiment. Their FBGs were taken. Group I served as untreated controls and received vehicle (distilled water) only, and animals of groups II, III, IV, and V received aqueous seed extract suspended in distilled water at doses of 100, 200, 300, and 400 mg kg−1, respectively. Blood samples were collected from the tail vein at 2, 4, 6, and 8 h after treatment.
Assessment of hypoglycemic activity by GTT studies in normal healthy rats
Varied doses of aqueous extract and vehicle treatment were given orally to different groups of normal healthy rats in the same fashion as above, after taking their FBG. The BGL value at 2 h after treatment was considered as the “0” h value. The animals were then orally administrated with 2 g kg−1 of glucose, and their glucose tolerance was studied at 1 h intervals for another 3 h. Thus, the total period of blood collection was up to 5 h.
Assessment of anti-diabetic activity by GTT in sub-diabetic rats
The rats were divided into six groups of six rats each. Group I was the control group, and received vehicle (distilled water) only, whereas varied doses of 100, 200, 300, and 400 mg kg−1 of extract were given orally to groups II, III, IV, and V, respectively, after taking their FBG. Blood glucose levels were then checked after 2 h of treatment, considered as the 0 h value, and then 2 g kg−1 glucose was given orally to all the groups. Blood glucose levels were further checked up to 3 h at regular intervals of 1 h each, considered as 1, 2, and 3 h values. The results were compared with those of group VI that served as the positive control group, treated with 250 mg kg−1 of tolbutamide, a reference drug.
Assessment of anti-diabetic activity by GTT in mild-diabetic rats
Varied doses of aqueous extract and vehicle treatment were given orally to different groups of mild-diabetic rats in the same fashion as above after taking their FBG. Blood glucose levels were then checked after 2 h of treatment, considered as the 0 h value, and then 2 g kg−1 glucose was given orally to all the groups. Blood glucose levels were further checked up to 3 h at regular intervals of 1 h each, considered as 1, 2, and 3 h values. The results were compared with those of group VI of rats, which were treated with 250 mg kg−1 of tolbutamide (hypoglycemic agent).
LD50 experiment
The toxic effect of the water extract was also studied by LD50 experiment. Two groups of rats of both sexes (six animals per group, three females and three males), weighing about 180–200 g, were orally administered a single dose of 3 and 4.5 g, respectively, of the aqueous extract. Then the rats were observed for gross neurologic (CitationCartmell et al., 1991) and toxic effects continuously. Food consumption, feces, and urine were also examined at 2 h and then at 6 h intervals for 24 h.
Statistical analysis
Data were statistically evaluated using two-way analysis of variance (ANOVA), followed by post hoc Scheffe’s test using the statistical package PRISM version 3.0. The values were considered significant when p < 0.05.
Results
Effect on normal healthy rats
describes the hypoglycemic effect of a single oral administration of varied doses of 100, 200, 300, and 400 mg kg−1 of aqueous seed extract in normal healthy rats. Treated rats showed a regular increase in percentage fall of 18.3 and 27.3% (p < 0.001) with the doses of 100 and 300 mg kg−1, respectively, after 6 h. However, a fall of only 22.8% (p < 0.001) was observed with the dose of 400 mg kg−1 after the same interval of time, indicating thereby a slight decrease in the maximum fall observed with the dose of 300 mg kg−1.
Table 1. Effect of varied doses of E. officinalis seed aqueous extract on blood glucose level (BGL) during fasting blood glucose (FBG) test of normoglycemic rats (mean ± SD).
Effect on normal rats during GTT
deals with the study of the aqueous extract of E. officinalis seeds on BGL levels and glucose tolerance of normal healthy rats. Different doses of 100, 200, 300, and 400 mg kg−1 of extract were given orally to overnight fasted healthy rats, after taking their FBG sample. The dose of 300 mg kg−1 produced a maximum fall of 25.3% (p < 0.001) in BGL of rats after 3 h of glucose administration, whereas falls of 20.9, 22.9, and 22.1% (p < 0.001) were observed in BGL with the doses of 100, 200, and 400 mg kg−1, respectively.
Table 2. Effect of varied doses of E. officinalis seed aqueous extract on blood glucose level (BGL) during glucose tolerance test (GTT) of normoglycemic rats (mean ± SD).
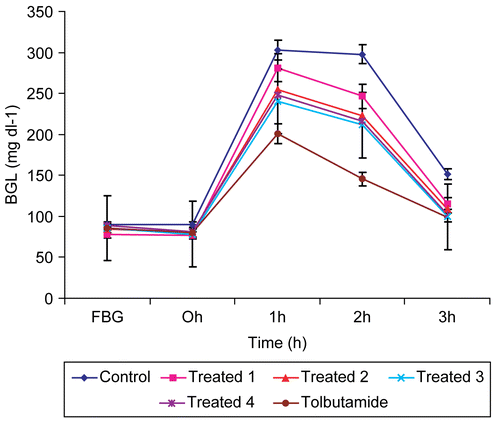
Effect on sub-diabetic rats during GTT
demonstrates the anti-diabetic effect of varied doses of aqueous extract of E. officinalis seeds on sub-diabetic rats. The standard drug tolbutamide 250 mg kg−1 was also given as a reference drug. The fall observed was 23.6, 28.5, 34.1, and 32.4% (p < 0.001) after 3 h of glucose administration with doses of 100, 200, 300, and 400 mg kg−1, respectively. However, the dose of tolbutamide showed the highest fall of 51.2% (p < 0.001) at 2 h. Moreover, the fall observed at 3 h with this reference drug was 34.7% (p < 0.01), which is comparable to the fall shown by the 300 mg kg−1 dose of extract. Hence, this dose of extract can be identified as the most effective dose.
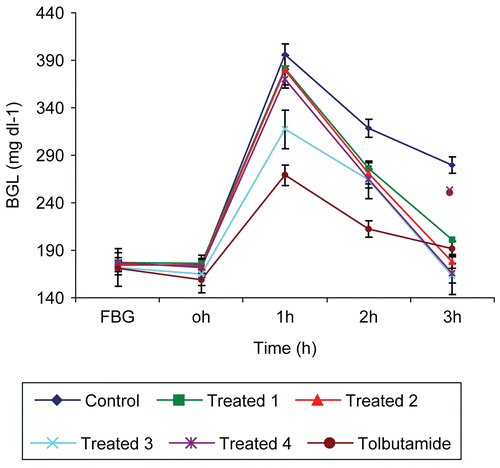
Effect on mild-diabetic rats during GTT
depicts the anti-diabetic potential of graded doses of aqueous extract of E. officinalis seeds and tolbutamide at a dose of 250 mg kg−1 in mild-diabetic animals. The maximum fall observed after 3 h of glucose administration was 27.9, 36.3, 41.6, and 40.7% (p < 0.01) with the doses of 100, 200, 300, and 400 mg kg−1, respectively. Tolbutamide produced a maximum fall of 33.2% (p < 0.001) within 2 h, which came down further to 31.3% (p < 0.01) after 3 h. Hence, in mild-diabetic cases, the fall shown by the 300 mg kg−1 dose of extract was much higher than that of the reference drug at 3 h.
LD50
The experiment was carried out on normal healthy rats. The behavior of the treated rats appeared normal. No toxic effect was reported at doses up to 10 and 15 times the effective dose of aqueous extract and there was no death in any of these groups.
Phytochemical studies
The preliminary studies indicated the presence of flavonoids and gallic acid. The total phenolic content in terms of gallic acid equivalent (mg g−1 of dry mass) was 21 mg g−1 in the extract powder.
Discussion
E. officinalis fruits are well known for their pharmacological activities (CitationAhmad et al., 1998; CitationBhattacharya et al., 1999; CitationJeena et al., 1999; CitationJose et al., 2001; CitationSairam et al., 2002) as well as antidiabetic properties (CitationSalu & Kuttan, 2002; CitationSuryanarayana et al., 2007). However, there are no reports on hypoglycemic and anti-diabetic effects of the seeds of E. officinalis, though it is well documented that the seeds have much more anti-diabetic activity than the fruits (CitationKamalakkannan & Prince, 2003; CitationKesari et al., 2006; CitationRai et al., 2008).
Thus, the present study based on FBG and GTT was carried out with graded doses of 100, 200, 300 and 400 mg kg−1 of aqueous extract of E. officinalis seeds given to normal as well as sub- and mild-diabetic rats. Results indicate that each dose of E. officinalis aqueous extract reduces the blood glucose level and improves glucose tolerance in both normal and diabetic animals.
It is evident from and that the dose of 300 mg kg−1 is the most effective dose as it showed the maximum reduction in FBG within 6 h and maximum improvement in glucose tolerance after 3 h of glucose administration, respectively, in normal rats. Since the animals were fasted overnight before treatment, therefore the hypoglycemic action of the extract by inhibiting glucose absorption (CitationAdamson & Okafor, 1990; CitationAnderson & Akanji, 1991) can be ruled out. Moreover, in the case of sub- and mild-diabetic animals the maximum glucose tolerance was also associated with the same dose of 300 mg kg−1 at 3 h ( and ). These results are comparable with those of the standard drug tolbutamide, which showed practically the same percentage of fall in BGL at the same interval of time.
Since it is well documented that the administration of STZ selectively destroys the β cells of the pancreas and causes hyperglycemia in rats (CitationGoldner & Gomori, 1943; CitationHofteizer, 1973; CitationRai et al., 2008), therefore the antihyperglycemic effect of the aqueous extract of the seeds might be due to the activation of existing β cells of the pancreas (CitationKar et al., 1999; CitationRai et al., 2008). This plausible action of the extract may be confirmed by the well established mechanism of action of the standard drug tolbutamide, i.e. activation of β cells (CitationHenquin, 1980), in STZ-induced diabetic rats.
Preliminary phytochemical screening revealed the presence of flavonoids and gallic acids in the extract. The phenolic content in terms of gallic acid equivalent is 21 mg g−1. Moreover, flavonoids (CitationAnila & Vijayalakshmi, 2000, Citation2002) and tannoides (CitationSuryanarayana et al., 2007) isolated from the fruits of E. officinalis are also reported to manage diabetes and its complications.
Further exploration of the most effective dose (300 mg kg−1) in STZ-induced severely diabetic models (type 1) along with the isolation of bioactive principles from active fractions is in progress.
Declaration of interest: Financial assistance to one of the authors (S.M.) in the form of a Junior Research Fellowship from the University Grant Commission, New Delhi, India is acknowledged.
References
- Adamson, Okafor (1990): Dietary fiber an overview. Diabetes care 14: 1126–1131.
- Ahmad J, Mehmood Z, Mohammad F (1998): Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol 62: 183–193.
- Anderson JW, Akanji AO (1991): Dietary fiber: An overview. Diabetes Care 14: 1126–1131.
- Anila L, Vijayalakshmi NR (2000): Beneficial effects of flavonoids from Sesamum indicum, Emblica officinalis and Momordica charantia. Phytother Res 17: 592–595.
- Anila L, Vijayalakshmi NR (2002): Flavonoids from Emblica officinalis and Mangifera indica: Effectiveness for dyslipidemia. J Ethnopharmacol 79: 81–87.
- Arulmozhi DK, Portha B (2006): GLP-I based therapy for type 2 diabetes. Eur J Pharm Sci 28: 96–108.
- Babu PS, Stanely Mainzen Prince P (2004): Antihyperglycaemic and antioxidant effect of hyponidd, an ayurvedic herbomineral formulation in streptozotocin-induced diabetic rats. J Pharm Pharmacol 56: 1435–1442.
- Barham D, Trinder P (1972): An improved colour reagent for the determination of blood glucose by glucose oxidase system. Analyst 97: 142–145.
- Bhattacharya A, Chatterjee A, Ghosal S, Bhattacharya SK (1999): Antioxidant activity of active tannoid principles of Emblica officinalis (amla). Indian J Exp Biol 37: 676–680.
- Cartmell SM, Gelgor L, Mitchell D (1991): A revised rotarod procedure for measuring the effect of antinociceptive drugs on motor function in the rat. J Pharmacol Methods 26: 149–159.
- Chaudhuri RK (2004): Standardization extract of Phyllanthus emblica: a skin lightener with antiaging benefits. In: Proceedings of the PCIA Conference, Guangzhou, China, 9–11 March 2004, p. 38.
- Damodaran M, Nair KR (1936): A tannin from the Indian gooseberry (Phyllanthus emblica) with a protective action on ascorbic acid. Biochem J 30: 1014–1020.
- El-Fiky FK, Abou-Karam MK, Afify A (1996): Effect of Luffa aegyptiaca (seeds) and Carisa edulis (leaves) extracts on blood glucose level of normal and streptozotocin diabetic rats. J Ethnopharmacol 60: 43–47.
- Ghosal S, Tripathi VK, Chauhan S (1996): Active constituents of Emblica officinalis: Part I. The chemistry and antioxidant effect of two new hydrolysable tannins, emblicanin A and B. Indian J Chem 35B: 941–948.
- Goldner M, Gomori N (1943): Alloxan induced diabetes. Endocrinology 33: 297–299.
- Harborne JB (1998): Methods of extraction and isolation. In: Phytochemical Methods. London, Chapman & Hall, pp. 60–66.
- Henquin JC (1980): Mechanisms of tolbutamide-stimulation of pancreatic β cells – a reply. Diabetologia 19: 162–163.
- Hofteizer V (1973): Comparison of streptozotocin-induced diabetes in the rat inducing volumetric quantitation of the pancreatic islets. Diabetologia 9: 178–184.
- Jeena KH, Joy KL, Kuttan R (1999): Effect of Emblica officinalis, Phyllanthus amarus and Picrorhizia kurroa on N-nitrosodiethyl amine induced hepatocarcinogenesis. Cancer Lett 136: 11–16.
- Jose JK, Kuttan Y, Kuttan R (2001): Antitumour activity of Emblica officinals. J Ethnopharmacol 82: 1–9.
- Kamalakkannan N, Prince PS (2003): Hypoglycemic effect of water extract of Aegle marmelos fruits in Streptozotocin diabetic rats. J Ethnopharmacol 87: 207–210.
- Kar A, Choudhary BK, Bandopadhyay NG (1999): Preliminary studies on the inorganic constituents of some indigenous hypoglycemic herbs on oral glucose tolerance test. J Ethnopharmacol 64: 179–184.
- Kesari AN, Gupta RK, Singh SK, Diwakar S, Watal G (2006): Hypoglycemic and antihyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. J Ethnopharmacol 107: 374–379.
- Kokate CK (1994): Preliminary Phytochemical Screening, Practical Pharmacognosy. New Delhi, Vallabh Prakashan, pp. 107–113.
- Kumar KBH, Sabu MC, Lima PS, Kuttan R (2004): Modulation of haematopoietic system and antioxidant enzymes by Emblica officinalis Gaertn and its protective role against γ-radiation induced damages in mice. J Radiat Res 45: 549–555.
- McDonald S, Renzler PD, Autolovich M, Robards K (2001): Phenolic content and antioxidant activity of olive extracts. Food Chem 73: 73–84.
- Rage NN, Thatte UM, Dahanukar SA (1999): Adaptogenic properties of six Rasayana herbs used in Ayurvedic medicine. Phytother Res 13: 275–291.
- Rai PK, Rai NK, Rai AK, Watal G (2007): Role of LIBS in elemental analysis of P. guajava responsible for glycemic potential. Instrum Sci Technol 35: 507–522.
- Rai PK, Jaiswal D, Diwakar S, Watal G (2008): Antihyperglycemic profile of Trichosanthes dioica seeds in experimental models. Pharm Biol 46: 360–365.
- Rao TP, Sakaguchi N, Juneja LR, Wada E, Yokozawa T (2005): Amla (Emblica officinalis Gaertn.) extracts reduce oxidative stress in streptozotocin-induced diabetic rats. J Med Food 8: 362–368.
- Sairam K, Raoch V, Dora Babu M, Vijay Kumar K, Agarwal VK, Goel RK (2002): Antiulcerogenic effect of methanolic extract of Emblica officinalis: an experimental study. J Ethnopharmacol 82: 1–9.
- Salu MC, Kuttan K (2002): Antidiabetic activity of medicinal plants and its relationship with their antioxidant properly. J Ethnopharmacol 81: 155–160.
- Shukla R, Anand K, Prabhu KM, Murthy PS (1994): Hypoglycemic effect of the water extract of Ficus bengalensis in alloxan recovered, mildly diabetic and severely diabetic rabbits. Int J Diabetes Dev Ctries 14: 78–81.
- Singh SK, Rai PK, Jaiswal D, Watal G (2007): Assessment of antidiabetic potential of Cynodon dactylon in streptozotocin diabetic rats. J Ethnopharmacol 11: 174–179.
- Suryanarayana P, Saraswat M, Petrash JM, Reddy GB (2007): Emblica officinalis and its enriched tannoids delay streptozotocin- induced diabetic cataract in rats. Mol Vis 13: 1291–1297.