Abstract
Turnera ulmifolia Linn. (Turneraceae) is an herb commonly found in northeastern Brazil, frequently employed in folk medicine, including by pregnant woman, for many afflictions due to it expectorant, tonic, anti-inflammatory, antiulcerogenic, and antioxidant effects. This work studied the infusion commonly used by the population, obtained by maceration of fresh leaves of T. ulmifolia in filtered water, to evaluate if the same may promote alterations in rat gestation and exposed offspring. Pregnant rats received, by gavage, the aqueous extract (0, 1, 2, or 3 g/kg/day) from gestation day (GD) 1 to GD 21. The treatment was not able to promote maternal toxicity: body weight gain, food and water intake were not altered during gestation period. The offspring presented normal physical and reflexological development. No alterations were observed in the histopathological study and sexual hormone levels of the dams and offspring at 30, 60, and 90 days of age. The sexual behavior was evaluated in male and female offspring at adult age (GD 90) and no alterations were observed. These results suggest that the infusion of T. ulmifolia, employed in folk medicine, at these doses, is not able to promote alterations to pregnant rats, to impair gestation, or to damage the exposed offspring.
Introduction
Turnera ulmifolia Linn. (Turneraceae), popularly known as chanana, is an herb commonly found in northeast Brazil (CitationLorenzi, 1991). This plant is employed in folk medicine for many afflictions due to it expectorant, tonic, anti-inflammatory, antiulcerogenic, and antioxidant effects (CitationCorrea, 1984; CitationAntônio & Souza-Brito, 1998; CitationNascimento et al., 2006; CitationGalvez et al., 2006). Studies detected phenolic compounds, particularly flavonoids and glycosylated flavones derived from apigenine and luteonine, β-sitosterol, stigmasterol, and 24-metilenocicloartenol in T. ulmifolia leaves (CitationGracioso et al., 2002).
The flavonoids observed in T. ulmifolia are an important field to study and help elucidate the actions of this plant, since many references attribute to these compounds a range of biologic activities, including hormonal and antioxidant effects (CitationLe March, 2002; CitationNestel, 2003; CitationNascimento et al., 2006; CitationGalvez et al., 2006). Alterations in the sexual behavior of animals exposed perinatally to hormonal active compounds, such as endocrine disruptors and phytoestrogens, may occur as a consequence of the absorption of the compound through the placental barrier and action at the fetuses, promoting morphophysiological changes on the central nervous system, during the sex differentiation period, that occurs in rat fetuses from gestation day 18 to post-natal day 10, approximately. These alterations can promote impaired sexual behavior in the exposed rats at adult age (CitationMcCluscky & Naftolin, 1981).
Considering that T. ulmifolia is a plant largely employed in folk medicine including pregnant woman, the aim of this study was to investigate the effects of an infusion obtained from T. ulmifolia leaves in pregnant rats and their offspring. This study has implications for pregnant woman, who might be employing phytotherapeutic formulations under the impression that they are harmless when used during gestation.
Materials
Plant
The Turnera ulmifolia leaves used in this study were collected in April 2006 in Natal city (Rio Grande do Norte, Northeast Brazil; GPS location: latitude -5.900000, longitude -35.246333). The plant was identified by Iracema Loiola and a voucher specimen was deposited at the Herbarium “Parque das Dunas” of the Universidade Federal do Rio Grande do Norte (registration no. 674).
At the toxicology laboratory of the Universidade Federal do Rio Grande do Norte, the fresh leaves were oven dried at 40°C and then milled. The dried and milled leaves (400 g) were boiled in distilled water (1,000 mL) for 10 min. After filtration the aqueous extract (40% m/v) was obtained.
Animals
Pregnant Wistar rats (n = 30) from our colony, weighing approximately 170-230 g each, were used (gestation day (GD 1) = spermatozoa in the vaginal smear). The dams were housed in pairs in polypropylene cages (40 × 50 × 20 cm) on a 12/12 h light-dark cycle (lights on at 6 a.m.). The dams were randomly distributed into control and experimental groups (n = 6/group). The groups received the infusion at 0 (filtered water), 1, 2 or 3 g/kg/day, by gavage, from gestational day (GD) 1 to GD 21. These doses were adopted considering a toxicity study developed previously (Antonio & Souza-Brito, 1998) with a hydroalcoholic extract obtained from the leaves of this plant, where oral doses up to 5 g/kg did not shown toxicity and where the LD50 = 7.82 g/kg intra peritoneal (for mice) was established. Water and food were available ad libitum. The animals used in this study were maintained in accordance with the Ethical Principles in Animal Research adopted by the National Ethic Research Committee (CONEP/MS) and approved by the Onofre Lopes University Hospital Research Ethical Committee (protocol no. 007/06).
Reproductive parameters and maternal data
The pregnant rats (6/group) were weighed daily. Food and water ingestion were measured daily until parturition. The parameters length of gestation, litter size and weight, sex ratio, post-natal death, body length and anogenital distance of one female and one male pup per litter were assessed at post-natal day (PND) 1.
At PND 21 (weaning day), the dams were sedated by ketamine and xilazine association. Blood (5 mL) was collected by cardiac punction for posterior measurement of estradiol, progesterone, follicule stimulant and luteinizing hormones in serum, by quimioluminescence. The serum obtained by blood centrifugation was reserved in polypropylene microtubules and reserved under -20°C, until analysis. Ovaries, uterine horns, kidney, liver, pancreas and spleen were then removed, weighed and analyzed, and specimens (6/group) were fixed in 10% neutral buffered formaldehyde and routinely processed for light microscopic evaluation.
Offspring studies
All the pregnant rats (6/group) were allowed to give birth and nurture their offspring normally. No cross-fostering procedure was used. At parturition day (PND 1) all the litters were examined externally and sexed. Litters were organized into groups of eight pups each, four males and four females, and the remaining pups were culled. The anogenital distance (mm) and offspring body length (cm) were obtained at birth (PND 1). The anogenital distance was considered as the length from the anal opening to the genitals. The ratio between the anogenital distance and the cube root of body weight at PND 1 was adopted as the appropriate method for measuring anogenital length (CitationGallavan et al., 1999). The litter was considered the unit of analysis. One male and one female pup from each litter (6 males and 6 females/group) were marked with colored felt tip pens for body weight accompaniment (once a week from post natal day (PND) 1 to PND 90 and for observation of the physical and reflexological development, evaluated by the parameters palmar grasp reflex (pup grasped a paper clip with forepaws if stroked; pups are born with this reflex that usually disappears between PND 8 and PND 10), coat appearance (beginning at PND 2), pinna detachment (beginning at PND 2), eruption of incisor teeth (beginning on PND 3), day of appearance of the surface righting reflex (first day when the normal ventral position is assumed successfully within a period of time not exceeding 15 sec after the pup is placed on its back, beginning on PND 5), adult gait (when the pups walk without propping their ventral portion on the floor, beginning on PND 10), ears opening (determined by the visualization of the open auditory meatus, beginning on PND 10), eye opening (determined by the visualization of a longitudinal eyelid fissure, beginning on PND 10), testis descent (considered as the scrotum purse touching the testis, beginning on PND 16) and vaginal opening (when the vaginal hole is visualized, beginning on PND 30).
At PND 30, 60, and 90, 6 male and 6 female pups per group, obtained from distinct litters, were euthanized. The male sexual accessory organs were removed through an incision in the venter-pubic region: testis, seminal vesicle and epididymis (the last at PND 90 only) were weighed. Female ovaries and uterine horns were removed and weighed. In addition, the liver, kidney, pancreas and spleen of these animals were weighed. The organ weight data were normalized, expressed as a ratio of organ weight/body weight. Specimens of these organs were fixed in 10% formaldehyde, routinely processed, embedded in paraffin and sectioned for light microscopic evaluation.
Sexual behavior studies
For male and female sexual behavior evaluations, the animals were housed in a room with reverse light/dark cycle for at least 15 days (time required for adaptation). During observation, a lamp of 40 W provided room illumination with a red filter. A rectangular glass box ( 56 cm long × 35 cm wide × 31 cm high) was employed. The inside of the box was covered with a 3 cm sawdust layer.
Female sexual behavior
At PND 75, 6 females of each group, obtained from distinct litters, were left in their cages (3 females/cage) in a room with reverse light/dark cycle, for 15 days, a period sufficient for habituation. The females (now at PND 90) received, 24 h before the test, 200 mg/kg of conjugated estrogens (Premarin®-Wyeth). At the moment of the test, conducted during the dark cycle, the females were in an induced pseudo-estrous phase and therefore, sexually receptive for adult experienced males of our stock colony. In the mating box, the male was allowed to adapt for 5 min before one rendered sexually receptive female was introduced. The lordosis quotient (LQ%) was calculated as the percent of lordosis postures by the female (a position that allows intromission) in response to 10 mounts by the male, in a maximal time of 10 min (LQ% = number of lordosis/number of mounts × 100) (CitationBeach, 1967).
Male sexual behavior
On PND 90, 6 males of each group, obtained from different litters, were employed. The male was placed in the mating box 5 min before introducing a female, from our stock colony (not any of the unexposed control females of the experiment), which was rendered sexually receptive as described above. If the male did not mount within the next 10 min, the test was considered negative and the male was removed. The following measures were recorded in a 40 min session: mount and intromission latencies, defined as the times from introduction of the female in the cage to the first mount and intromission, respectively; mount and intromission frequencies, the number of mounts and intromissions preceding the first ejaculation; ejaculation latency, the time from introduction of the female in the cage to first ejaculation; both mount and intromission latencies post-ejaculation, the time for the first mount and intromission after the first ejaculation; intromission frequency post-ejaculation, the number of intromissions after the first ejaculation; and total number of ejaculations. This technique was based on that described by CitationBitran and Hull (1987), for evaluation of sexual motivation. Hand operated counters and stop watches were employed to score these parameters.
The parameters copulatory efficiency (ratio between the number of intromissions that precedes the ejaculation and total number of mounts and intromissions that precedes the ejaculation × 100) (CitationMalmnas, 1973), frequency of mounts and intromissions per minute (CitationBitran & Hull, 1987) and sexual activity score (CitationAgmo, 1987) were also evaluated for the male rat sexual potency study. The sexual activity score (SAS) reunites the most important variables observed during the male sexual behavior evaluation. It is calculated by mathematical transformations of the latency values for the first mount (LFM), first intromission (LFI), first ejaculation (LFE) and the total number of mounts (TM), by the expression: SAS = log[(1/LFM) × 40] + log[(1/LFI) × 40] + log[(1/LFE) × 40] + TM + Y (Y = 0 in the absence of ejaculation and Y = 4 in the presence of ejaculation). The number 40 refers to the duration of the analysis/animal, in minutes.
Statistical analyses
The data were analyzed by the analysis of variance ANOVA and Tukey Kramer post hoc test when necessary. In all cases, results were considered significant for p < 0.05.
Results
Reproductive parameters and maternal data
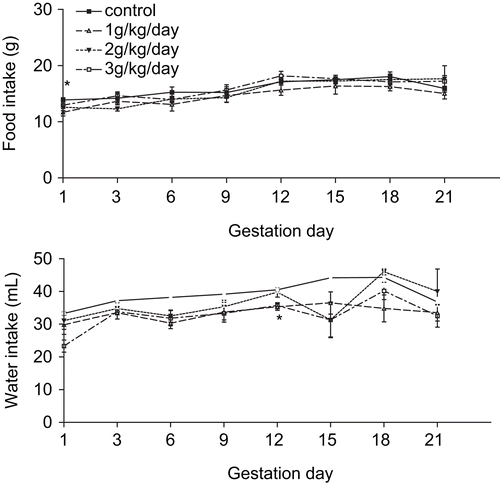
Statistically significant differences were not observed in body weight and body weight gain. However, reduced food consumption at GD 1 by the experimental 3 group (F(3/20) = 3.253; p = 0.0433) and reduced water intake at GD 12 by experimental 1 and 2 groups (F(3/20) = 5.812; p = 0.005) were observed, when compared to control group, as shown in .
Figure 1. Food consumption and water intake of dams treated with an aqueous extract obtained from T. ulmifolia fresh leaves (n = 6/group).
No significant organ weight or hormonal () alterations were observed in the experimental dams. The absence of histopathological alterations in liver and kidney of the treated dams, for example, are well depicted in . Also, no alterations were observed in the parameters employed to evaluate the reproductive performance, as pregnancy duration, litter weight, number of male and female pups, number of dead pups, body length and anogenital distance of pups at birth (), showing that the aqueous extract, at these concentrations and administered during gestation, was not able to promote maternal toxicity and to impair their reproductive performance.
Table 1. Serum hormone levels of dams at weaning day (n = 6/group).
Figure 2. Tissue portions of kidney (x10) and liver (x40) of control (photos A and C) and experimental (photos B and D) dams. Observe absence of alterations.
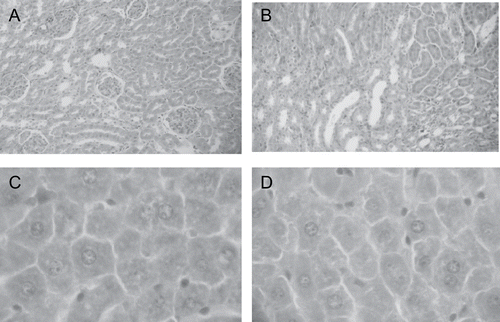
Table 2. Reproductive performance of dams treated, during gestation, with an aqueous extract obtained from fresh leaves of T. ulmifolia (n = 6/group).
Offspring studies
The statistical analysis showed that the treatment was not able to promote alterations in experimental pups body weight and body weight gain from birth (PND 1) to adult age (PND 90), as observed in . Also, no alterations were observed in the parameters employed to evaluate the physical and reflexological development of the pups (). Finally, no histological alterations or significant organ weight/body weight ratio were observed in the male and female pups, except by the increased heart weight/body weight ratio [F(3/20) = 4.337; p = 0.0192] observed in experimental 1 male pups at PND 60, reduced heart weight/body weight ratio [F(3/20) = 8.339; p = 0.0017] observed in experimental 2 male pups at PND 90, reduced spleen weight/body weight ratio [F(3/20) = 19.460; p < 0.0001]observed in experimental 1 and 2 male pups at PND 90 and reduced pancreas weight/body weight ratio [F(3/20) = 14.628; p < 0.0001] observed in experimental 2 and 3 female pups at PND 90.
Figure 3. Body weight gain of female (A) and male (B) pups exposed perinatally to an aqueous extract obtained from T. ulmifolia fresh leaves (n = 6 litter/group).
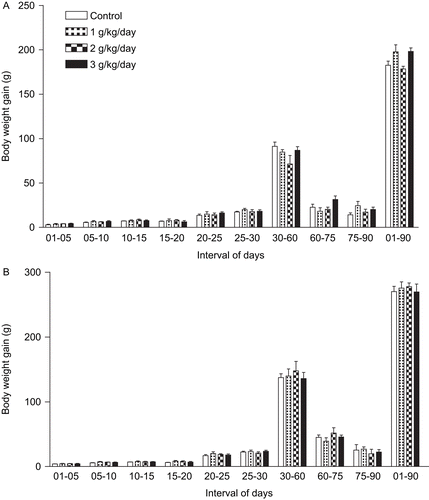
Table 3. Physical and reflexological development of pups exposed or not (control group) to the aqueous extract obtained from T. ulmifolia fresh leaves (n = 6 litters/group).
Sexual behavior studies
The ANOVA revealed absence of alterations in the sexual behavior of exposed females. The lordosis quotient (LQ%), measured for the different groups, was (mean ± SEM): control (65 ± 12.04), experimental 1 (65 ± 9.92), experimental 2 (58.33 ± 10.14) and experimental 3 (63.33 ± 9.55). Also, the ANOVA showed no significant alterations in the male sexual behavior (). Also, 3 males of the experimental groups 1 and 3 did not realize any mount or intromission in the first 20 min of the test. These animals were discarded and then these groups were constituted of 3 animals, each.
Table 4. Sexual behavior of male adult rats exposed perinatally to T. ulmifolia aqueous extract.
Discussion
The aqueous extract obtained by infusion of T. ulmifolia fresh leaves was employed in this study because this is the preparation used by population in folk medicine. The objective of this work was to verify possible effects of this aqueous extract if the same is taken during gestation, bearing in mind that medicinal plants are commonly employed by pregnant women in folk medicine assuming that they are harmless, frequently in the form of infusions, for the treatment of diseases.
In this study the three doses employed (1, 2, and 3 g/kg/day) were adopted considering a toxicity study developed previously (Antonio & Souza-Brito, 1998) with a hydroalcoholic extract obtained from the leaves of this plant, where oral doses up to 5 g/kg did not shown toxicity to mice, since no significant changes in daily body weight or organ weight occurred during the next 14 days after treatment. This same study evaluated the LD50 value (7.82 g/kg i.p.) for the hydroalcoholic extract in mice. Our results indicate that the treatment of pregnant rats with the aqueous extract at 1, 2, or 3 g/kg/day was not able to promote alterations during gestation or for the exposed fetuses.
Absence of alterations at dams body weight, body weight gain, food consumption and water intake indicate that the treatment was not able to promote maternal toxicity. Also, the parameters of pregnancy duration, number of pups/litter, number of male and female pups/litter, body length and anogenital distance of pups at PND 1, indicate absence of alterations in reproductive performance. The reproductive hormonal patch way, analyzed by measurement of the estradiol, progesterone, follicule stimulant and luteinizing hormones in serum, was not altered, confirming the absence of alterations at reproductive performance.
The histopathological study is a tool employed to help elucidate the organ site and the mechanism of action of xenobiotics and toxicants once inside the organism. In this study no alterations and lesions were detected after detailed observation of tissue portions of liver, lung, pancreas, spleen, ovaries, uterus, testis, epidydimis of the treated dams (PND 21) and of the exposed pups at adult age (PND 30, 60, and 90).
The sexual behavior evaluated in the adult pups was not altered. However, the 3 g/kg/day exposed males showed reduced (but not statistically significant) copulatory efficiency. With these data we can speculate the possibility of statistically significant alterations if the dams be exposed to higher doses. However, the absence of alterations observed with the 3 g/kg/day dose, is sufficient to speculate about the possible absence of toxicity of this infusion.
This study showed that the infusion obtained from fresh leaves of T. ulmifolia in distilled water, was not able to promote toxic effects in dams or to impair gestation and fetal development. However, it is known that compounds like β-sitosterol, for example, are hydrophobic, and probably was not extracted by water. To confirm whether this plant possesses hormonal activity or not, more studies have to be realized, employing organic solvents such as ethanol as extraction agents.
We the authors would like to thank the Universidate federal do Rio Grande do Norte, for provide animals, food and adequate conditions for animals maintenance.
Declaration of interest: This study was supported by CNPq and is referent to the project developed by the first author at Universidate Federal do Rio Grande do Norte. The authors alone are responsible for the content and writing of the paper.
References
- Agmo A (1987): Male rat sexual behavior. Brain Res Protoc 1: 203–209.
- Antônio MA, Souza Brito ARM (1998): Oral anti-inflammatory and anti-ulcerogenic activities of a hydroalcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae). J Ethnopharmacol 61: 215–228.
- Beach FA (1967): Cerebral and hormonal control of reflexive mechanisms involved in copulatory behavior. Physiol Rev 47: 289–316.
- Bitran D, Hull E (1987): Pharmacological analysis of male rat sexual behavior. Neurosci Biobehav Rev 11: 365–389.
- Gallavan RH, Holson JF, Stump DG, Knapp JF, Reynolds VL (1999): Interpreting the toxicologic significance of alterations in anogenital distance: Potential for confounding effects of progeny body weights. Reprod Toxicol 13: 383–390.
- Galvez J, Gracioso JS, Camuesco D, Galvez J, Vilegas W, Souza Brito ARM, Zarzuelo A (2006): Intestinal antiinflammatory activity of a lyophilized infusion of Turnera ulmifolia in TNBS rat colitis. Fitoterapia 77: 515–520.
- Gracioso JS, Vilegas W, Hiruma-Lima CA, Souza Brito AR (2002): Effects of tea from Turnera ulmifolia L. on mouse gastric mucosa support the Turneraceae as a new source of antiulcerogenic drugs. Biol Pharm Bull 25: 87–91.
- Le March L (2002): Cancer preventive effects of flavonoids - A review. Biomed Pharmacother 56: 296–301.
- Lorenzi H (1991). Plantas daninhas do Brasil: Terrestres, aquáticas, parasitas, tóxicase medicinais. Nova Odessa, Plantarum Press, pp. 392.
- Malmnas CO (1973): Monoaminergic influence on testosterone activated copulatory behavior in castrated male rat. Acta Physiol Scand 395: 1–12.
- McCluscky NJ, Naftolin F (1981): Sexual differentiation of the central nervous system. Science 211: 1294–1303.
- Nascimento MA, Silva AK, França LCB, Quignard ELJ, López Já, Almeida MG (2006): Turnera ulmifolia L. (Turneraceae): Preliminary study of its antioxidant activity. Bioresour Technol 97: 1387–1391.
- Nestel P. (2003): Isoflavones: Their effects on cardiovascular risk and functions. Curr Opin Lipidol 14: 3–8.
- Pio Correa M (1984): Dicionário das plantas úteis do Brasil e das exóticas cultivadas. Rio de Janeiro, Imprensa Nacional, pp. 49–50.
