Abstract
Microbial transformation of natural products is a well established model for mammalian metabolism. Salvinorin A, a diterpenoid isolated from the hallucinogenic mint Salvia divinorum Epling & Játiva-M (Lamiaceae), is a potent non-nitrogenous κ-opioid receptor agonist. The metabolism of salvinorin A has still not yet been well established. Thirty fungal species were screened for the ability to metabolize salvinorin A. We observed that salvinorin A undergoes fast hydrolysis of the acetate group at carbon atom C2, resulting in formation of the pharmacologically inactive product, salvinorin B. Ex vivo experiments were also performed using organelle fractions isolated from rat liver and brain. Crude tissue homogenate and individual organelles show that the primary route of salvinorin A metabolism is hydrolysis to salvinorin B. No metabolic transformation of salvinorin B was observed in these studies.
Introduction
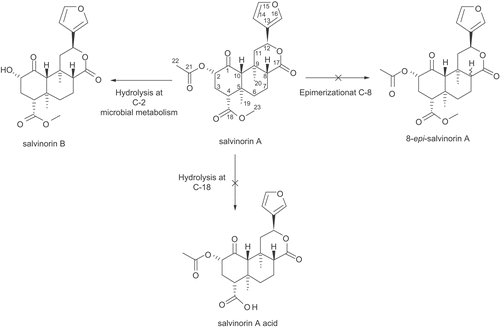
The diterpenoid salvinorin A is a natural product with hallucinogenic properties (CitationValdes et al., 1983). It has been isolated from Mexican sage Salvia divinorum Epling & Játiva-M (Labiatae), the only known hallucinogenic species of the Salvia genus. Salvinorin A attracted interest after CitationRoth and co-workers (2002) reported that it is a selective and highly potent naturally occurring κ-opioid receptor agonist. It has become a lead compound in searches for new drugs to combat psychiatric disorders such as schizophrenia, or Alzheimer’s or other dementias, in which κ-opioid receptors are believed to be involved (CitationSheffler & Roth, 2003). In experiments with monkeys addicted to cocaine, κ-opioid agonists reduce cocaine craving (CitationMello & Negus, 2000). Recent results show that salvinorin A, when given to rats along with cocaine, attenuates cocaine-induced locomotor activity (CitationChartoff et al., 2008).
The in vivo metabolism of salvinorin A is not fully understood (CitationBabu et al., 2008). Although studies with isolated body fluids from primates and humans incubated with salvinorin A revealed salvinorin B as a metabolite (CitationSchmidt et al., 2005b), in vivo pharmacokinetic experiments with nonhuman primates failed to detect salvinorin B or any other metabolite in the bloodstream (CitationSchmidt et al., 2005a). Prompted by these conflicting observations, we undertook studies of the fungal transformation and ex vivo metabolism with salvinorin A.
Smith and Rosazza were the first to propose microbes (fungi) as a model for mammalian metabolism (CitationSebek, 1982). Metabolites from fermentation are similar to those released in phase I (functionalization), or more rarely phase II (conjugation), of xenobiotic modification by mammalian enzymes (CitationAbourashed et al., 1999; CitationHanson, 1992). Microbial transformation of organic compounds is also a convenient method of obtaining structurally novel leads for drug discovery. The classes of chemicals being enzymatically modified range from simple monoterpenoids (CitationMiyazawa et al., 2003), sesquiterpenoids (artemisinin) (CitationParshikov et al., 2006), and aromatic compounds (CitationOrabi et al., 1999) to structurally more complex molecules, such as alkaloids (morphine) (CitationStabler et al., 2001), higher terpenoids, antibiotics, or marine natural products (e.g., sarcophine) (CitationEl Sayed et al., 1998). ent-Kaurenes and gibbererellins are among the most studied terpenoids utilizing microbial systems (CitationHanson, 1992). Xenobiotic inactivation in mammalian systems usually occurs in the liver, where hydroxylation, reduction, oxidation, or hydrolysis leads to increased polarity of compounds for better water solubility and eventual excretion (CitationVenisetty & Ciddi, 2003). The brain is also a place of drug metabolism, where efflux pumps effectively remove drugs from its space. For these reasons our ex vivo studies focused on these organs.
The goal of this study was to find the metabolites of salvinorin A using an analytical model for mammalian metabolism. Classical methods of searching for drug metabolites such as tracking down the radioactive label, or analysis of urine and other body fluids, still suffer from several constraints. In this case, one of them is lack of appropriately radiolabeled salvinorin A, still a challenging problem. The potency and unknown side effects of this psychedelic agent also limit the amounts that can be administered to animals or human volunteers, making a search for metabolites difficult. We are reporting here microbial transformation as a potential method of identifying salvinorin A metabolites.
Materials and methods
General experimental procedures
Mass spectra of crude extracts were generated by liquid chromatography-mass spectrometry (LC-MS) (Bruker Daltonik microTOF; Leipzig, Germany). Analysis was performed using a 150 × 4. 6 mm C8 column (Phenomenex Luna; Torrance, CA) with 3 μm particle size and a linear gradient ranging from 20% of MeCN to 100% over 20 min. The spectrometer was furnished with a photodiode array detector (Agilent 1100; Palo Alto, CA). Positive ionization mode was used in high-resolution electrospray ionization (HR-ESI)-MS analysis. Nuclear magenetic resonance (NMR) spectra were recorded on a 600 MHz Varian Innova instrument with a 3 mm Nalorac dual broadband probe. The NMR sample of salvinorin B was dissolved in acetonitrile-d3. Chemical shifts were calibrated to residual solvent resonances. NMR resonance assignments of salvinorin B were according to CitationGiner et al. (2007). Thin layer chromatography (TLC) of transformation products was performed with Merck HF254 silica gel plates, developed using a mobile phase composed of hexanes and ethyl acetate 1:1, v/v, and visualized by dipping in a 10% solution of vanillin–sulfuric acid reagent (1 g of vanillin in 40 mL of EtOH and 0.5 mL of conc. H2SO4), followed by heating at 120°C.
Chemicals
All chemicals were purchased from Fisher Scientific except for Difco potato dextrose broth (Becton, Dickinson and Co., Sparks, MD). Salvia divinorum was purchased from the Salvia divinorum Research Center, Malibu, CA, USA, and deposited in the Department of Pharmacognosy plant repository (voucher specimen number FH4-408-07). Crude plant extract was obtained through exhaustive extraction of 500 g of dry plant material with 6000 mL of acetone (3 × 2000 mL). Solvent was removed in vacuo at 35°C and reconstituted in ethanol for subsequent crystallization. After 24 h, crystals were filtered and washed with hexane to yield 95% pure salvinorin A. Salvinorin B was prepared as described elsewhere (CitationChavkin et al., 2004). Briefly, 1 g of salvinorin A was dissolved in 500 mL of MeOH, 1 g of K2CO3 was added, and the mixture was stirred for 36 h at room temperature. The precipitate of 95% pure salvinorin B was then filtered and dried under vacuum for further use.
Chromatographic conditions
Residues obtained from liquid extraction of microbial cultures were dissolved in acetonitrile (MeCN) and filtered through a 0.2 μm high-performance liquid chromatography (HPLC) filter (Millex®-GN; Bedford, MA) prior to analysis. Analytical profiling of extracts was performed using HPLC (Waters Alliance; Waters Corp., Milford, MA) furnished with a Synergi Fusion-RP C18 column (150 × 10 mm, 4 μm particle size) (Phenomenex). The mobile phase composition consisted of phase A – MeCN, and phase B – water. The gradient started at 20% of phase A and increased to 80% over 30 min, and further to 100% of MeCN in the next 10 min, followed by 10 min of wash with 100% MeCN. Salvinorin A retention time was 20.7 min and salvinorin B 15.6 min. Isolation of metabolites was performed also by HPLC, where the crude extract was loaded onto a Phenomenex C8 (250 × 10 mm, 5 μm particle size) column connected to an HPLC (Delta Prep 4000; Waters Corp.) system equipped with a dual wavelength detector (Model 2487; Waters Corp.). The mobile phase composition consisted of an MeCN:H2O (40:60) isocratic system. Retention time for salvinorin A was 11.3 min and salvinorin B 4.1 min. For detection, ultraviolet (UV) radiation, λ = 210 and 254 nm, was used. For analysis of extracts from ex vivo experiments, a Waters C18 XTerra column (100 × 7. 6 mm, 5 μm particle size) was used, where salvinorin A and B retention times were 16.3 min and 12.9 min, respectively. The mobile phase composition was the same as for the analytical method presented above.
Microorganisms
The following fungal strains were chosen for the preliminary screening and were purchased from the American Type Culture Collection (ATCC, Rockville, MD): Mucor plumbeus ATCC 8773, Aspergillus niger ATCC 9142, Aspergillus flavus ATCC 9170, Cunninghamella bainieri ATCC 9244, Cunninghamella echinulata ATCC 9245, Mucor ramannianus ATCC 9628, Saccharomyces cerevisiae ATCC 9763, Rhizopus oryzae ATCC 11145, Rhodococcus rhodochrous ATCC 12674, Fusarium solani ATCC 12823, Beauveria bassiana ATCC 13144, Mortierella zonata ATCC 13309, Curvularia lunata ATCC 13633, Rhizopus stolonifer ATCC 14037, Aspergillus niger ATCC 16888, Aspergillus ochraceus ATCC 18412, Aspergillus ochraceus ATCC 18500, Mucor mucedo ATCC 20094, Rhodotorula mucilaginosa ATCC 20129, Absidia glauca ATCC 22752, Aspergillus ochraceus ATCC 22947, Rhizopus stolonifer ATCC 24795, Verticillium lecanii ATCC 28300, Rhizopus oryzae ATCC 34121, Curvularia lunata ATCC 34690, Cunninghamella echinulata ATCC 36190, Cunninghamella echinulata ATCC 10028b, Cunninghamella echinulata ATCC 11585a, Rhizopus stolonifer ATCC 6227a, and Rhizopus stolonifer ATCC 6227b.
Microbial transformation screening and scale-up
For all stages of fermentation the same medium containing: peptone, yeast extract, potassium phosphate, sodium chloride, and dextrose in weight ratio 1:1:1:1:4 dissolved in double deionized water was used, and the pH was adjusted to 7.2 by the addition of 0.1 M potassium hydroxide. Medium was autoclaved at 121°C for 15 min. Cultures were grown according to the standard two-stage procedure (CitationAbourashed et al., 1999). Fermentation was inoculated to 125 mL conical Bellco Delong steel capped flasks containing 25 mL of broth, where the initial stage was run for 72 h, followed by transfer to a fresh medium. After 1 day of culture, 5 mg of salvinorin A was added, with the final concentration 0.25 mg/mL in dimethylsulfoxide (DMSO) (stage II). The second stage was allowed to proceed for 14 days at 28°C on a rotary shaker (C24KC refrigerated shaker; New Brunswick Scientific, Edison, NJ) at 200 rpm. Control experiments with the same set of microorganisms containing vehicle only were conducted simultaneously. After 14 days of incubation the culture broth was filtered and extracted with ethyl acetate. The organic solvent was evaporated in vacuo at 35°C and samples were later re-dissolved in acetonitrile for analysis. Scale-up experiments were performed under the same experimental conditions with a 10-fold increase in broth volume and substrate content per selected microorganism. The kinetics of salvinorin A conversion was measured by sampling 1 mL of culture broth per day, followed by extraction with ethyl acetate. The organic extract was subsequently injected into an analytical HPLC apparatus and the peak area was measured with regard to standard curves of salvinorins A and B.
Ex vivo assays
Adult Sprague-Dawley rats (Harlan, 250–300 g), maintained in a 12 h light/dark cycle and fed ad libitum, were used for the study. The protocol for the study was approved by the University of Mississippi Institutional Animal Care and Use Committee (IACUC). Rat brain and liver were excised upon decapitation. After dissection, the tissues were weighed and immediately homogenized in ice-cold buffer (10 mM Tris and 0.25 M sucrose, pH 7.2). Homogenates were further fractionated by centrifugation at 1000 × g for 10 min, 10,000 × g for 20 min (Allegra 25R centrifuge; Beckmann Coulter, Fullerton, CA), and 100,000 × g for 60 min (Optima Max ultracentrifuge; Beckmann Coulter) to obtain fractions of nuclei, mitochondria, and microsomes, respectively. Assays were carried out with homogenates of each organ or respective organelle fractions in 1 mL of 10 mM Tris buffer (150 mM NaCl, pH 7.2). Treatment was done in triplicate whereas controls were done in duplicate. Treatment samples were incubated with 4 μL (10 mmol) salvinorin A dissolved in DMSO (50 mM stock). Two types of controls were used in the study: the first comprised only buffer and salvinorin A to evaluate the stability of salvinorin A in blank Tris buffer, and the second contained the respective tissue/organelle fraction and vehicle. The assay was carried out for 1 h at 37°C on a rotary shaker (Thermomixer® R; Eppendorf, Westbury, NY) at 800 rpm. The reaction was terminated by centrifugation at 4°C at 10,000 × g for 5 min. The supernatant was then collected and stored at −20°C for further HPLC analysis.
Salvinorin B
Salvinorin B was isolated as a white solid, HR-MS m/z [M + H]+ 391.1518 (calcd for C21H26O7 390.1679); NMR spectroscopic data matched those previously published (CitationGiner et al., 2007).
Results and discussion
Microbial transformation of salvinorin A
Microorganisms used for the experiment were previously reported capable of transforming diterpenoids in reactions such as hydroxylation, oxidation, reduction, or epoxidation (CitationHanson, 1992). A preliminary screening of salvinorin A (5 mg) showed that most of the microorganisms transformed the substrate into salvinorin B (). TLC monitoring of the transformation process revealed that some microbes were capable of transforming approximately 50% of the substrate within the first 24 h after addition of salvinorin A into a medium (data not shown). However, the conversion efficiency varied between organisms, so, for example, C. lunata, M. zonata, R. rhodochrous, M. plumbeus, A. niger, C. bainieri, and R. stolonifer were capable of complete hydrolysis of salvinorin A to salvinorin B within 14 days of incubation. Approximately 70% of salvinorin A was metabolized into salvinorin B by A. glauca, A. ochraceus, and B. bassiana. M. mucedo, M. ramannianus, and R. oryzae hydrolyzed approximately 50% of the substrate in this time period. By contrast, V. lecanii, C. lunata, and C. echinulata poorly transformed salvinorin A (only about 10–15% of conversion to salvinorin B) over 14 days of incubation.
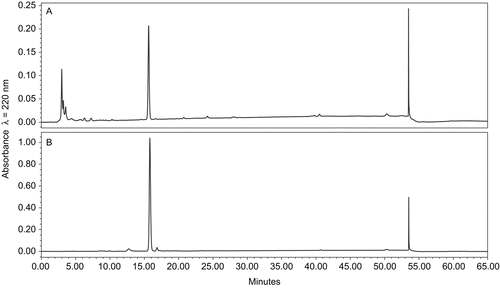
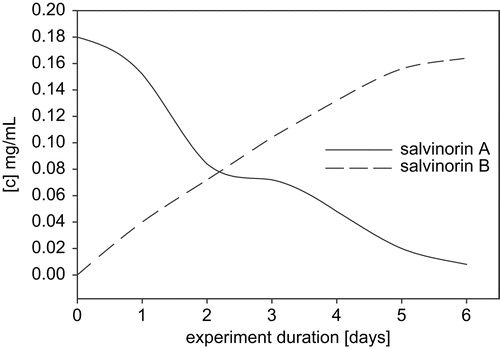
Scale-up experiments involved four species and all were carried out for 14 days: C. bainieri, M. zonata, R. stolonifer, and A. glauca, which were chosen based on their TLC and analytical HPLC chromatographic profiles. LC-MS analysis was performed to confirm the presence of salvinorin B in fermentation extracts. HPLC profiles of extracts from scale-up experiments showed the exact rate of transformation of salvinorin A into salvinorin B. C. bainieri converted salvinorin A to salvinorin B in 100% yield (), and was used later on to determine the kinetics of the conversion. The presented data () indicate that 50% of salvinorin A was hydrolyzed to salvinorin B within 48 h, and that it was almost completely converted 6 days after adding substrate to the culture. M. zonata, which also gave 100% conversion of salvinorin A to salvinorin B (~45 mg), was used to isolate and identify the metabolite. NMR spectra of the metabolite were in full agreement with published data, and confirmed that the obtained product was indeed salvinorin B (CitationGiner et al., 2007). R. stolonifer produced salvinorin B in 90% yield, but no other products were detected, comparing with the control strains. The preparative yield of A. glauca did not correlate with that of the screening scale, with only 37% conversion of salvinorin A to salvinorin B.
To make sure that salvinorin B was indeed the final product of microbial metabolism, we performed another experiment where we incubated all 30 microorganisms that we used to biotransform salvinorin A with pure salvinorin B. Attempts to demonstrate further transformation of this compound were unsuccessful. The screening experiment revealed that none of the 30 species was able to further metabolize salvinorin B. HPLC analysis of additional scale-up experiments involving C. echinulata and A. glauca confirmed these results. Both salvinorin A and salvinorin B proved to be stable in the medium with the absence of microorganisms (substrate control), ruling out any possibility of medium-influenced hydrolysis or decomposition of the substrate.
There has been only one report indicating that salvinorin A is rapidly hydrolyzed in vitro to its inactive metabolite, salvinorin B. CitationSchmidt and co-workers (2005a) described the formation of salvinorin B within monkey plasma incubated with salvinorin A at 37°C. The authors reported that within the first 15 min of incubation, approximately 20% of salvinorin A was converted to salvinorin B, where blood esterases were mainly responsible for that conversion. They did not observe any other metabolites (CitationSchmidt et al., 2005a). Such a short presence of salvinorin A under in vitro conditions was also confirmed by in vivo experiments conducted by CitationPichini and co-workers (2005). The average recovery of salvinorin A from saliva, 1 h post-treatment, was about 18.0 ng/mL, and about 6.7 ng/mL from urine (n = 2). The total amount of salvinorin A extracted from urine after that time was about 0.8% of the administered dose (0.5 mg). Blood samples were not analyzed in that study (CitationPichini et al., 2005). An in vivo experiment performed by CitationSchmidt et al. (2005b) also showed rapid elimination of salvinorin A in the rhesus monkey, which varied with gender. For the male rhesus monkey, the elimination half-life was 37.9 ± 5.6 min, whereas for the female it was much slower, 80.0 ± 13.1 min. In none of these in vivo experiments, however, did the authors observe the formation of salvinorin B, or any other metabolites of salvinorin A. These should have been detectable in the analyzed matrices (CitationPichini et al., 2005; CitationSchmidt et al., 2005b).
In our experiments we focused on the microbial model as that which could potentially reflect a wider variety of the phase I enzymes, resembling the situation present in more specific organs such as the liver. Although the use of a variety of microbial species was aimed to assess possible diverse enzymatic catalysis of salvinorin A into more oxidized products than salvinorin B, we were unable to show such transformations. At this point we conclude that rapid decay of the hallucinogenic effect of salvinorin A, after administration, is most probably due to a fast and non-specific hydrolysis of the acetate group at C-2 to form salvinorin B. Recent experiments with 2-methoxymethyl salvinorin B, which has an ether instead of an ester bond, shows that its metabolism is greatly slowed by the presence of a hydrolysis-resistant group at C-2 (CitationWang et al., 2008).
Ex vivo metabolism of salvinorin A
We carried out ex vivo metabolism studies using rat liver crude homogenate. Incubation of salvinorin A with liver crude homogenate resulted in complete hydrolysis of substrate to salvinorin B. Further fractionation of the homogenate to nuclei, mitochondria, and microsomes confirmed the same reaction, with 100% conversion in all fractions. Incubation of salvinorin A with cytosol also gave a similar result. The ubiquity of hydrolases in liver tissue gave the expected deacetylation product of salvinorin A. While the liver is the primary site of drug metabolism in many organisms, we were expecting to observe additional routes of metabolism, such as oxidation by cytochrome P450, or conjugation with glucuronic acid. However, none of these reactions were detected. Brain tissue is also a place of drug metabolism, but unlike the liver, it has a different enzyme composition. In our experiment, enzymes from brain homogenate metabolized salvinorin A as efficiently as those from the liver. The incubation of individual organelles with salvinorin A resulted in full hydrolysis to salvinorin B. Incubation with the nuclear and mitochondrial fractions as well as with microsomes and cytosol gave the same result. Although the cytosol is rich in phase II enzymes, we did not observe any transformation product of salvinorin A, characteristic of phase II metabolism.
There is no noticeable difference in metabolism of salvinorin A between brain and liver, two functionally distinct tissues. Although both the liver and the brain contain enzymes of phase I of drug metabolism (cytochrome P450, epoxyhydrolases, dehydrogenases, various types of oxidases, and oxidoreductases), the brain has an effective way of removing drugs from its space, for example by using the P-glycoprotein efflux pump (CitationAllt & Lawrenson, 2000; CitationCordon-Cardo et al., 1989). Whether there is any deposition of salvinorin B in vivo in any of the analyzed tissues remains unknown.
Conclusions
In conclusion, we are reporting here that the biotransformation of salvinorin A, using two independent models of mammalian metabolism (fungi/rat liver and brain), yielded salvinorin B as the same metabolite. The metabolite concentration, however, varied between the models. While the substrate is more available to free enzyme(s) in ex vivo experiments, fungi, on the other hand, vary in mechanisms of substrate uptake and enzyme composition, which may have affected the rate of salvinorin B production. Attempts to demonstrate metabolism of salvinorin B did not yield any further detectable metabolites in either model. Although microbial transformation in general is a good method of derivatization of natural products, it was of limited utility in this case. There is a need for appropriately radiolabeled salvinorin A, which would be a highly valuable tool for tracking metabolites in in vivo models.
Acknowledgements
The authors would like to acknowledge Ms. Kelly Thomas for her help during experiments with microbes, Ms. Anna Kochanowska for recording mass spectra, Dr. Jiangnan Peng for 600 MHz NMR of salvinorin B, and Dr. Asok Dasmahapatra for helpful suggestions during ex vivo experiments.
Declaration of interest: Financial support for one of the authors (L.M.K.) came from NIUST grant NA16RU1496, another (V.T.K.) was supported by the Peptide Radioiodination Service Center of the University of Mississippi, and research funds came from NIH Grant P20 RR 021929-01 from the National Center for Research Resources and CDC Grant U50/CCU418839-01. The work was conducted in a facility constructed with support from research improvement program grant C06 RR-14503-01 from the National Institutes of Health, Bethesda, MD, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- Abourashed EA, Clark AM, Huford CD (1999): Microbial models of mammalian metabolism of xenobiotics: An updated review. Curr Med Chem 6: 359–374.
- Allt G, Lawrenson JG (2000): The blood-nerve barrier: enzymes, transporters and receptors – a comparison with the blood-brain barrier. Brain Res Bull 52: 1–12.
- Babu KM, McCurdy CR, Boyer EW (2008): Opioid receptors and legal highs: Salvia divinorum and Kratom. Clin Toxicol 46: 146–152.
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WAJ (2008): Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology 33: 2550–2562.
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL (2004): Salvinorin A, an active component of the hallucinogenic sage Salvia divinorum is a highly efficacious kappa-opioid receptor agonist: Structural and functional considerations. J Pharmacol Exp Ther 308: 1197–1203.
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR (1989): Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA 86: 695–698.
- El Sayed KA, Hamann MT, Waddling CA, Jensen CL, Sang K, Dunstan CA, Pezzuto JM (1998): Structurally novel bioconversion products of the marine natural product sarcophine effectively inhibit JB6 cell transformation. J Org Chem 63: 7449–7455.
- Giner JL, Kiemle DJ, Kutrzeba L, Zjawiony JK (2007): Unambiguous NMR spectral assignments of salvinorin A. Magn Reson Chem 45: 351–354.
- Hanson JR (1992): The microbiological transformation of diterpenoids. Nat Prod Rep 9: 139–151.
- Mello NK, Negus SS (2000): Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann NY Acad Sci 909: 104–132.
- Miyazawa M, Kawazoe H, Hyakumachi M (2003): Biopreparation of (–)-(1S,3R,4S,6S)-6-hydroxymenthol and (–)-(1S,3R,4S)-1- hydroxymenthol from 1-menthol by Rhizoctonia solani AG-1-IA and IB. Nat Prod Res 17: 307–311.
- Orabi KY, Li E, Clark AM, Hufford CD (1999): Microbial transformation of sampangine. J Nat Prod 6: 988–992.
- Parshikov IA, Miriyala B, Muraleedharan KM, Avery MA, Williamson JS (2006): Microbial transformation of artemisinin to 5-hydroxyartemisinin by Eurotium amstelodami and Aspergillus niger. J Ind Microbiol Biotechnol 33: 349–352.
- Pichini S, Abanades S, Farre M, Pellegrini M, Marchei E, Pacifici R, Torre RD, Zuccaro P (2005): Quantification of the plant-derived hallucinogen salvinorin A in conventional and non-conventional biological fluids by gas chromatography/mass spectrometry after Salvia divinorum smoking. Rapid Commun Mass Spectrom 19: 1649–1656.
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB (2002): Salvinorin A: A potent naturally occurring nonnitrogenous κ opioid selective agonist. Proc Natl Acad Sci USA 99: 11934–11939.
- Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, Kreek MJ, Prisinzano TE (2005a): Pharmacokinetics of the plant-derived κ-opioid hallucinogen salvinorin A in nonhuman primates. Synapse 58: 208–210.
- Schmidt MS, Prisinzano TE, Tidgewell K, Harding W, Butelman ER, Kreek MJ, Murry DJ (2005b): Determination of salvinorin A in body fluids by high performance liquid chromatography-atmospheric pressure chemical ionization. J Chromatogr B 818: 221–225.
- Sebek OK (1982): Microbial models of mammalian metabolism. In: Rosazza JP, ed., Microbial Transformation of Bioactive Compounds. Boca Raton, FL, CRC Press, pp. 1–8.
- Sheffler DJ, Roth BL (2003): Salvinorin A: The “magic mint” hallucinogen finds a molecular target in the kappa opioid receptor. Trends Pharmacol Sci 24: 107–109.
- Stabler PJ, Holt PJ, Bruce NC (2001): Transformation of 2,2′-bimorphine to the novel compounds 10-α-S-monohydroxy-2,2′-bimorphine and 10,10′-α,α′-S,S′-dihydroxy-2,2′-bimorphine by Cylindrocarpon didymum. Appl Environ Microbiol 67: 3716–3719.
- Valdes LJ, Diaz JL, Paul AG (1983): Ethnopharmacology of Ska Maria Pastora (Salvia divinorum, Epling and Játiva-M.). J Ethnopharmacol 7: 287–312.
- Venisetty RK, Ciddi V (2003): Application of microbial biotransformation for the new drug discovery using natural drugs as substrates. Curr Pharm Biotechnol 4: 153–167.
- Wang Y, Chen Y, Xu W, Lee DYW, Ma Z, Rawls SM, Cowan A, Liu-Chen, Lee-Yuan (2008): 2-Methoxymethyl-salvinorin B is a potent κ-opioid receptor agonist with longer lasting action in vivo than salvinorin A. J Pharmacol Exp Ther 324:1073–1083.