Abstract
Twenty compounds isolated from Calophyllum inophyllum L., C. inophylloides King, Garcinia opaca King, G. bancana Miq., and G. parvifolia Miq. (Guttiferae) were evaluated for their ability to inhibit platelet aggregation in human whole blood induced by arachidonic acid (AA), collagen, and adenosine diphosphate (ADP). The compounds inhibited platelet aggregation in a dose-dependent manner. Among the compounds tested, 2-(3-methylbut-2-enyl)-1,3,5-trihydroxyxanthone and 2-(3-methylbut-2-enyl)-1,3,5,6- tetrahydroxyxanthone showed strong inhibitory activity on platelet aggregation induced by AA with IC50 values of 115.9 and 113.0 μM, respectively. Rubraxanthone showed inhibitory activity against aggregation caused by the three inducers, and was the most effective antiplatelet compound against collagen-induced platelet aggregation with an IC50 value of 47.0 μM. Macluraxanthone, GB-1a, pyranoamentoflavone, and a neoflavonoid showed selective inhibitory activity on platelet aggregation induced by ADP.
Introduction
Platelets play an important role in hemostasis during tissue injury. They interact with activated plasma clotting factors at the site of blood vessel injury, forming a mechanical plug which blocks the defect and terminates blood loss (CitationHarker & Mann, 1992). They have also been implicated in the formation of rapidly progressing atherosclerotic lesions, and play a key role in acute arterial thrombosis (CitationGibbins, 2004). Platelet aggregation is induced by the action of endogenous agonists such as arachidonic acid (AA), adenosine diphosphate (ADP), platelet activating factor (PAF), thrombin, and collagen (CitationArita et al., 1989).
Aspirin has been the drug of choice for the long-term treatment of platelet hyperactivity, especially to reduce the risk of serious ischemic events in several cardiovascular disease states including stroke, myocardial infarction, unstable angina, and following coronary artery bypass surgery. Aspirin usage is associated with aspirin resistance and serious side effects such as gastric hemorrhage (CitationLloyd & Bochner, 1996). Clopidogrel and ticlopidine still have considerable limitation in their mode of action and efficacy (CitationGresele & Agnelli, 2002). Thus, the search for more potent and safer non-aspirin platelet inhibitors has continued.
In recent years, phenolic compounds (CitationNurtjahja-Tjendraputra et al., 2003), oxygenated xanthones (CitationChung et al., 2002), coumarins (CitationTsai et al., 1998), isothiocyanates (CitationMorimitsu et al., 2000), a diterpene (CitationShen et al., 2000), quinines (CitationLiao et al., 2000), prenylflavonoids (Lin et al., Citation1993a), and alkaloids of diverse chemical structures (CitationJantan et al., 2006), which have been isolated from various plants, have shown potent antiplatelet activity.
The ability of compounds from several Guttiferae species to displace [3H]PAF-specific binding from washed rabbit platelets was reported earlier (Jantan et al., Citation2001a, Citation2001b, Citation2002). In the present study, the methanol extracts of these plants showed strong antiplatelet activity in human whole blood in vitro. The authors investigated the antiplatelet activity of compounds from five Guttiferae species, namely, Calophyllum inophyllum L., C. inophylloides King, Garcinia opaca King, G. bancana Miq., and G. parvifolia Miq.
Materials and methods
The compounds were previously isolated from Calophyllum inophyllum, C. inophylloides, Garcinia opaca, G. bancana, and G. parvifolia (CitationGoh & Jantan, 1991; Goh et al., Citation1992a, Citation1992b; CitationJantan & Goh, 1995; CitationJantan et al., 2002). Collagen, ADP, and AA were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The use of human whole blood in this study was approved by the Ethics Committee of the Universiti Kebangsaan Malaysia. Healthy volunteers were recruited based on the criteria that they were non-smokers and had not taken any medications within the last 2 weeks, including aspirin, and had not taken any food within the last 8 h.
Whole blood (20 mL) of a subject was collected in a vacutainer containing 3.8% sodium citrate (9:1, v/v) and thoroughly mixed by inverting the vacutainer several times. The blood sample was then diluted with normal saline in the ratio of 1:1. The dried methanol extracts and the isolated compounds were each dissolved in dimethyl sulfoxide (DMSO) to obtain concentrations of 20, 10, 5, and 2.5 μg/μL. Each sample (5 μL) was added to a cuvette containing diluted whole blood, and the mixture was incubated for 4 min at 37°C prior to the addition of AA (0.5 mM), ADP (10 μM), or collagen (2 μg/mL). The total volume of the mixture was 1 mL. The final concentrations of the sample in the mixture were 100, 50, 25, and 12.5 μg/mL. The platelet aggregation was measured by a whole blood Lumi-Aggregometer (Chrono-Log Corp., Havertown, PA) using an electrical impedance method (CitationIngerman-Wojenski & Silver, 1984). The mean platelet aggregation in whole blood was measured as a change in impedance over 6 min after the addition of inducer by comparison to that of a control group impedance (CitationChallen et al., 1982). A mixture containing 0.5% DMSO in diluted whole blood was used as control. Aspirin, a potent cyclooxygenase inhibitor, was used as a positive control in the bioassay (CitationLloyd & Bochner, 1996). The final concentration of DMSO in whole blood was 0.5% to eliminate the effect of the solvent on aggregation (CitationDong & Chen 1998).
The percentage inhibition of platelet aggregation was calculated as follows:
Each sample was measured in triplicate and the data are presented as mean ± SE. A one-way analysis of variance (ANOVA) was used for multiple comparison, and if significant variation occurred between treatment groups, the mean values for inhibitors were compared with those for controls by Student’s t-test; p values of 0.05 or less were considered to be statistically significant. The IC50 values, that is, the concentrations of the compounds required to inhibit aggregation by 50%, were obtained from at least three determinations.
Results and discussion
In the present study, the methanol extracts of Calophyllum inophyllum, C. inophylloides, Garcinia opaca, G. bancana, and G. parvifolia showed strong antiplatelet aggregation activity at 100 μg/mL in human whole blood in vitro, with all extracts exhibiting about 90–96% inhibition. Twenty compounds isolated from these species were investigated for their effects on platelet aggregation of human whole blood. shows the inhibitory effects of the compounds at various concentrations. The compounds inhibited platelet aggregation in a dose-dependent manner, i.e. as the concentration of the compound increased the percentage inhibition increased. The structures of compounds with significant antiplatelet effects are shown in .
Figure 1. Structures of compounds from Guttiferae species with significant antiplatelet aggregation effects.
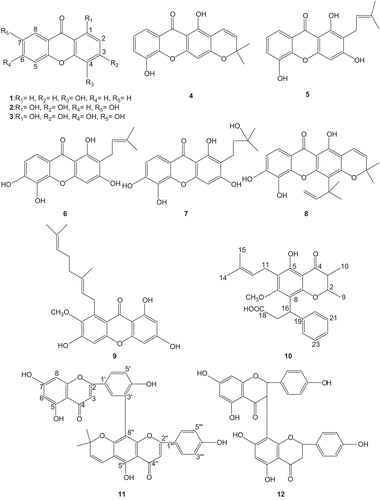
Table 1. Percentage inhibition of isolated compounds from Guttiferae species on platelet aggregation in human whole blood induced by arachidonic acid (AA) (0.5 mM), collagen (2 μg/mL), and adenosine diphosphate (ADP) (10 μM).
Except for isocowanol (13), which showed no obvious activity on platelet aggregation at 100 μg/mL, all the other xanthones (compounds 1–9) showed marked inhibitory effects on platelet aggregation caused by one, two, or all three inducers. The results of this study are in agreement with previous studies, which reported that many xanthones showed marked antiplatelet effects (CitationTeng et al., 1989; Lin et al., Citation1993b; CitationChung et al., 2002). Quercetin (15) moderately inhibited platelet aggregation with all the aggregant agents, whereas its 3-O- rhamnoside and kaempferol-3-O-rhamnoside showed slight antiplatelet effects. In fact, the antiplatelet activity of the flavonoid quercetin is well known, and has been widely investigated (CitationGuglielmone et al., 2005). Among the three biflavonoids, pyranoamentoflavone (11) and GB-1a (12) exhibited selective inhibitory activity on platelet aggregation induced by ADP, but amentoflavone (14) showed minimum activity. The neoflavonoid (10) also showed selective inhibitory activity on platelet aggregation induced by ADP.
The IC50 values of the compounds with antiplatelet activity are shown in . 2-(3-Methylbut-2-enyl)- 1,3,5-trihydroxyxanthone (5) and 2-(3-methylbut-2-enyl)-1,3,5,6-tetrahydroxyxanthone (6) showed strong inhibition on platelet aggregation induced by AA, with IC50 values of 115.9 and 113.0 μM, respectively. Structure–activity analysis of xanthones revealed that a prenyl group at C-2 may be important with regard to its relatively strong antiplatelet activity. However, hydroxylation of the prenyl group (i.e., compound 7) significantly increased the IC50 value. Cyclization of the prenyl group of compound 5 to form a chromene ring substituted at C-2 and C-3 resulted in an increase in IC50 value (i.e., compound 4). The IC50 values of all the compounds evaluated were higher than that of aspirin (27.3 μM).
Table 2. IC50 values (μM) of isolated compounds on platelet aggregation induced by arachidonic acid (AA) (0.5 mM), collagen (2 μg/mL), and adenosine diphosphate (ADP) (10 μM).
Inhibition of platelet aggregation induced by the agonists may be related to the hydroxylation pattern of the xanthone ring. Tetraoxygenated xanthone ( compound 3) showed a higher antiplatelet effect than the trioxygenated derivative (compound 2), whereas the monooxygenated xanthone (compound 1) was not effective toward AA-induced aggregation. However, against ADP-induced aggregation, 1,3,7- trihydroxyxanthone (2) showed significant inhibition with an IC50 value of 69.5 μM, 4- hydroxyxanthone (1) showed selective inhibition with an IC50 value of 136.2 μM, whereas 1,3,6,7- tetrahydroxyxanthone (3) showed no significant inhibition. The antiplatelet activity of compounds 1, 2, and 3 against AA- and ADP-induced aggregation was in agreement with the results of previous studies in which oxygenated xanthones, in general, were significantly active against aggregation induced by many agonists (Lin et al., Citation1993b).
Macluraxanthone (8) was the most effective antiplatelet compound against ADP-induced aggregation, as it exhibited an IC50 value of 56.0 μM. Rubraxanthone (9) showed inhibitory activity against aggregation caused by all three inducers and was the most effective antiplatelet compound against collagen-induced platelet aggregation with an IC50 value of 47.0 μM. The presence of a geranyl group substituted at C-8 may contribute to its relatively strong antiplatelet effect. However, the presence of a hydroxylated prenyl group at C-4 significantly reduced the antiplatelet effect. The neoflavonoid (10) and the biflavonoids, pyranoamentoflavone (11) and GB-1a (12), showed selective inhibitory activity on platelet aggregation induced by ADP with IC50 values of 88.0, 95.1, and 74.4 μM, respectively.
The results indicate that various xanthones and flavonoids isolated from Guttiferae species are relatively strong inhibitors of platelet aggregation. Further studies need to be carried out to investigate the mechanisms of action and structure–activity relationships of the active compounds and to find the lead structures with maximum inhibitory activity.
Declaration of interest: This work was supported by a grant (IRPA 09202065-EA165) from the Ministry of Science, Technology and Innovation, Malaysia.
References
- Arita H, Nakano T, Hansaki K (1989): Thromboxane A2, its generation and role in platelet activation. Prog Lipid Res 28: 273–301.
- Challen A, Branch WJ, Cummings JH (1982): Quantitation of platelet mass during aggregation in the electronic (Wellcome) whole blood aggregometer. J Pharmacol Meth 8: 115–122.
- Chung MI, Weng JR, Wang JP, Teng CM, Lin CN (2002): Antiplatelet and anti-inflammatory constituents and new oxygenated xanthones from Hypericum geminiflorum. Planta Med 68: 25–29.
- Dong H, Chen S-X (1998): A new antiplatelet diarylheptanoid from Alpinia blepharocalyx. J Nat Prod 61: 142–144.
- Gibbins JM (2004): Platelet adhesion signaling and the regulation of thrombus formation. J Cell Sci 117: 3415–3425.
- Goh SH, Jantan I (1991): A xanthone from Calophyllum inophyllum. Phytochemistry 30: 366–367.
- Goh SH, Jantan I, Gray AI, Waterman PG (1992a): Prenylated xanthones of Garcinia opaca. Phytochemistry 31: 1383–1386.
- Goh SH, Jantan I, Waterman PG (1992b): Neoflavonoid and biflavonoid constituents of Calophyllum inophylloide. J Nat Prod 55: 1415–1420.
- Gresele P, Agnelli G (2002): Novel approaches to the treatment of thrombosis. Trends Pharmacol Sci 23: 25–32.
- Guglielmone HA, Agnese AM, Nunez Montoya SC, Cabrera JL (2005): Inhibitory effects of sulphated flavonoids isolated from Flaveria bidentis on platelet aggregation. Throm Res 115: 495–502.
- Harker LA, Mann KG (1992): Thrombosis and fibrinolysis. In: Fuster V, Verstraete M, eds., Thrombosis in Cardiovascular Disorders. Philadelphia, PA, WB Saunders, pp. 1–16.
- Ingerman-Wojenski CM, Silver MJ (1984): A quick method for screening platelet dysfunctions using the whole blood lumi- aggregometer. Thromb Haemost 2: 154–156.
- Jantan I, Goh SH (1995): Flavonoids, xanthones and triterpenes of the leaves and heartwood of Garcinia bancana Miq. Sains Malaysiana 24: 23–30.
- Jantan I, Jalil J, Warif NA (2001a): Inhibitory effects of xanthones on platelet activating factor receptor binding in vitro. J Ethnopharmacol 75: 287–290.
- Jantan I, Jalil J, Warif NA (2001b): Platelet activating factor (PAF) antagonistic activities of compounds isolated from Guttiferae species. Pharm Biol 39: 243–246.
- Jantan I, Pisar M, Idris MS, Taher M, Mohd AR (2002): Inhibitory effect of rubraxanthone isolated from Garcinia parvifolia Miq. on platelet activating factor receptor binding. Planta Med 68: 1133–1134.
- Jantan I, Raweh SM, Yassin YH, Murad S (2006): Antiplatelet activity of aporphine and phenanthrene alkaloids from Aromadendron elegans Blume. Phytother Res 20: 493–496.
- Liao CH, Ko FN, Wu CL, Teng CM (2000): Antiplatelet effect of marchatinquinone, isolated from Reboulia hemisphaerica, in rabbit washed platelets. J Pharm Pharmacol 52: 353–359.
- Lin CN, Shieh WL, Ko FN, Teng CM (1993a): Antiplatelet activity of some prenylflavonoids. Biochem Pharmacol 45: 509–512.
- Lin CN, Liou SS, Ko FN, Teng CM (1993b): Gamma-pyrone compounds. IV: Synthesis and antiplatelet effects of mono- and deoxygenated xanthones and xanthonoxypropanolamine. J Pharm Sci 82: 11–16.
- Lloyd J, Bochner F (1996): Aspirin: how low is the dose? Aust Prescr 19: 79–81.
- Morimitsu Y, Hayashi K, Nakagawa Y, Fujii H, Horio F, Uchida K, Osawa T (2000): Antiplatelet and anticancer isothiocyanates in Japanese domestic horseradish, Wasabi. Mech Ageing Dev 116: 125–134.
- Nurtjahja-Tjendraputra E, Ammit AJ, Roufogalis BD, Tran VH, Duke CC (2003): Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res 111: 259–265.
- Shen Z, Chen Z, Li L, Lei W, Hao X (2000): Antiplatelet and antithrombotic effects of the diterpene spiramine Q from Spirae japonica var. incise. Planta Med 66: 287–289.
- Teng CM, Lin CN, Ko FN, Cheng KL, Huang TF (1989): Novel inhibitory actions on platelet thromboxane and inositolphosphate formation by xanthones and their glycosides. Biochem Pharmacol 38: 3791–3795.
- Tsai I-L, Wun M-F, Teng C-M, Ishikawa T, Chen I-S (1998): Antiplatelet aggregation constituents from Formosan Toddalia asiatica. Phytochemistry 48: 1377–1382.
