Abstract
This study investigated the effect of an aqueous extract of Tinospora cordifolia (Willd.) Miers (Menispermaceae) stem on mast cell mediated allergic reactions in vivo and in vitro and studied its possible mechanism. T. cordifolia (125 to 1000 mg/kg) dose-dependently inhibited compound 48/80 induced lethality in rats, histamine induced paw edema in mice and histamine induced bronchial asthma in guinea pigs. T. cordifolia significantly (p < 0.001) inhibited the cutaneous anaphylaxis reaction activated by histamine in a rat model and compound 48/80 induced ear swelling response in mice. T. cordifolia (2.5-160 μg/mL) also showed significant (p < 0.001) inhibition of histamine induced contraction of guinea-pig ileum in vitro implying the H1 antihistamine activity. T. cordifolia (0.01 to 10 mg/mL) significantly (p < 0.001) inhibited the histamine release from rat peritoneal mast cells activated by compound 48/80. In addition, T. cordifolia (0.01 to 10 mg/mL) significantly (p < 0.001) inhibited the secretion of tumor necrosis factor-α (TNF-α) in antidinitrophenyl (DNP) IgE-stimulated rat peritoneal mast cells. The level of cAMP in RPMC transiently and significantly increased compared with that of control cells when T. cordifolia was incubated with mast cells. T. cordifolia (0.01 to 10 mg/mL) showed concentration-dependent inhibition in compound 48/80 induced reactive oxygen species (ROS) generation. In addition, T. cordifolia decreased intracellular calcium levels of activated mast cells. These results show that T. cordifolia may be beneficial in the treatment of acute and chronic allergic disorders.
Introduction
Tinospora cordifolia (Willd.) Miers. (Menispermaceae) is a large, glabrous, climbing shrub. It is widely used in veterinary folk medicine/Indian system of medicine (Ayurvedic) for its general tonic, antispasmodic, anti-inflammatory, anti-arthritic, and antidiabetic properties. The antioxidant, antidiabetic, anti-inflammatory and immunomodulatory properties of novel polysaccharide and glucans from T. cordifolia have also been well documented (CitationNair et al., 2006; CitationPendse et al., 1981; CitationPrince et al., 2004; CitationSingh et al., 2003). In light of the aforementioned anti-inflammatory properties of T. cordifolia, it is hypothesized that the herb may be helpful in reducing the mast-mediated allergic reactions in rats via anti- histaminic activities since inflammatory mediators such as histamine are mainly involved in allergic reactions. The allergic reactions are thought to be driven by cross-linking of allergen-specific IgE bound to the surface of resident mast cells via the high-affinity IgE receptor, FcϵRI (CitationBeaven & Metzger, 1993; CitationBryce & Oettgen, 2005). Thus, the mast cell is the key effector cell in immediate hypersensitivity reactions, releasing histamine, inflammatory cytokines, chemokines and platelet-activating factor upon antigenic stimulation (CitationKim & Lee, 1999). Mast cell activation by IgE-dependent and IgE-independent stimuli brings about the process of degranulation which results in the fusion of the cytoplasmic granule membranes with the plasma membrane. This is followed by the fast release of granule-associated stored mediators such as histamine, cytokines, chemotactic factors, neutral proteases, proteoglycans, etc., as well as by the generation and release of newly generated mediators ( arachidonic acid metabolism products) (CitationKuehn & Gilfillan, 2007). At the later stage, mast cells start production and release of an array of cytokines (CitationChurch & Levi-Schaffer, 1997). Histamine remains the best-characterized and most potent vasoactive mediator implicated in the acute phase of immediate hypersensitivity among the inflammatory substances released from mast cells (CitationRobin et al., 2008).
Histamine stimulates nerve endings and dilates the blood vessels, causing itching and redness (CitationLeonardi, 2000). Mast cell degranulation can be elicited by a number of positively charged substances, collectively known as the basic secretagogues of mast cells (CitationPalomaki & Laitinen, 2006). Compound 48/80 is best known as a potent inducer of degranulation of mast cells and the release of chemical mediators of anaphylactic reactions (CitationNishikawa & Kitani, 2008). The secretory responses of mast cells can be also induced by aggregation of their cell surface-specific receptors for IgE by the corresponding antigen. Although mast cells also store small amounts of cytokines in their granules (CitationGordon & Galli, 1990), these cells dramatically increase their production of tumor necrosis factor-a (TNF-α) and other cytokines within 30 min after their surface FcϵRI are cross linked with specific antigen (CitationBurd et al., 1989). Therefore, the aim of our study was the effect of T. cordifolia on histamine or compound 48/84 or anti-DNP IgE induced in vivo and in vitro allergic reaction models. Further in vitro studies on measurement of cAMP levels, intracellular Ca2+ levels and intracellular reactive oxygen species were also performed to find out the mechanism of action of T. cordifolia on allergic disorders.
Materials and methods
Reagents
Compound 48/80, histamine diphosphate, anti-DNP IgE, DNP-human serum albumin (HSA), dichlorofuoroscein diacetate (DCF-DA), Fura-2/AM and metrizamide were purchased from Sigma Chemical (St Louis, MO). Evans blue and o-phthaldialdehyde (OPT) were purchased from HiMedia, Mumbai, India. All other chemicals were of analytical grade.
Preparation of T. cordifolia extract
The stem parts of T. cordifolia were collected from the Himalayas during March and were authenticated by P. S. N. Rao, at the Botanical Survey of India, Ministry of Environment and Forests, Government of India, Pune, where the voucher specimen (no. JVV1) was deposited at the herbarium unit of the institute. The plant sample was extracted with distilled water at 70°C for 5 h. The extract was filtered through a 0.45 μm filter and the filtrate was lyophilized, and kept at 4°C. The yield of dried extract from starting crude materials was about 10%. The dried extract was dissolved in saline before use.
Experimental protocol
All experiments and protocols described in the present study were approved by the Institutional Animal Ethics Committee of The Maharaja Sayajirao University of Baroda, and with permission from the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India. Animals were housed in groups of three and maintained under standardized conditions (12 h light/dark cycle, 22° ± 2°C and relative humidity of 55% ± 5%) and provided free access to pelleted Chakkan diet (Nav Maharashtra Oil Mills, Pune, India) and purified drinking water ad libitum. All the experimental animals were fasted overnight before the experiment. For in vivo studies animals were randomly divided into six groups as follows: Control animals (CON) group 1 treated with vehicle by oral gavage. Groups 2, 3, 4, and 5 were treated with extract of T. cordifolia orally at the dose of 125, 250, 500, 1000 mg/kg, respectively (TC 125, TC 250, TC 500, TC 1000). Animals of group 6 (cetirizine) were treated orally with cetirizine (0.25/0.3 mg/kg) and served as positive control.
For the histamine induced contraction of guinea-pig ileum study, the drug under investigation (T. cordifolia) was studied individually. For all other in vitro efficacy studies, rat peritoneal mast cells (RPMC) were isolated and incubated in the absence or presence of the test sample (T. cordifolia, cetirizine) at different concentrations ranging from 0.01 to 10 mg/mL before the addition of compound 48/84 or anti-DNP IgE. For the histamine release assay, sodium chromoglycate was studied as the reference drug as a mast cell stabilizer (0.0625 to 0.5 mg/mL).
The following groups were involved in the in vitro assay.
Group I: Control group (saline treated)
Group II: RPMC treated with herbal drug (T. cordifolia) at the concentration of 0.01 mg/mL
Group III: RPMC treated with herbal drug (T. cordifolia) at the concentration of 0.1 mg/mL
Group IV: RPMC treated with herbal drug (T. cordifolia) at the concentration of 1 mg/mL
Group V: RPMC treated with herbal drug (T. cordifolia) at the concentration of 10 mg/mL
Group VI: RPMC treated with cetirizine at the concentration of 0.0625 mg/mL
Group VII: RPMC treated with cetirizine at the concentration of 0.125 mg/mL
Group VIII: RPMC treated with cetirizine at the concentration of 0.25 mg/mL
Group IX: RPMC treated with cetirizine at the concentration of 0.5 mg/mL
In vivo studies
Protective effect of T. cordifolia against compound 48/80 induced lethality in rats
The study of compound 48/80 induced lethality in rats was carried out as previously described (CitationPandey et al., 1978). Briefly, male Sprague-Dawley rats (200 ± 25 g) were given an intravenous injection of compound 48/80 (0.75 mg/kg) through the caudal vein. Mortality was observed for 4 h after induction of the systemic anaphylactic reaction. Test drugs were orally administered 1 h before the injection of compound 48/80. Percentage protection from lethality was recorded after 48/80 injection with reference to the control group (vehicle treated) animals. Percentage protection and dose, was converted into Probit and log dose, respectively. ED50 calculations were performed by plotting the graph of log dose versus Probit.
Inhibitory effect of T. cordifolia on histamine induced paw edema in mice
This study was carried out as previously described (CitationKreutner et al., 2000). Male CD-1 mice (25 ± 5 g) were administered with test drug at different doses. After 1 h of dosing, animals were injected with 30 μg histamine diphosphate (in 25 μL physiological saline) into the sub-plantar region of the right hind paw and the same volume of saline into the left hind paw. The difference in the weight of paws was the index of the edema. After 30 min of histamine injection, the animals were sacrificed and both the hind paws were cut immediately at the tarsal joint. Right and left paws were weighed separately and differences in the weight of paws were recorded. Percentage inhibition was calculated with reference to control group animals. ED50 calculations were performed by plotting the graph of dose versus percentage inhibition.
Inhibitory effect of T. cordifolia on anti-DNP IgE induced cutaneous reaction in rats
Male Sprague-Dawley rats (200 ± 25 g) were anesthetized and the rats were injected intradermally with 0.5 μg (50 μL) of anti-DNP IgE into a dorsal skin site that had been shaved earlier. After 48 h, each rat received an injection of 100 μg of DNP-HSA in PBS containing 4% Evans blue (1:4) via the tail vein. The animals were sacrificed after 30 min after the anti-DNP IgE injection and reaction sites were removed. Evans blue was extracted from tissues by immersing the tissues in an extracting solution of 3 mL 0.5% sodium sulfate and 7 mL acetone kept at room temperature and vigorously shaken. Twenty-four hours later, the solutions were centrifuged at 400 g for 10 min and the color intensity of the supernatant was evaluated by spectrophotometer (Model UV-1601, Shimadzu) at 620 nm. Standard curves were performed to transform absorbance units into μg of Evans blue/mL of solution. Test drugs were given orally 1 h before anti-DNP IgE injection. The inhibitory effects of test drugs were expressed as the percentage reduction in dye leakage compared with that determined in vehicle treated animals. ED50 calculations were performed by plotting the graph of dose versus percentage inhibition (CitationShin et al., 2005).
Inhibitory effect of T. cordifolia on compound 48/80 induced ear swelling response in mice
The study of compound 48/80 induced ear swelling response was carried out as previously described (CitationJin et al., 2002). Briefly, male CD-1 mice (25 ± 5 g) were treated with T. cordifolia at different doses as described earlier. After 1 h of dosing, animals were injected intradermally with 25 μL of compound 48/80 (5 mg/mL) into the dorsal aspect of a mouse ear using a microsyringe with a 28-gauge hypodermic needle. Within a 40 min interval, ear swelling response evoked by physiologic saline returned nearly to baseline thickness. Therefore, ear swelling response was determined 40 min after the injection of compound 48/80. After 40 min of compound 48/80 injection the animals were sacrificed and both the ears were cut immediately. Right and left ears were weighed separately and differences in the weight of ears were recorded. Percentage inhibition was calculated with reference to control group animals. ED50 calculations were performed by plotting the graph of dose versus percentage inhibition.
Histamine induced experimental bronchial asthma
Either sex of Dunkin Hartley guinea pigs (350 ± 25 g) were kept on fasting for 12 h before the experiments, only drinking water was available ad libitum. The animals were treated orally with the vehicle/test samples/reference 2 h before bronchial challenge in an inhalator chamber (Dolphin Instruments, Mumbai, India). The appearance of the first evidence of symptoms of asphyxia (the bronchospastic reaction) was monitored and the time for onset of convulsions (preconvulsion) was recorded. In the control groups, an aerosol of histamine (0.5% histamine diphosphate in 0.9% NaCl solution) provoked a bronchospastic reaction in 100% of the animals within three minutes. The delay in the appearance of the bronchospastic reaction was considered as a protective effect. The mean preconvulsion time of animals treated with the test compounds, was compared to the control and is expressed in terms of percentage protection (CitationCzarnecki et al., 2001).
Percentage protection = [1-(T1/T2)] × 100
where T1 is the preconvulsive time of control and T2 is the preconvulsive time of test sample.
In vitro studies
Inhibition of histamine induced contraction of guinea pig ileum in vitro for evaluation of the H1 antihistamine activity of T. cordifolia
Dunkin Hartley guinea-pigs of either sex weighing 350 ± 50 g were fasted overnight before the experiment. The abdomen was cut open after sacrificing the animal and a good length of the ileum was placed on a Petri dish containing Tyrode solution at 37°C. A 2.5 cm long piece of the distal part of the ileum was taken for the study. Experiments were performed in 24 mL organ bath containing Tyrode solution at 37°C and bubbled with a mixture of 95% O2 and 5% CO2. The contractions of the ileum strips to histamine were recorded using a two-channel Gemini recorder (UGO-BASILE, Comerio VA, Italy) and isotonic force displacement transducer and 1 g tension. The preparation was allowed to equilibrate for 30 min during which the Tyrode solution was changed at intervals of 10 min. The non-cumulative stimuli with submaximal dose histamine (5.5 × 10−7 M) were given. The contraction in the absence and presence of (10 min incubation) of the test compounds was recorded. The responses of the T. cordifolia (2.5-160 μg/mL) or cetirizine (0.061-1.22 μg/mL) were recorded. The percentage inhibition of the test drugs on contractions induced by histamine was calculated (CitationHarish et al., 2001). EC50 calculations were performed by plotting the graph of log dose versus percentage inhibition. In another set of experiments, dose-response curves for histamine were obtained in the absence and presence of T. cordifolia at various concentrations (2.5 to 20 mg/mL). Data were analyzed using the method of Schild slope to find out the pA2 value.
Assay of histamine release from rat peritoneal mast cells (RPMC)
RPMC were isolated as previously described (CitationJippo-Kanemoto et al., 1993). In brief, rats were anesthetized by ether and injected with 20 mL of Tyrode buffer B (137 mM NaCl, 5.6 mM glucose, 12 mM NaHCO3, 2.7 mM KCl, 0.3 mM NaH2PO4 and 0.1% gelatin), into the peritoneal cavity and the abdomen was gently massaged for about 90 sec. The peritoneal cavity was carefully opened and the fluid containing peritoneal cells was aspirated by a Pasteur pipette. Thereafter, the peritoneal cells were sedimented at 150 g for 10 min at room temperature and resuspended in Tyrode buffer B. Mast cells were separated from the major components of rat peritoneal cells, i.e., macrophages and small lymphocytes, according to the method previously described (CitationJippo-Kanemoto et al., 1993). In brief, peritoneal cells suspended in 1 mL of Tyrode buffer B were layered on 2 mL of metrizamide (22.5% w/v) and centrifuged at room temperature for 15 min at 400 g. The cells remaining at the buffer-metrizamide interface were aspirated and discarded; the cells in the pellet were washed and resuspended in 1 mL Tyrode buffer A. Purified RPMC were resuspended in Tyrode buffer A for the treatment of compound 48/80. RPMC suspensions (2 × 105 cells/mL) were preincubated for 10 min at 37°C before the addition of compound 48/80 (5 mg/mL). The cells were preincubated without or with the T. cordifolia (0.01 to 10 mg/mL) or sodium chromoglycate (0.0625 to 0.5 mg/mL) preparations and then incubated (10 min, 37°C) with the compound 48/80. The cells were separated from the released histamine by centrifugation at 400 g for 5 min at 4°C. Residual histamine in cells was released by disrupting the cells with perchloric acid and centrifugation at 400 g for 5 min at 4°C. After centrifugation, histamine content in the supernatant was determined by a fluorometric assay as % emission (CitationAnton & Sayre, 1969). Briefly, samples were incubated in the presence of NaOH (1 M), and OPT (5 mg/mL) for exactly 4 min. The reaction was quenched by the addition of 200 μL of citric acid (2 M), and the fluorescence measured on a Shimadzu RF-540 Spectrofluorophotometer, with λexcitation = 345 nm and λemission = 441 nm. Standard curves were performed to transform percentage emission units into μg histamine per mL of solution. The percentage inhibition of histamine release was calculated using the following equation:
% Inhibition = (a – b) × 100/a
where “a” is histamine release without test drug and “b” is histamine release with test drug.
Assay of TNF-α production from rat peritoneal mast cells (RPMC)
TNF-α production was measured with the quantitative enzyme immunoassay technique, using a commercial kit (Endogen, Pierce Biotechnology, Inc., Rockford, IL, USA). RPMC (3 × 105 cells/mL) were sensitized with anti-DNP IgE (1 mg/mL) and incubated for 18 h in the absence or presence of T. cordifolia (0.01 to 10 mg/mL) or cetirizine (0.0625 to 0.5 mg/mL) preparations before the DNP-HAS (0.1 mg/mL) challenge. TNF-α production was measured by ELISA. The ELISA was performed in an anti-mouse TNF-α precoated 96-well strip plate. Fifty μL standard, controls, and samples were pipetted into the wells in duplicate. An aliquot of 50 μL biotinylated antibody reagent was added to each well. The plate was covered and incubated at room temperature for 2 h. After incubation the plate was washed five times with wash buffer. An aliquot of 100 μL dilute Streptavidin-HRP concentrate was added to each well. The plate was covered and incubated at room temperature for 30 min. After incubation the plate was washed five times. Thereafter, 100 μL premixed TMB substrate solution was added to each well. The plate was developed in the dark at room temperature for 30 min and the reaction was stopped by adding 100 μL stop solution to each well. Absorbance was measured on a plate reader at 450 minus 550 nm. The intensity of the color measured was in proportion to the amount of TNF-α bound in the initial step. The sample values were then read off the standard curves.
Measurement of cAMP level
The cAMP level was measured as previously described (CitationPeachell et al., 1988) In brief, purified mast cells were resuspended in prewarmed (37°C) Tyrode buffer A. Typically, an aliquot of cells (2 × 105 cells) was added to an equivalent volume (50 μL) of prewarmed buffer containing the T. cordifolia (1 mg/mL) in a micro-centrifuge tube. The reaction was allowed to proceed for discrete time intervals, terminated by the addition of ice-cold acidified ethanol (0.9 mL of 86% ethanol/1M HCl, 99:1) with brief vigorous vortexing and was then snap frozen in liquid nitrogen. The sample was later thawed and vortexed, then the debris was sedimented in a centrifuge (400 g at 4°C, for 5 min), and an aliquot (0.9 mL) of the supernatant was removed and evaporated to dryness under reduced pressure. The dried sample was reconstituted in assay buffer (150 mL) and stored frozen. The cAMP level was determined by enzyme immunoassay kit (Cayman).
Intracellular Ca2+ measurement
The RPMC (2 × 105 cells/mL) were preincubated with Fura-2/AM (2 μM) for 30 min at 37°C. After washing the dye from the cell surface, T. cordifolia was pretreated 10 min prior to the compound 48/80 treatment. Cells were incubated for another 10 min at 37°C with compound 48/80 (5 mg/mL). Fluorescence was measured from the supernatant on a Shimadzu RF-540 spectrofluorophotometer, with λexcitation = 340 nm and λemission = 500 nm.
Measurement of intracellular reactive oxygen species
The measurement of intracellular reactive oxygen species was carried out as previously described (CitationLebel & Bondy, 1990). Briefly, purified RPMC suspensions (2 × 105 cells/mL) were preincubated for 10 min at 37°C before the addition of compound 48/80 (5 mg/mL). The cells were preincubated without or with the T. cordifolia (0.01 to 10 mg/mL) preparations and then incubated (10 min, 37°C) with the compound 48/80. After the challenge of cells with compound 48/80, the cells were incubated with dichlorofluoroscein diacetate (DCF-DA; 1.25 μM final concentration) in 0.1% methanol for 15 min at 37°C.The cells were separated from the released ROS by centrifugation at 400 × g for 5 min at 4°C. Basal ROS generation was measured from cells incubated in vehicle alone. Fluorescence was measured from supernatant on a Shimadzu RF-540 spectrofluorophotometer, with λexcitation = 488 nm and λemission = 525 nm and intracellular ROS generation was expressed as the percentage ROS generated above the basal.
Statistical analysis
All the data are expressed as mean ± SEM. Statistical significance between more than two groups was tested using one-way ANOVA followed by the Bonferroni multiple comparisons test or unpaired two-tailed Student’s t-test as appropriate using a computer-based fitting program (Prism, GraphPad). Differences were considered to be statistically significant when p < 0.05.
Results
Effect of T. cordifolia treatment on compound 48/80 induced lethality in rats
Compound 48/80 induced 100% mortality in an orally administered vehicle-treated control group of animals, indicating degranulation of mast cells by compound 48/80. There was a dose dependent protection in compound 48/80-induced lethality in TC (125-1000 mg/kg)-treated groups as compared with the CON group which indicates the protective effect of T. cordifolia against mast cell degranulation. The maximal protective effect shown by TC 1000 was 80%. ED50 of T. cordifolia was found to be 517.81 mg/kg, while cetirizine, a known H1 antihistaminic, showed 70% protection in the dose of 0.3 mg/kg ().
Table 1. The effect of TC (125- 1000 mg/kg, p.o.) treatment on compound 48/80 induced lethality in rats.
Effect of T. cordifolia treatment on histamine induced paw edema in mice
T. cordifolia treatment showed significant (p <0.001) reduction in the paw volume which was increased after histamine injection as compared with the CON group. ED50 of T. cordifolia was found to be 672.32 mg/kg while cetirizine-treated mice showed 73.58% inhibition in histamine induced edema at the dose of 0.25 mg/kg ().
Table 2. The effect of TC (125- 1000 mg/kg, p.o.) treatment on histamine induced paw edema in mice.
Effect of T. cordifolia on anti-DNP IgE induced cutaneous reaction in rats
In this experiment, the effects of T. cordifolia on anti-DNP IgE induced cutaneous reactions in rats were evaluated by extravasation of intravenously administered Evans blue. In the control group, mean Evans blue extravasation was 3.11 ± 0.44 μg/mL, which was found to be maximal among all groups. This reaction was inhibited dose-dependently by oral administration of T. cordifolia (ED50 = 520.07 mg/kg) as compared with CON group. The maximal inhibitory effect shown by T. cordifolia was 67.61%. Cetirizine showed a 76.82% inhibition on anti-DNP IgE induced cutaneous reactions at the dose of 0.3 mg/kg ().
Table 3. The effect of TC (125- 1000 mg/kg, p.o.) treatment on anti-DNP IgE induced cutaneous reaction (vascular permeability of Evans blue) in rats.
Effect of T. cordifolia on compound 48/80 induced ear swelling response
The pretreatment of TC 125 to 1000 mg/kg showed dose-dependent inhibition in ear swelling response. The inhibition was significant (p < 0.001) at the doses of 500 and 1000 mg/kg as compared to CON group. ED50 of T. cordifolia was found to be 423.93 mg/kg. Cetirizine also significantly (p < 0.001) inhibited compound 48/80 induced ear swelling response at the dose level of 0.25 mg/kg ().
Table 4. The effect of TC (125-1000 mg/kg, p.o.) treatment on compound 48/80 induced ear swelling response in mice.
Effect of T. cordifolia on histamine induced experimental bronchial asthma
The effect of T. cordifolia on guinea pigs exposed to histamine spray is shown in . The extract produced a dose-dependent prolongation of pre-convulsive breathing and showed significant (p < 0.05) 69.97% inhibition in allergic bronchoconstriction at 1000 mg/kg. ED50 of T. cordifolia was found to be 374.33 mg/kg. Cetirizine was found to exhibit 68.91% inhibition at 0.3 mg/kg.
Table 5. The effect of TC (125-1000 mg/kg, p.o.) or cetirizine (0.3 mg/kg) alone on allergic bronchoconstriction in guinea pigs.
Inhibition of histamine induced contraction of guinea-pig ileum in vitro
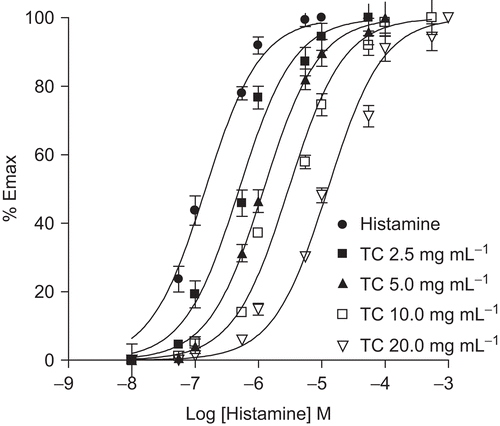
Histamine (5.5 × 10−7 M) produced sub-maximal contractile responses on guinea-pig ileum. Addition of T. cordifolia (2.5-160 μg/mL) resulted in concentration-dependent significant (p < 0.001) inhibition in the contractions as compared to control (sub-maximal response of histamine) value. T. cordifolia in these concentration ranges did not show any effect on the ileum when given alone. Cetirizine (1- 625 nM) significantly (p < 0.001) inhibited histamine induced contraction of guinea pig ileum. EC50 was found from the plot of log dose versus percentage inhibition of histamine response. EC50 of T. cordifolia and cetirizine was found to be 13.48 and 0.04 μg/mL, respectively (). Dose-response curves for histamine induced contraction of guinea-pig ileum are shown in . Schild plot analysis showed a pA2 value of 7.5.
Table 6. The effect of TC (2.5-160 μg/mL) treatment on histamine induced contraction of guinea pig ileum in vitro.
Figure 1. Effect of TC on dose-response curves for histamine- induced contraction of guinea pig ileum. Dose-response curves for histamine-induced contraction of guinea pig ileum were obtained in the absence of (•) or presence of 2.5 (▪), 5 (▴), 10 (□) and 20 (▾) mg/mL TC. Each point is the mean percentage of response (n = 4-5) at a given concentration of histamine with respect to the maximum contraction obtained in the control curve (•). pA2 = 7.5.
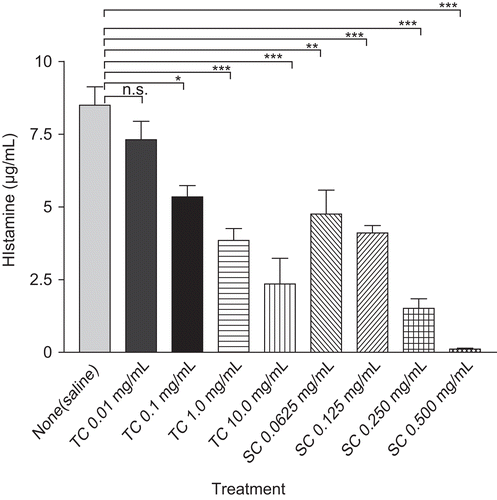
Effect of T. cordifolia on histamine release from RPMC
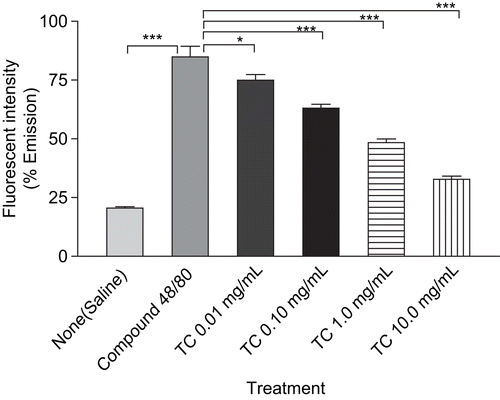
The inhibitory effects of T. cordifolia on compound 48/80 induced histamine release from RPMC are shown in . T. cordifolia (0.01- 10 mg/mL) and sodium chromoglycate (0.0625-0.5 mg/mL) showed concentration-dependent inhibition in compound 48/80 induced mediated histamine release from RPMC as compared to saline value. At the highest concentration, T. cordifolia and sodium chromoglycate showed 72.33% and 98.73% inhibition, respectively, in histamine assay.
Figure 2. The effect of TC (0.01-10 mg/mL) and sodium chromoglycate (SC) treatment on compound 48/80-induced histamine release from mast cells. RPMC (2 × 105 cells/mL) were preincubated with test samples at 37°C for 10 min prior to incubation with compound 48/80. Each data represents the mean ± SEM of 3 independent experiments. ***p < 0.001; **p < 0.01; *p < 0.05; n.s., not significant compared to saline value.
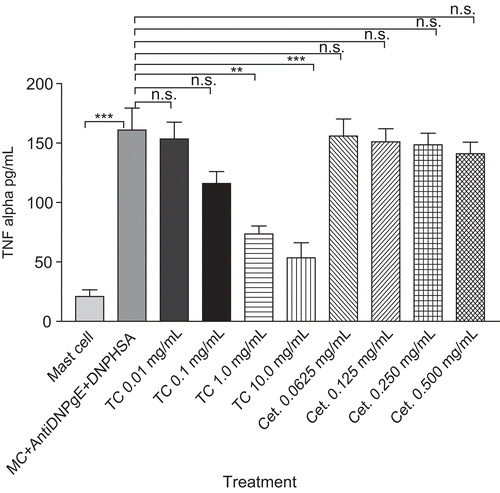
Effect of T. cordifolia on TNF-α secretion from RPMC
T. cordifolia showed significant 54.35% (p < 0.01) and 66.77% (p <0.001) inhibition at a concentration level of 1 and 10 mg/mL respectively, in anti-DNP IgE induced TNF-α production in RPMC as compared with mast cell + anti-DNP IgE + DNP-HSA group. The cetirizine (0.0625-0.5 mg/mL) treatment had no significant activity on TNF-α release from RPMC as compared with mast cell + anti-DNP IgE + DNP-HSA group ().
Figure 3. The effect of TC (0.01-10 mg/mL) and cetirizine (Cet.) treatment on anti-DNP IgE antibody-induced TNF-α secretion from mast cells (MC). RPMC (3 × 105 cells/mL) were sensitized with anti-DNP IgE (1 mg/mL) and incubated for 18 h in the absence or presence of test samples before the challenge with DNP-HSA (0.1 mg/mL). Each data represents the mean ± SEM of 3 independent experiments. ***p < 0.001; **p < 0.01; *p < 0.05; n.s., not significant compared to mast cell+ anti-DNP IgE+DNP-HSA group.
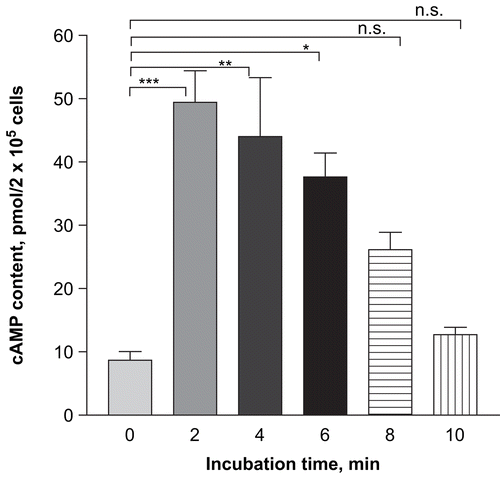
Effect of T. cordifolia on cAMP level of RPMC
To understand the mechanism of inhibition of histamine secretion from mast cells, we studied the effect of T. cordifolia incubation on the cAMP content. Incubation of T. cordifolia (1 mg/mL) with RPMC significantly (p < 0.001) increased the cAMP content at 2 min and decreased the cAMP content to basal value at 10 min ().
Figure 4. Time-dependent effect of TC (1 mg/mL) on cAMP level of RPMC. RPMC (2 × 105 cells/mL) were preincubated with test samples at 37°C for 10 min prior to incubation with compound 48/80 (5 mg/mL). Each datum represents the mean ± SEM of three independent experiments. ***p < 0.001; **p < 0.01; *p < 0.05; n.s., not significant compared to saline value.
Effect of T. cordifolia on intracellular Ca2+
To investigate the mechanisms of T. cordifolia on the reduction of histamine release, we analyzed the intracellular Ca2+. shows the increased fluorescent intensity when the RPMC are treated with compound 48/80 (5 mg/mL). Preincubation of T. cordifolia (10 mg/mL) with RPMC, decreased compound 48/80 induced intracellular Ca2+ levels ().
Figure 5. Effect of TC on the intracellular Ca2+ levels in RPMC. RPMC (2 × 105 cells/mL) were preincubated with test samples at 37°C for 10 min before adding compound 48/80 (5 mg/mL) and then another 10 min with compound 48/80. Each data represents the mean ± SEM of 3 independent experiments. ***p < 0.001; **p < 0.01; *p < 0.05; n.s., not significant for the compound 48/80 value.
Effect of T. cordifolia on generation of intracellular ROS
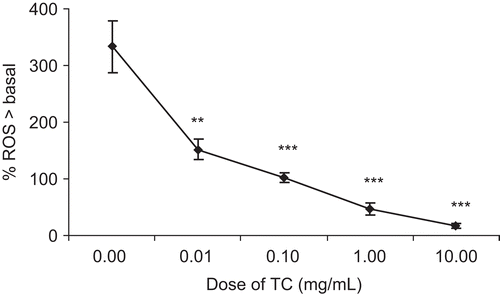
Unstimulated RPMC were found to generate a low level of basal ROS, and this was used as a baseline to which all other results were compared. Compound 48/80 caused maximal of 333.23% ± 44.96% generation of ROS over basal (n = 6) at 5 mg/mL. T. cordifolia (0.01-10 mg/mL) showed significant (p < 0.001) inhibition of ROS generation over basal level ().
Figure 6. Effect of TC on concentration-response relationships for intracellular reactive oxygen species. Concentration-response relationships for intracellular reactive oxygen species generation in the absence and presence of TC (0.01- 10 mg/mL) from rat peritoneal mast cells (2 × 105 cells/mL) in response to compound 48/80. Each point is the mean ± SEM of six experiments. ***p < 0.001; **p < 0.01; *p < 0.05; n.s., not significant compared to saline value.
Discussion
Mast cell mediated diseases can be treated by immunomodulation, competitive inhibition of released mediators or prevention of release of mediators (mast cell stabilization) (CitationDubuske, 2007; Hisbergues et al., 2007). Compound 48/80 has been used as direct secretagogues and the compound 48/80 induced increase in membrane permeability is an essential trigger for the release of the mediator from mast cells (CitationShin & Kim, 2000). From the present study in animal models, T. cordifolia showed an inhibitory effect on compound 48/80 induced lethality (systemic anaphylaxis) and compound 48/80 induced ear swelling response while T. cordifolia stabilized the lipid bilayer membrane in the in vitro assay of histamine release from rat peritoneal mast cells. Histamine is known to cause edema and has also been a well-known inflammatory mediator for a long time (CitationGrega et al., 1972; CitationRowley & Benditt, 1956). In the early phase of allergic response, histamine initiates edema formation (CitationWhite, 1990). Supporting these observations, treatment with T. cordifolia displayed an inhibitory effect on edema induced by histamine. These results suggest that the T. cordifolia can be much more effective in inhibiting reactions whose mechanism depends on the release of histamine. In addition, anti-DNP IgE induced local allergic cutaneous reaction is a well-known animal model for anaphylaxis in rats. Our results suggest the inhibitory effect of T. cordifolia in anaphylaxis. The role of histamine in asthma disease is well established (CitationNelson, 2003). The present study indicates that administration of T. cordifolia can reduce the histamine induced bronchospasm. Since bronchospasm occurs after H1-receptor activation of the PLC–IP3–DAG cascade and intracellular calcium mobilization (CitationSmit et al., 1999), many possibilities of antagonistic intervention for the plant extracts may apply.
T. cordifolia also attenuated histamine induced contraction of guinea-pig ileum and TNF-α secretion from RPMC in vitro. Reduction of pro-inflammatory cytokines from mast cells is one of the key indicators of reduced allergic symptom (CitationThomas, 2001). Thus, from the in vitro studies it can be proved that T. cordifolia can competitively inhibit the released mediators as well as it can prevent the release of mediators. The role of cAMP and calcium on histamine release is controversial but the cAMP and Ca2+ pathways to the degranulation of mast cells are critical. The agents that induce elevations in intracellular cAMP attenuate the stimulated release of mediators from mast cells (CitationNorn et al., 2007). The study results showed that the incubation of T. cordifolia increased the intracellular cAMP content of the mast cells from that of basal cell cAMP level. The present study observations showed an attenuation of compound 48/80 induced intracellular Ca2+ in mast cells with T. cordifolia treatment and proved that the decreased intracellular Ca2+ is involved in the inhibitory effect of T. cordifolia on histamine release. Further, cAMP inhibits the secretion of mediators by reducing the membrane permeability to Ca2+ (CitationAlfonso et al., 2000). It means that inhibitory action of T. cordifolia is by elevation of cAMP as well as by decrease in intracellular Ca2+. Mast cells are known to generate intracellular ROS in response to antigen challenge, which may be involved in histamine release. It could also be suggested that the anti-allergic action of T. cordifolia is partly due to antioxidant potential of T. cordifolia as anti-oxidants can reduce ROS levels in antigen-IgE activated mast cells and concomitantly inhibited histamine release (CitationChen et al., 2000; CitationMatsui et al., 2000).
It is also confirmed from our study that cetirizine a well known antiallergic drug, possesses only H1-antihistamine activity and it has no effect on mast cell stabilization, while T. cordifolia showed H1-antihistamine activity as well as mast cell stabilization.
Conclusion
It can be concluded that T. cordifolia has mast cell stabilizing effect as well as H1 antihistamine effect on allergic disorders. From all these observations of the study involving different models of allergic reactions with distinct mechanism of action, it can be said that effects of herbals drugs alone on allergic disorders is encouraging. Herbal drug treatment provides a unique opportunity to develop a therapy to reduce the side effects of currently marketed antiallergenics and can achieve better management of patients suffering from allergic disorders.
Acknowledgements
The authors would like to thank Sun Pharma Advanced Research Centre and Alembic Research Centre, Vadodara for help in the ELISA studies.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alfonso A, Cabado A, Vieytes M, Botana L (2000): Functional compartments in rat mast cells for cAMP and calcium on histamine release. Cell Signal 12: 343–350.
- Anton AH, Sayre DF (1969): A modified fluorometric procedure for tissue histamine and its distribution in various animals. J Pharmacol Exp Ther 166: 285–292.
- Beaven MA, Metzger H (1993): Signal transduction by Fc receptors: The Fc epsilon RI case. Immunol Today 14: 222–226.
- Bryce P, Oettgen H (2005): Antigen-independent effects of immunoglobulin E. Curr Allergy Asthma Rep 5: 186–190.
- Burd PR, Rogers HW, Gordon JR, Martin CA, Jayaraman S, Wilson SD, Dvorak AM, Galli SJ, Dorf ME (1989): Interleukin 3-dependent and independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med 170: 245–257.
- Chen S, Gong J, Liu F, Mohammed U (2000): Naturally occurring polyphenolic antioxidants modulate IgE-mediated mast cell activation. Immunology 100: 471–480.
- Church MK, Levi-Schaffer F (1997): The human mast cell. J Allergy Clin Immunol 99: 155–160.
- Czarnecki R, Librowski T, Pawlowski M (2001): Antianaphylactic and antiasthmatic properties of new piperazinyl 7-(beta-hydroxypropyl)-theophylline derivatives in guinea pigs. Pol J Pharmacol 53: 131–136.
- Dubuske L (2007): Levocetirizine: The latest treatment option for allergic rhinitis and chronic idiopathic urticaria. Allergy Asthma Proc 28: 724–734.
- Gordon JR, Galli SJ (1990): Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature 346: 274–276.
- Grega GJ, Kline RL, Dobbins DE, Haddy FJ (1972): Mechanisms of edema formation by histamine administered locally into canine forelimbs. Am J Physiol 223: 1166–1171.
- Harish MS, Nagu RM, Badami S (2001): Antihistaminic and mast cell stabilizing activity of Striga orobanchioides. J Ethnopharmacol 76: 197–200.
- Hisbergues M, Magi M, Rigaux P, Steuve J, Garcia L, Goudercourt D, Pot B, Pestel J, Jacquet A (2007): In vivo and in vitro immunomodulation of Der p 1 allergen-specific response by Lactobacillus plantarum bacteria. Clin Exp Allergy 37: 1286–1295.
- Jin MY, Seung HH, Hyo JL, Jin HW, Joong MK, Dong MJ, Seung HB, Jong PL, Hyung MK (2002): Ixeris dentata green sap inhibits both compound 48/80-induced anaphylaxis-like response and IgE-mediated anaphylactic response in murine model. Biol Pharm Bull 25: 5–9.
- Jippo-Kanemoto TJ, Kasugai T, Yamatodani A, Ushio H, Mochizuki T, Tohya K, Kimura M, Nishimura M, Kitamura Y (1993): Supernormal histamine release and normal cytotoxic activity of beige (Chédiak-Higashi syndrome) rat mast cells with giant granules. Int Arch Allergy Immunol 100: 99–106.
- Kim HM, Lee YM (1999): Role of TGF-beta 1 on the IgE-dependent anaphylaxis reaction. J Immunol 162: 4960–4965.
- Kreutner W, Hyo JL, Anthes J, Barnett A, Young S, Tozzi S (2000): Preclinical pharmacology of desloratadine, a selective and nonsedating histamine H1 receptor antagonist. 1st communication: Receptor selectivity, antihistaminic activity, and antiallergenic effects. Arzneimittelforschung 50: 345–352.
- Kuehn H, Gilfillan A (2007): G protein-coupled receptors and the modification of Fc epsilon RI-mediated mast cell activation. Immunol Lett 113: 59–69.
- Lebel C, Bondy S (1990): Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem Int 17: 435–440.
- Leonardi A (2000): Role of histamine in allergic conjunctivitis. Acta Ophthalmol Scand 230: 18–21.
- Matsui T, Suzuki Y, Yamashita K, Yoshimaru T, Suzuki-Karasaki M, Hayakawa S, Yamaki M, Shimizu K (2000): Diphenyleneiodonium prevents reactive oxygen species generation, tyrosine phosphorylation and histamine release in RBL-2H3 mast cells. Biochem Biophys Res Commun 276: 742–748.
- Nair PK, Melnick SJ, Ramachandran R, Escalon E, Ramachandran C (2006): Mechanism of macrophage activation by (1,4)-alpha-D-glucan isolated from Tinospora cordifolia. Int Immunopharmacol 6: 1815–1824.
- Nelson HS (2003): Prospects for antihistamines in the treatment of asthma. J Allergy Clin Immunol 112: S96–S100.
- Nishikawa H, Kitani S (2008): Tea catechins have dual effect on mast cell degranulation induced by compound 48/80. Int Immunopharmacol 8: 1207–1215.
- Norn S, Geisler A, Stahl P, Klysner R (2007): Cyclic AMP and allergic histamine release. Influence of methylxanthines on rat mast cells. Allergy 32: 183–191.
- Palomaki V, Laitinen J (2006): The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase d-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br J Pharmacol 147: 596–606.
- Pandey SC, Awouters F, Nueten V, Nollin S, Janssen P (1978): Protection of rats from compound 48/80-induced lethality: a simple test for inhibitors of mast cell mediated shock. Arch Int Pharmacodyn 234: 164–176.
- Peachell PT, MacGlasham DW, Lichtenstein LM, Schleimer RP (1988): Regulation of human basophil and lung mast cell function by cyclic adenosine monophosphate. J Immunol 140: 571–579.
- Pendse VK, Mahavar MM, Khanna KC, Somani SK (1981): Anti-inflammatory and related activity of water extract of Tinospora cordifolia, “Neem- Giloe”. Indian Drugs 19: 14–21.
- Prince PS, Kamalakkannan N, Menon VP (2004): Restoration of antioxidants by ethanolic Tinospora cordifolia in alloxan-induced diabetic Wistar rats. Acta Pol Pharm 61: 283–287.
- Robin L, Erwin W, Paul J (2008): The role of histamine H1 and H4 receptors in allergic inflammation: The search for new antihistamines. Nat Rev Drug Discov 7: 41–53.
- Rowley DA, Benditt EP (1956): 5-Hydroxytryptamine and histamine as mediators of the vascular injury produced by agents which damage mast cells in rats. J Exp Med 103: 399–412.
- Shin TY, Kim DK (2000): Inhibitory effect of mast cell-dependent anaphylaxis by Gleditsia sinensis. Arch Pharm Res 23: 401–406.
- Shin TY, Kim SH, Suk K, Ha JH, Kim I, Lee MG, Jun CD, Kim SY, Lim JP, Eun JS, Shin HY, Kim HM (2005): Anti-allergic effects of Lycopus lucidus on mast cell-mediated allergy model. Toxicol Appl Pharmacol 209: 255–262.
- Singh SS, Pandey SC, Srivastava S, Gupta VS, Patro B, Ghosh AC (2003): Chemistry and medicinal properties of Tinospora cordifolia (guduchi). Indian J Pharm 35: 83–91.
- Smit MJ, Hoffmann H, Timmerman H, Leurs R (1999): Molecular properties and signalling pathways of the histamine H1 receptor. Clin Exp Allergy 29: 19–28.
- Thomas PS (2001): Tumour necrosis factor-alpha: The role of this multifunctional cytokine in asthma. Immunol Cell Biol 79: 132–140.
- White MV (1990): The role of histamine in allergic diseases. J Allergy Clin Immunol 84: 599–605.
