Abstract
Three new sesquiterpene coumarins, namely, farnesiferone B (1), flabellilobin A (2) and flabellilobin B (3), together with nine known compounds, ligupersin A, 7-epi-gamma-eudesmol, persicasulfide A, conferdione, umbelliprenin, conferone, feselol, lehmferin and farnesiferol B were isolated from the roots of Ferula flabelliloba Rech. f. & Aell. (Apiaceae). The structures of these compounds were elucidated by various 1- and 2-D NMR techniques as well as HREIMS.
Introduction
The genus of Ferula belongs to the tribe Peucedaneae, subfamily of Apioideae, family of Umbelliferae with 133 species distributed throughout the Mediterranean area and central Asia, especially in the former USSR and neighboring countries such as Iran (CitationEvans, 1989; CitationMozaffarian, 1983; CitationHeywood, 1985). More than 70 species of Ferula have already been investigated phytochemically (CitationDiab et al., 2001; Iranshahi et al., Citation2004a; CitationAbd El-Razek et al., 2003). Several species of this genus have been used in folk medicine (CitationChen et al., 2000). The Iranian flora comprises 30 species of Ferula, some of which are endemic (CitationMozaffarian, 1983, Citation1996). The popular Persian name of the most of these species is “koma” (CitationMozaffarian, 1996).
The chemistry of this genus has been studied by many investigators and is well documented as a good source of biologically active compounds such as sesquiterpene derivatives (CitationAhmed et al., 2001; CitationAhmed, 1999; CitationValle et al., 1987; Iranshahi et al., Citation2004b, Citation2007, Citation2008; CitationShahverdi et al., 2006). Sesquiterpene derivatives, especially sesquiterpene coumarins, were stored in the roots of the plants; therefore the roots are a better source for isolating sesquiterpene coumarins than the aerial parts. Ferula flabelliloba Rech. F. et Aell. is a plant endemic to Iran (CitationHedge et al., 1982) and no phytochemical studies of this species have been reported to date. In the present study we report the isolation and the structure elucidation of three new sesquiterpene coumarins, together with nine known compounds from the roots of Ferula flabelliloba.
Materials and methods
Plant material
The roots of F. flabelliloba were collected from the Hezarmasjed mountains, Khorasan Razavi province, Iran, in April 2006. The plant material was identified by Mohammad Reza Joharchi, Ferdowsi University of Mashhad Herbarium (FUMH). A voucher specimen (No. 1004) has been deposited at the herbarium of School of Pharmacy, Mashhad University of Medical Sciences.
General experimental procedures
NMR spectra were measured on a Bruker DRX 500 (Bruker Biospin, Rheinstetten, Germany). 1H NMR, 13C NMR, DEPT, 1H-1H COSY, HMBC, HSQC, and NOESY spectra were measured using an inverse-detection probe (5 mm). The operating frequencies were 500.13 MHz for acquiring 1H NMR and 125.75 MHz for 13C NMR spectra. Samples were measured at 300 K in CDCl3 with TMS as the internal standard. Column chromatography was conducted with silica gel 230-400 mesh (Merck). Preparative Thin Layer Chromatography (TLC) was performed on GF254s plates (20 × 20 cm, Merck, Berlin) and observation of plates was carried out under UV CAMAG spectrometer (254 nm).
Electron impact (EIMS) and high resolution (HREIMS) mass spectra were obtained on a Micromass MasSpec (Micromass, Manchester) sector-field mass spectrometer in positive ion mode using 70 eV ionization energy. The spectrometer was coupled to a HP 6980 series gas chromatograph (Agilent, Berlin). A capillary ZB-5ms column (length 30 m, internal diameter 0.25 mm, film thickness 0.25 μm, Phenomenex, Berlin) was used. Helium was used as a carrier gas at a constant flow of 1.0 mL/min. The column oven temperature was programmed at 40°C for 2 min, before being elevated to 280°C at 15°C/min and then held at this temperature for another 5 min.
Extraction and isolation
Dried, powdered roots of F. persica (500 g) were extracted with CH2Cl2 using a Soxhlet apparatus. The extract was concentrated in vacuo to give a red extract (47.4 g) and a part of that (20 g) was subjected to column chromatography on silica gel (5 × 50 cm) using petroleum ether with increasing volumes of EtOAc (petrol (360 mL), petrol:EtOAc (20:1, 1.05 L), (15:1, 960 mL), (10:1, 990 mL), (9:1, 1.9 L), (8:1, 2.7 L), (7:1, 800 mL), (6:1, 3.71 L), (5:1, 1.8 L), (4:1, 2.5 L), (3:1, 6.27 L) and (2:1, 3.6 L), and EtOAc (2 L)). The fractions were compared by TLC, and those giving similar spots were combined. Nine fractions were finally obtained. Fractions 5, 7 and 8 afforded conferone (438 mg), feselol (772 mg) and conferdione (951 mg), respectively. Fraction 6 afforded lehmferin (448 mg) and farnesiferol B (224 mg). Fractions 1, 2, 3, 4 and 9 needed more purification with PTLC (silica gel using petrol:EtOAc, 3:1, 20 × 20 cm, glass plates, each plate was run four times). After further purification fraction 1 afforded 7-epi-gamma- eudesmol (268 mg) and persicasulfide A (419 mg). Fractions 2, 4 and 9 afforded umbelliprenin (235 mg), compound 1 (14.9 mg) and ligupersin A (258 mg), respectively. Fraction 3 afforded 18 mg of a mixture of compounds 2 (8.2 mg) and 3 (12.4 mg). The latter two compounds were isolated as an inseparable mixture and their 1H NMR and 13C NMR spectral data were very helpful to identify their slight structural differences and also to specify their ratio in the mixture (compound 2/compound 3 = 2/3).
Results and discussion
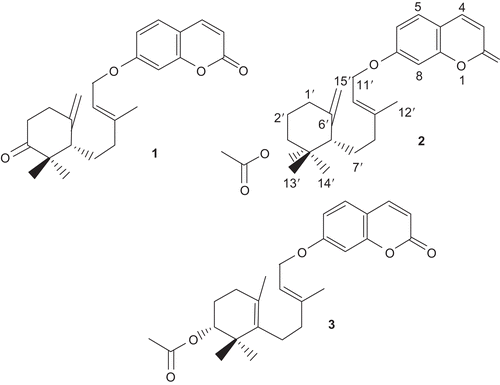
Normal-phase column chromatography of the dichloromethane extract of roots, followed by preparative TLC, afforded three new natural products (compounds 1-3, ), and nine known compounds, namely ligupersin A, 7-epi-gamma-eudesmol, persicasulfide A, conferdione, umbelliprenin, conferone, feselol, lehmferin and farnesiferol B. The structures of the mentioned known compounds were confirmed according to the literature (Iranshahi et al., Citation2003a, Citation2003b, Citation2007; CitationAbd El-Razek et al., 2003; CitationRaharivelomanana et al., 1998; CitationNabiev et al., 1982).
The molecular formula of compound 1, C24H28O4, was established by HREIMS (m/z 380.2003, calcd 380.1987). The structure of 1 was established from analysis of the 1H and 13C NMR spectra (). The 13C NMR of compound 1 resonances displayed 24 carbon signals, nine being typical of an umbelliferone skeleton and the other 15 signals were ascribable to a sesquiterpene moiety. The downfield signals at δC 161.2 and 215 were assigned to the carbonyls of the coumarin and the sesquiterpene respectively. HSQC spectrum classified the carbon signals to six aliphatic methylenes at δC 37.3 (C-8’), 30.6 (C-1’), 25.4 (C-7’), 37.6 (C-2’), 65.3 (C-11’; characteristic for an oxygenated methylene) and 113.4 (C-15’), to seven methines, five of them for umbelliferone moiety at δC 101.6 (C-8), 113 (C-3), 113.2 (C-6), 128.7 (C-5) and 143.4 (C-4), and to three methyls at δC 16.8 (C-12’), 21.3 (C-13’) and 27.1 (C-14’). The 1H NMR spectrum of 1 showed resonances characteristic for three methyl singlets at δH 1.02 (H-13’), 1.16 (H-14’) and 1.7 (H-12’), and five olefinic resonances at δH 4.8 (H-15’a), 5 (H-15’b), 5.4 (H-10’), 6.23 (H-3) and 7.61 (H-4). Three aromatic protons at δH 7.34 (H-5), 6.78 (H-8) and 6.82 (H-6) suggested the presence of a 7,9,10-trisubstituted benzene ring, which was supported by the 13C NMR spectrum. In the HMBC spectrum, the correlations of H-2’ (δH 2.28 and 2.6) with C-3’; H-5’ (δH 2.11) with C-4’ (δC 49); H-11’ (δH 4.56) with C-7 and C-9’ (δC 141.9); H-12’ with C-8’ (δC 37.3), C-9’ and C-10’; H-13’ with C-3’, C-5’ (δC 55.9), C-14’ (δC 27.1) and C-4’; H-14’ with C-3’, C-13’ and C-4’; and H-15’ with C-1’, C-5’, and C-6’ confirmed the structure of compound 1. The proposed structure was further supported by 1H-1H COSY data. The stereochemistry of the proton at C-5’ was determined as β on the basis of the NOESY experiment, in which cross-peaks were observed from H-5’/H-13’ pairs and H-7’/H-14’ pairs. Therefore, the structure of compound 1 was elucidated as shown in and was named farnesiferone B.
Table 1. 1H NMR (500 MHz) and 13C NMR (125 MHz) data for compounds 1-3 (CDCl3)a.
The isomers flabellilobin A (2) and flabellilobin B (3) were isolated as an inseparable mixture and showed the molecular formula, C26H32O5, by HREIMS (m/z 424.223, calcd. 424.2249). The NMR spectroscopic data of 2 were similar to those of 1, except for some differences in the signals assigned to the sesquiterpene unit which was due to the replacement of the ketone group at C-3’ (δC 78.4) with an acetoxy group [δH 2.03, H-17’; δC 21.3 (C-17’); 170.6 (C-16’)]. The signals at δC 28.6/δH 1.79; 1.55 and δC 39.2 were assigned to C/H-2’ and C-4’ according to their homo- and heteronuclear correlations. Other 1H and 13C NMR spectral data of 2 were closely comparable to those of 1 (). The location of the acetoxy group at C-3’ was confirmed by a HMBC correlation of H-3’ (δH 4.62) with C-16’ carbonyl.
The 1H and 13C NMR data of 3 were very similar to those of 2, except signals indicating a different position of the double bond in the sesquiterpene unit. Thus, the differences between the 1H and 13C NMR of 2 and 3 appeared in the signals due to C-5’, C-6’, C-15’ and their corresponding protons. In 3, the signal for the H-15’ appeared at δH 1.61 and those for the C-5’, C-6’ and C-15’ at δC 134.9, 127.0 and 19.6, respectively. Other 1H and 13C NMR spectral data of 3 were closely comparable to those of 2 (). The position of double bond in the sesquiterpene unit compound 3 was established from the HMBC spectrum: the tertiary methyl H-15’ showed correlations with C-6’ and C-5’, and H-13’ (δH 0.78) and H-14’ (δH 0.92) correlated with C-5’.
The stereochemistry at C-3’ and C-5’ in flabellilobin A (2) was determined as β on the basis of the NOESY experiment, in which a cross-peak was observed between H-3’ and H-14’. It seems that the stereochemistry of the proton at C-3’ in flabellilobin B (3) is identical to that of 2. However, it was impossible to distinguish whether the cross-peak between H-3’ and the geminal methyl groups at C-4’ is due to H-13’ (δH 0.99) or H-14’ (δH 1) because of overlapping resonances in the spectrum of compound 3.
Acknowledgements
This research was supported by a grant from Mashhad University of Medical Sciences Research Council. We thank Sybille Lorenz (Jena) for mass spectrometric measurements.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abd El-Razek MH, Ohta S, Hirata T (2003): Terpenoid coumarins of the genus Ferula. Heterocycles 60: 689–716.
- Ahmed AA (1999): Sesquiterpene coumarins and sesquiterpenes from Ferula sinaica. Phytochemistry 50: 109–112.
- Ahmed AA, Abd El-Razek MH, Nassar MI, Izuma S, Ohta S, Hirata T (2001): Sesquiterpene coumarins from the roots of Ferula assa-foetida. Phytochemistry 58: 1289–1295.
- Chen B, Teranishi R, Kawazoe K, Takaishi Y, Honda G, Itoh M, Takeda Y, Kodzhimatov OK (2000): Sesquiterpenoids from Ferula kuhistanica. Phytochemistry 54: 717–722.
- Diab Y, Dolmazon R, Bessiere JM (2001): Daucane aryl esters composition from the Lebanese Ferula hermonis Boiss. (zallooh root). Flav Fragr J 16: 120–122.
- Evans WC (1989): Trease and Evans’ Pharmacognosy, 13th edition. London, Bailliere Tindall, pp. 205–206.
- Hedge IC, Lamond JM, Rechinger KH (1982): Ferula, in: Flora Iranica. Umbelliferae, No. 162. Graz-Austria, Akademische Druck-u.Verlagsanstalt, p. 406.
- Heywood VH (1985): Flowering Plants of the World, London, Croom Helm, pp. 219–221.
- Iranshahi M, Amin GR, Amini M, Shafiee A (2003a): Sulfur containing derivatives from Ferula persica var. latisecta. Phytochemistry 63: 965–966.
- Iranshahi M, Amin GR, Jalalizadeh H, Shafiee A (2003b): New germacrane derivative from Ferula persica Willd. var. latisecta Chamberlain. Pharm Biol 41: 431–433
- Iranshahi M, Amin GR, Shafiee A (2004a): A new coumarin from Ferula persica. Pharm Biol 42: 440–442.
- Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, Bassarello C, Piacente S, Pizza C (2007): Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry 68: 554–561.
- Iranshahi M, Kalategi F, Rezaee R, Shahverdi AR, Ito C, Furukawa H, Tokuda H, Itoigawa M (2008): Cancer chemopreventive activity of terpenoid coumarins from Ferula species. Planta Med 74: 147–150.
- Iranshahi M, Shahverdi AR, Mirjani R, Amin GR and Shafiee A (2004b): Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z. Naturforschung 59c: 506–508.
- Mozaffarian V (1983): The Family of Umbelliferae in Iran: Keys and Distribution, Tehran, Research Institute of Forests and Rangelands Press, pp. 114–116.
- Mozaffarian V (1996): A Dictionary of Iranian Plant Names, Tehran, Farhang-e Moaser, pp. 228–230.
- Nabiev AA, Khasanov TKH, Malikov VM (1982): A new terpenoid coumarin from Ferula kopetdaghensis. Khim Prir Soedin 1: 48–51.
- Raharivelomanana P, Bianchini JP, Ramanoelina RP, Rasoarahona Faure R, Cambon A (1998): Eudesmane sesquiterpenes from Laggera alata. Phytochemistry 47: 1085–1088.
- Shahverdi AR, Saadat F, Khorramizadeh MR, Iranshahi M, Khoshayand MR (2006): Two matrix metalloproteinase from Ferula persica var. persica. Phytomedicine 13: 712–717.
- Valle GM, Appendino G, Nano GM, Picci V (1987): Prenylated coumarins and sesquiterpenoids from Ferula communis. Phytochemistry 26: 253–258.