Abstract
Proteins from Parkia speciosa Hassk. (Fabaceae) seeds were extracted and stepwise precipitated using ammonium sulfate. Proteins precipitated with 25% ammonium sulfate were separated by affinity chromatography on Affi-Gel Blue gel followed by protein liquid chromatography on Superdex 200. The protein Gj, which was identified as a protein similar to putative aristolochene synthase, 3′-partial from Oryza sativa L. (Poaceae), had hemagglutinating activity of 0.39 μg/μL. Moreover, fraction C2 from the proteins precipitated with 60% ammonium sulfate, separated by lectin-specific adsorption chromatography using Con A Sepharose, had hemagglutinating activity of 1.17 μg/μL. Using gel electrophoresis, two proteins C2a and C2b were separated, having molecular weights of 45 kDa and 23 kDa, respectively. From protein identification, C2a was found to be similar to the hypothetical protein B1342F01.11 from Oryza sativa, and C2b was similar to the hypothetical protein At1g51560 from Arabidopsis thaliana (L.) Heynh. (Brassicaceae).
Introduction
Parkia speciosa Hassk. (Fabaceae) has many common names, including sator, petai, pete, peuteuy, petai gede, segobang, and petai pare. Sator is found in many countries in Southeast Asia such as the northern area of Malaysia, the southern part of Thailand, Indonesia, and Brunei. Sator is a perennial plant that is 30 m in height; a large tree can reach 40–50 m tall. Leaves are twice pinnate, and each pinnate leaf, 15–30 cm long, has many pairs of small, sessile leaflets. Flowers, which are small and creamy white, are found in densely crowded heads. Pods are large, 40–55 cm long and 4–5 cm wide, and are straight or more commonly twisted, dangling in small bundles, green becoming black. Each pod contains 10–18 large seeds. The pods taste like garlic and have a very strong odor. The immature seeds, young leaves, and fresh parts of the flower stalks can be eaten raw. Half-ripe pods are pickled in salt. In addition, sator is a well known vegetable and an economic plant because it has high nutrient value as well as many medicinal properties (CitationAli et al., 2006; CitationJamaluddin et al., 1994; CitationSuvachittanont & Jaranchavanapet, 2000; CitationTaungbodhitham, 1995). For example, it has a mitogenic effect, is antidiabetic, has antithiamine factor activity, and can reduce blood pressure.
Parkia speciosa seed lectins (molecular weights of 46,700 Da and 47,300 Da) were determined by denaturizing gel electrophoresis (CitationSuvachittanont & Peutpaiboon, 1992). The lectins agglutinated rat erythrocytes but not those of humans. Sugars which specifically bind with the lectin were tested by hemagglutination inhibition with d-glucose, d-mannose, d-fructose, maltose, N-acetyl-d-glucosamine, and α-methyl-d-glucopyranoside. Five polysulfides have been isolated from Parkia speciosa seeds (CitationGmelin et al., 1981). The structures of four of these have been established as 1,2,4- trithiolane, 1,2,4,6-tetrathiepane, 1,2,3,5,6-pentathiepane (lenthionine), and 1,2,4,5,7,8-hexathionane. A further product has been tentatively assigned either the 1,2,4,6,7- or the 1,2,4,5,7-pentathiocane structure. Two compounds have antimicrobial and antifungal activities (1,2,4,5,7,8-hexathionane and 1,2,4-trithiolane). Moreover, thiazolidine- 4-carboxylic acid (thioproline) (CitationSuvachittanont et al., 1996) is present in Parkia speciosa seeds. Thioproline is an effective nitrite-trapping agent in the human body, thereby inhibiting the endogenous formation of carcinogenic N-nitroso compounds. Additionally, the uncooked Parkia speciosa seeds contain substantial amounts of formaldehyde and thiol compounds that decrease after boiling.
Affinity chromatography is a method that overcomes the above limitations by exploiting the unique interaction of one molecule with a second, complementary binding molecule (ligand) (CitationRozycki et al., 2000). It is one of the most useful and effective fractionations that can be applied, and often proteins can be purified in a single affinity chromatography step. Affi-Gel Blue is a gel matrix for affinity chromatography column which contains a reactive blue dye molecule (Cibacron Blue F3GA) linked to agarose beads, and binds many nucleotide- requiring enzymes, albumin, and other proteins (CitationBio-Rad Laboratories, 2000). Concanavalin A (Con A) coupled to Sepharose™ 4B via cyanogen bromide activation binds molecules that contain α-d-mannose, α-d-glucose, and sterically related residues with available C-3, C-4, or C-5 hydroxyl groups, such as glycoprotein and lectin (CitationAmersham Biosciences, 2002). Moreover, Con A is generally applicable as a group-specific adsorbent for molecules containing sugars. These two media are successfully used as the stationary phase for protein purification in an affinity chromatography step (CitationKonozy et al., 2003; CitationLi & Kushad, 2005; CitationOhtsubo & Richardson, 1992; CitationWong & Ng, 2005; CitationYe & Ng, 2001). In this study, Affi-Gel Blue gel and Con A were used as the media for affinity chromatography to purify specific proteins with hemagglutinating activity.
Materials and methods
Chemicals
Concanavalin A (Con A) and dextrose agar were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Methyl-α-d-mannopyranoside, ethylenediaminetetraacetic acid (EDTA), amd guanidine-HCl were purchased from Fluka (Germany). Affi-Gel Blue gel was obtained from Amersham Biosciences (USA). Most reagents and the staining kit used in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were obtained from Plusone Pharmacia Biotech (Uppsala, Sweden). The low molecular weight calibration kit used for standard molecular weight marker proteins was purchased from Amersham Pharmacia Biotech (UK). Brilliant Blue G and trichloroacetic acid were products of Sigma Chemical Co. (St. Louis, MO, USA) and Merck (Germany). Potassium hydrogen phosphate (KH2PO4), disodium hydrogen phosphate (Na2H2PO4), ammonium sulfate (NH4)2SO4, and sodium chloride (NaCl) were obtained from Merck.
Protein extraction and precipitation
Parkia speciosa seeds, 2 kg, were obtained from Chatujak Market, Bangkok, Thailand. A voucher specimen (BK59664) was deposited by Kongkanda Chayamarit at Bangkok Herbarium (BK) of the Plant Variety Protection Division, Department of Agriculture, Thailand. It was defatted with acetone at −20°C and extracted overnight at 4°C with 50 mM phosphate buffer, pH 7.5, containing 0.1 M NaCl and a chelating agent such as EDTA (0.1 mM) (CitationBains et al., 2005). After centrifugation (8500 × g, 15 min, 4°C), proteins in the supernatant were precipitated with 25, 40, 60, and 90% ammonium sulfate (CitationLi & Kushad, 2005; CitationOhtsubo & Richardson, 1992), respectively. The proteins were dissolved and dialyzed in distilled water and then were freeze-dried. Dried crude extract was dissolved in binding buffer (20 mM Tris-HCl, pH 7.4, containing 0.5 M NaCl, 1 mM CaCl2·2H2O, 1 mM MnCl2).
Protein isolation
For protein separation, three chromatography techniques were chosen: affinity chromatography, gel filtration chromatography, and high performance liquid chromatography (HPLC).
Affinity chromatography
Two types of stationary phase were used in this experiment, Con A and Affi-Gel Blue gel (CitationDass, 2001; CitationHolme & Peck, 1998).
Con A. Insoluble crude proteins were removed by centrifugation and the solution applied to an affinity column (0.8 × 5 cm) of concanavalin A (Amersham Biosciences) equilibrated in the same buffer. The bound proteins were eluted with 20 mM Tris-HCl, pH 7.4, containing 0.1–0.5 M methyl-α-d-mannopyranoside and regenerated with 0.5 M NaCl in 20 mM Tris-HCl buffer, pH 8.5, and 20 mM acetate buffer, pH 4.5 (CitationAmersham Biosciences, 2002; CitationKonozy et al., 2003).
Affi-Gel Blue gel. Crude protein solution was applied to an affinity column (0.8 × 5 cm) of Affi-Gel Blue gel (Bio-Rad) (0.8 × 10 cm).The protein bound to the matrix was eluted with 10 mM Tris-HCl, pH 7.2, containing 0.5 mM NaCl and regenerated with 1.5 M guanidine-HCl in binding buffer. The unabsorbed fraction was eluted by 10 mM Tris-HCl, pH 7.2 (CitationBio-Rad Laboratories, 2000; Ye & Ng, 2002).
Gel filtration chromatography
Protein was dissolved in double distilled water and the solution applied to a column (1.6 × 60 cm) of Superdex 200 equilibrated in double distilled water using an AKTAprime system (Amersham Pharmacia Biotech). The column was eluted at flow rate of 0.5 mL/min and maintained at 4°C. Protein absorption was monitored at 280 nm. Fractions were collected and concentrated using a freeze-dryer, and then kept at −20°C (CitationPerSeptive Biosystems, 1996; CitationPingoud et al., 2002).
One-dimensional gel electrophoresis
The proteins were subjected to sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) to determined components of the proteins and their molecular weights (CitationAmersham Biosciences, 1999; CitationCreighton, 1997; CitationWang, et al., 2004). Proteins (10 μL) were applied onto 12.5% SDS-PAGE gel. SDS-PAGE was run in a Hoefer™ miniVE (mini-vertical) apparatus: 8 × 9 cm gel, 1 mm thick, 10 wells (Amersham Pharmacia Biotech, Uppsala, Sweden) were prepared as a discontinuous gel. The stock solutions were 30% acrylamide, 0.8% bis-acrylamide, stacking gel buffer (0.5 M Tris-HCl, pH 6.8, 0.4% SDS), separating gel buffer (1.5 M Tris-HCl, pH 8.8, 0.4% SDS), 10% ammonium persulfate solution (APS), and electrophoresis buffer (25 mM Tris-HCl, 192 mM glycine, 0.1% SDS) (CitationWalker, 2002). After electrophoresis, proteins in the gel were stained by Coomassie blue (CitationRozycki, et al., 2000). The polyacrylamide gel from between the glass plates was removed and immersed in 50 mL of Coomassie blue staining solution (1 L: 1.0 g Coomassie blue R-250, 450 mL methanol, 450 mL water, 100 mL glacial acetic acid), placed on a rotary shaker, and gently shaken for 20 min. The staining solution was removed and the gel rinsed with Coomassie blue destaining solution (1 L: 100 mL methanol, 100 mL glacial acetic acid, 800 mL water) for 30 min, then the Coomassie blue destaining solution was changed and the mixture agitated overnight.
Determination of protein concentration
Protein concentration was determined by the method of CitationBradford (1976) using bovine serum albumin as a standard and color reagent from Amersham Biosciences. The assay is based on the observation that the absorbance maximum for an acidic solution of Coomassie Brilliant Blue G-250 shifts from 465 nm to 595 nm when binding to protein occurs. Both hydrophobic and ionic interactions stabilize the anionic form of the dye, causing a visible color change. The assay is useful since the extinction coefficient of a dye–albumin complex solution is constant over a 10-fold concentration range.
Biological activities
Hemagglutinating activity assay
A solution of two-fold diluted lectin (50 μL) was mixed with 2% of rabbit and goat erythrocytes in 10 mM phosphate buffer, pH 7.2, at room temperature. The result was recorded after approximately 1 h, when the blank had fully settled. The hemagglutination titer was defined as the reciprocal of the highest dilution exhibiting hemagglutination, equivalent to one hemagglutination unit. Specific hemagglutinating activity is expressed as the number of hemagglutinating units per mg protein (CitationWong & Ng, 2005; CitationYe & Ng, 2001).
α-Glucosidase inhibition assay
α-Glucosidase (0.0075 mL) was mixed with protein. p-Nitrophenyl glucopyranoside (pNPG) (3 mM) as a substrate in phosphate buffer was added to the mixture to start the reaction. The reaction was incubated at 37°C for 30 min and stopped by adding 2 mL of 0.1 M Na2CO3. The α-glucosidase activity was determined by measuring the p-nitrophenol release from pNPG at 400 nm (CitationKim et al., 2004; CitationWang et al., 2004).
Protein characterization using MALDI-ToF mass spectrometry
Molecular weight determination
Molecular weight determination was performed by mixing approximately 1 μL of sample with 10 μL of α-cyano-4-hydroxy-cinnamic acid (CCA) in 50% acetonitrile/0.1% trifluoroacetic acid 0.5 mL in an Eppendorf tube, and spotting on the target. Myoglobin 1 μM was used as external standard for calibration.
Protein analysis using peptide mass mapping
The peptides used in this study were obtained from the proteolytic digestion (CitationKinter & Sherman, 2000) of proteins in gel. The digested peptides were analyzed using matrix assisted laser desorption/ionization time-of-fight (MALDI-ToF) spectrometry. An Ultraflex (Bruker) spectrometer equipped with a nitrogen laser of wavelength 337 nm was used to obtain peptide mass spectra, and amino acid sequencing was acquired using a Biflex spectrometer (Bruker) (CitationGovorun & Archakov, 2002; CitationHoffmann & Stroobant, 2007). Peptide mass spectra were searched against protein databases via the Mascot search engine (CitationMatrix Science, 2007).
Results and discussion
Four precipitation fractions with ammonium sulfate at 25, 40, 60, and 90% were subjected to tests for biological activity, i.e., hemagglutination and α-glucosidase inhibition. Only two fractions possessed biological activities: 25 and 60% ammonium sulfate saturation. Consequently, these two fractions were selected for further analysis.
Proteins precipitated with 25% ammonium sulfate solution
Crude proteins precipitated with 25% saturated ammonium sulfate were tested for hemagglutinating activity and α-glucosidase inhibition. The results suggested that these proteins had moderate hemagglutinating activity ().
Table 1. Hemagglutinating activities and yields at various stages of purification of lectin from Parkia speciosa.
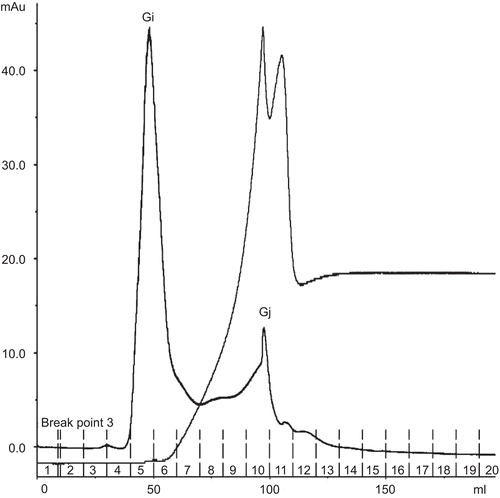
Affinity chromatography of this crude protein extract on Affi-Gel Blue gel produced unbound fraction A1 and bound fraction A2 (). All fractions were subjected to determination of biological activities. Fraction A2 did not show any biological activity, while fraction A1 revealed α-glucosidase inhibition at 65% (). Consequently, fraction A1 was chosen for further analysis by gel filtration. Gel filtration chromatography of fraction A1 on Superdex 200 yielded two peaks, Gi and Gj (). These two fractions were subjected to biological activity tests, the results of which suggested that Gj possessed high hemagglutinating activity with an IC50 value of 0.39 μg/μL. Surprisingly, the α-glucosidase activity of Gj was non-existent. It might have been in other fractions, or activity may have been lost due to the separation process. Fraction Gi did not possess any biological activity.
Figure 1. Affinity chromatogram of crude protein of Parkia speciosa on Affi-Gel Blue gel column (1.6 × 10 cm) in binding buffer 1 mM Tris-HCl, pH 7.2. Dashed lines indicate use of 0–100% linear gradient of 0.5 mM NaCl in binding buffer. Flow rate 1.5 mL/min.
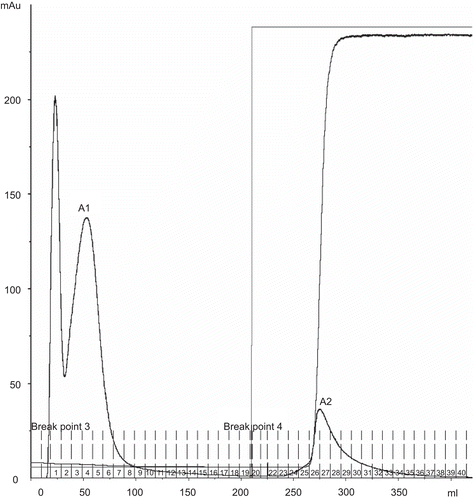
Figure 2. Gel filtration chromatogram of A1, A2 fractions from Affi-Gel Blue gel chromatography on Superdex 200 column (1.6 × 60 cm) in double distilled water. Flow rate 0.5 mL/min.
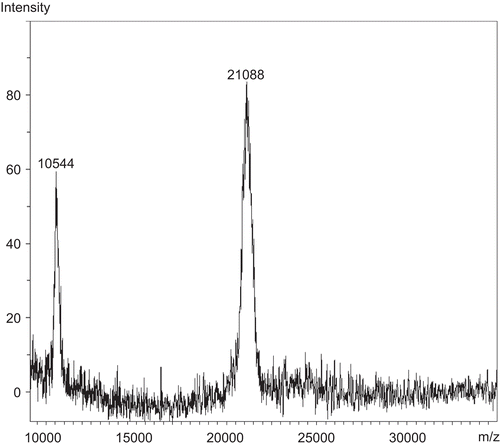
Due to the low amount of fraction Gj (31 μg), the molecular weight was measured by MALDI-ToF mass spectrometry. The result indicated that Gj had a molecular weight of 21 kDa (). Fraction Gj was subjected to protein identification. It was digested by the enzyme trypsin. Tryptic fragments of Gj were measured for molecular weight by MALDI-ToF mass spectrometry and subjected to peptide mass mapping followed by database searching using the Mascot search engine (CitationMatrix Science, 2007). The search results () indicated that protein Gj was similar to putative aristolochene synthase, 3′-partial (MW 23 kDa) from Oryza sativa L. var. Japonica (Poaceae) (Japanese rice) (CitationBuell, 2002). This enzyme cleaves between C-C, C-O, C-N, and other bonds by hydrolysis or oxidation, or conversely by adding a group to a double bond. It differs from other enzymes in that two substrates are involved in one reaction direction, but only one in the other direction. When acting on a single substrate, a molecule is eliminated, and this generates either a new double bond or a new ring.
Table 2. Proteins identified by MALDI-ToF mass spectrometry followed by peptide mass fingerprinting via the Mascot search engine.
Proteins precipitated with 60% ammonium sulfate solution
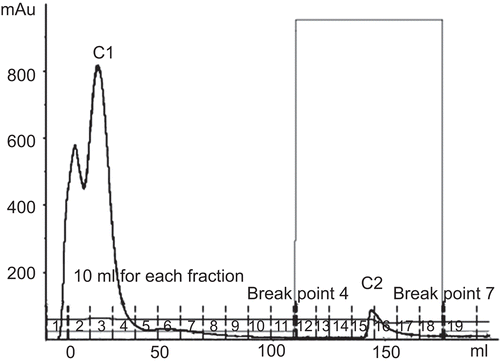
The proteins precipitated with 60% ammonium sulfate saturation from Parkia speciosa seeds also possessed some hemagglutinating activity (IC50 2.51 μg/μL), as could be seen by their ability to agglutinate rabbit erythrocytes ().
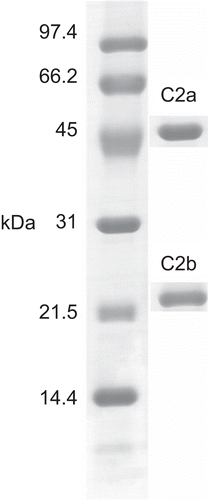
Crude proteins obtained with 60% saturated ammonium sulfate were subjected to separation by Con A affinity chromatography. The results indicated two fractions, unbound fraction C1 and bound fraction C2 (). Hemagglutinating activity was located only in fraction C2 (). Hence, the protein components of fraction C2 were investigated by one-dimensional SDS-PAGE. The results indicated the existence of two bands, C2a and C2b, with molecular weights of 45 kDa and 23 kDa, respectively (). The amount of fraction C2 was very low, so a further separation could not be performed. C2a and C2b were subjected to in-gel digestion and analysis by MALDI-ToF mass spectrometry. Peptide mass mapping techniques were performed and the results are shown in . C2a was identified as similar to hypothetical protein B1342F01.11 (MW 14 kDa) from Oryza sativa var. Japonica (CitationSasaki et al., 2002). C2b was identified as hypothetical protein At1g51560 (MW 43 kDa) from Arabidopsis thaliana (L.) Heynh. (Brassicaceae) (mouse-ear cress) (CitationYamada et al., 2002). The Mowse score confirmed the results for these two identified proteins to be significant. An earlier report from CitationSuvachittanont and Peutpaiboon (1992) indicated that there are two lectins (MW 46.7 and 47.3 kDa) containing proteins from Parkia speciosa seeds. Furthermore, the lectin from Parkia speciosa seeds has mitogenic activity, which increases the incorporation of [3H]thymidine into the DNA of human lymphocytes. Such activity of the lectin was indicated by the fact that it stimulated incorporation of [3H]thymidine into DNA of rat thymocytes (CitationSuvachittanont & Jaranchavanapet, 2000).
This is the first report on the identification of lectins from Parkia speciosa seeds which have hemagglutinating activity. Information on the activity and identification of proteins from this plant might be advantageous for future tentative drug development from alternative natural resources.
Figure 4. Affinity chromatogram of crude protein from Parkia speciosa on Con A Sepharose column (1.6 × 5 cm). Flow rate 1.5 mL/min. The equilibration buffer was 20 mM Tris-HCl, pH 7.4, containing 0.5 M NaCl, 1 mM CaCl2·2H2O, 1 mM MnCl2. The eluted lectin buffer was 20 mM Tris-HCl, pH 7.4, containing 0.1–0.5 M methyl-α-d-mannopyranoside.
Declaration of interest: We thank the Thailand Research Foundation for financial support and Chulalongkorn University for a Graduate Scholarship. The authors alone are responsible for the writing of this paper.
References
- Ali H, Houghton PJ, Soumyanath A (2006): Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol 107: 449–455.
- Amersham Biosciences (1999): Protein Electrophoresis Technical Manual. Manufacturer’s Instructions. Piscataway, NJ, Amersham Biosciences, pp. 5–25.
- Amersham Biosciences (2002): Con A Sepharose 4B. Manufacturer’s Instructions. Piscataway, NJ, Amersham Biosciences, pp. 1–16.
- Bains JS, Singh J, Kamboj SS, Nijjar KK, Agrewala JN, Dhuna V (2005): Novel lectins from rhizomes of two Acorus species with mitogenic activity and inhibitory potential towards murine cancer cell lines. Int Immunopharmacol 5: 1470–1478.
- Bio-Rad Laboratories (2000): Affi-Gel Blue Gel Instruction Manual. Hercules, CA, Bio-Rad, pp. 1–9.
- Bradford MM (1976): A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254.
- Buell R (2002): Nucleotide sequence: Oryza sativa chromosome 10, P0031G09, EMBL/GenBank/DDBJ databases [Online]. Available at: http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nuccore&id= 22539121.
- Creighton TE (1997): Protein Structure, 2nd ed. New York, Oxford University Press, pp. 110–135.
- Dass C (2001): Principles and Practice of Biological Mass Spectrometry. New York, John Wiley& Sons, pp. 2–28.
- Gmelin R, Susilo R, Fenwick GR (1981): Cyclic polysulphides from Parkia speciosa. Phytochemistry 20: 2521–2523.
- Govorun VM, Archakov AI (2002): Proteomic technologies in modern biomedical science. Biochemistry (Moscow)67: 1109–1123.
- Hoffmann ED, Stroobant V (2007): Mass Spectrometry. Principles and Applications, 3rd ed. London, John Wiley & Sons, pp. 60–75.
- Holme DJ, Peck H (1998): Analytical Biochemistry, 3rd ed. New York, Longman, pp. 128–140.
- Jamaluddin F, Mohamed S, Lajis MN (1994): Hypoglycemic effect of Parkia speciosa seeds due to the synergistic action of beta- sitosterol and stigmasterol. Food Chem 49: 339–345.
- Kim YM, Wang MH, Rhee HI (2004): A novel α-glucosidase inhibitor from pine bark. Carbohydr Res 339: 715–717.
- Kinter M, Sherman NE (2000): Protein Sequencing and Identification using Tandem Mass Spectrometry. New York, John Wiley & Sons, pp. 152–160.
- Konozy EHE, Bernardes ES, Rosa C, Faca V, Greene LJ, Ward RJ (2003): Isolation, purification, and physicochemical characterization of a d-galactose-binding lectin from seeds of Erythrina speciosa. Arch Biochem Biophys 410: 222–229.
- Li X, Kushad M (2005): Purification and characterization of myrosinase from horseradish (Aromracia rusticana) roots. Plant Physiol Biochem 43: 503–511.
- Matrix Science (2007): Mascot Search Engine. Available at: http://www.matrixscience.com.
- Ohtsubo K, Richardson M (1992): The amino acid sequence of a 20 kDa bifunctional subtilisin/α-amylase inhibitor from bran [correction of brain] of rice (Oryza sativa L.) seeds. FEBS Lett 309: 68–72.
- PerSeptive Biosystems (1996): The Busy Researcher’s Guide to Biomolecule Chromatography. USA, PerSeptive Biosystems, pp. 12–30.
- Pingoud A, Urbanke C, Hoggett J, Jeltsch A (2002): Biochemical Methods. Weinheim, Wiley-VCH Verlag GmbH, pp. 118–137.
- Rozycki DM, Edelstein JS, Bollag MD (2000): Protein Methods, 2nd ed. New York, John Wiley & Sons, pp. 126–128.
- Sasaki T, Matsumoto T, Katayose Y (2002): Nucleotide sequence: Oryza sativa nipponbare (GA3) genomic DNA, chromosome 2, BAC clone: B1342F01, EMBL/GenBank/DDBJ databases [Online]. Available at: http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nuccore&id= 32879800.
- Suvachittanont W, Jaranchavanapet P (2000): Mitogenic effect of Parkia speciosa seed lectin on human lymphocytes. Planta Med 66: 699–704.
- Suvachittanont W, Kurashima Y, Esumi H, Tsuda M (1996): Formation of thiazolidine-4-carboxylic acid (thioproline), an effective nitrite-trapping agent in human body, in Parkia speciosa seeds and other edible leguminous seeds in Thailand. Food Chem 55: 359–363.
- Suvachittanont W, Peutpaiboon A (1992): Lectin from Parkia speciosa seeds. Phytochemistry 31: 4065–4070.
- Taungbodhitham AK (1995): Thiamine content and activity of antithiamine factor in vegetables of southern Thailand. Food Chem 52: 285–288.
- Walker JM (2002): The Protein Protocols Handbook, 2nd ed. New Jersey, Humana Press, pp. 234–256.
- Wang HM, Kim MY, Jeong KY, Lee YW, Rhee IH (2004): Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition 21: 756–761.
- Wong JH, Ng TB (2005): Isolation and characterization of a glucose/mannose/rhamnose-specific lectin from the knife bean Canavalia gladiata. Arch Biochem Biophys 439: 91–98.
- Yamada K, Liu SX, Sakano H, Pham PK, Banh J, Chung MK, Goldsmith AD, Lee JM, Quach HL, Toriumi M, Yu G, Bowser L, Carninci P, Chen H, Cheuk R, Hayashizaki Y, Ishida J, Jones T, Kamiya A, Karlin-Neumann G, Kawai J, Kim C, Lam B, Lin J, Miranda M, Narusaka M, Nguyen M, Palm CJ, Sakurai T, Satou M, Seki M, Shinn P, Southwick A, Shinozaki K, Davis RW, Ecker JR, Theologis A (2002): Arabidopsis full length cDNA clones, EMBL/GenBank/DDBJ databases [Online]. Available at: http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nuccore&id= 18377804.
- Ye X, Ng TB (2001): Isolation of lectin and albumin from Pisum sativum var. macrocarpon ser. cv. sugar snap. Int J Biochem Cell Biol 33: 95–102.
- Ye XY, Ng TB (2002): Isolation of a novel peroxidase from French bean legumes and first demonstration of antifungal activity of a non-milk peroxidase. Life Sci 71: 1667–1680.