Abstract
The methanol extract of Vernonia cinerea Less (Asteraceae), which exhibited antimicrobial activity, was tested for toxicity. In an acute toxicity study using mice, the median lethal dose (LD50) of the extract was greater than 2000 mg/kg, and we found no pathological changes in macroscopic examination by necropsy of mice treated with extract. As well as the oral acute toxicity study, the brine shrimp lethality test was also done. Brine shrimp test LC50 values were 3.87 mg/mL (6 h) and 2.72 mg/mL (24 h), exhibiting no significant toxicity result. In conclusion, the methanol extract of V. cinerea did not produce toxic effects in mice and brine shrimp.
Introduction
Vernonia cinerea Less (Asteraceae), an annual herb, is reported to have many medicinal properties (CitationIwalewa et al., 2003). V. cinerea has many therapeutic uses in different traditional medicines of the world, including use for treatment of a number of disorders such as malaria fever, worms, pain, inflammation, infections, diuresis, cancer, abortion, and various gastrointestinal disorders (CitationJain & Puri, 1984; CitationJohn, 1984; CitationSingh & Ali, 1989; CitationBhattarai, 1991; CitationBajpai et al., 1995; CitationGrainger, 1996). Every part of the plant can be used medicinally (CitationChopra et al., 1985; CitationLatha et al., 1998). An extract of the plant is used to relieve cold and menstruation-related problems (CitationVerma et al., 1993). The plant has also been used as a tonic, stomachic, and astringent (CitationMisra et al., 1984). The flowers are used to treat conjunctivitis, fever, and rheumatism (CitationRastogi & Mehrotra, 1991). The leaf extracts of the plant are reported to be diuretic and antidiuretic (CitationAdeboye et al., 1997), anti-inflammatory (CitationMazumder et al., 2003), analgesic, and antipyretic (CitationIwalewa et al., 2003). Our previous study showed that the methanol extract of V. cinerea exhibited a good antibacterial and antiyeast activity (CitationYoga Latha et al., 2005).
Although medicinal plants may produce several biological activities in humans, generally very little is known about their toxicity (CitationMukinda & Syce, 2007), and the same applies for V. cinerea. Safety should be the principal criterion in the selection of medicinal plants for use in healthcare systems (CitationTomlinson & Akerele, 1998). Hence, formal toxicological evaluation of a plant is necessary as well as documented ethnomedicinal information. Therefore, the present study carried out basic toxicological tests and established the safety of the methanol extract of V. cinerea (whole plant part, including roots), focusing on its acute toxicity in mice and the brine shrimp lethality assay.
Materials and methods
Plant material
The whole plant part including roots of V. cinerea was obtained from the University Science of Malaysia campus, Penang, Malaysia in April 2004, and was authenticated by Associate Professor Dr. Shaida Fariza Sulaimana, a taxonomist at the University Science of Malaysia, Penang, Malaysia. A dried sample (with voucher number VC0404) was deposited at the herbarium of the School of Biological Sciences.
Preparation of the crude extract
Approximately 200 g of dried plant material was boiled in a Soxhlet apparatus with 200 mL of methanol [high performance liquid chromatography (HPLC) grade] for 48 h. The entire extract was further concentrated to dryness using a rotary evaporator, yielding 18 g extract (CitationNisha et al., 2008).
Animals
Mice (males and females) weighing 25 and 35 g from the Animal House of the University of Science Malaysia were used. Ten mice were kept in each matte plastic cage, measuring 17 × 27 × 14 cm. The cages with the mice were placed in a room with controlled cycles of 12 h of light and 12 h of darkness; the light went on at 7 a.m. Water and food were provided to animals ad libitum. Experiments were conducted in accordance with the internationally accepted principles for laboratory animal use and care (EEC Directive of 1986; 86/609/EEC).
Acute toxicity study of V. cinerea methanol crude extract
All mice of both sexes were fasted overnight before treatment and were given food 1 h after treatment. We expected that V. cinerea would be relatively safe because of its application in traditional medicine. Thus, a single high dose, as recommended by the CitationOrganization for Economic Co-operation and Development (OECD) guidelines (2000), of 2000 mg/kg of crude extract dissolved in water was administered by gavage to 10 male and 10 female mice, and water was given to 10 male and 10 female mice as a control group. After a single administration, signs of possible toxicity were observed every hour for the first 6 h and every day for 14 days. Surviving animals were weighed daily and observed for any signs or symptoms of toxicity and for mortality for up to 14 days, as described previously (CitationLee et al., 2002; CitationRyu et al., 2004). Visual observations included changes in the skin and fur, eyes, and mucous membranes, and also respiratory, circulatory, autonomic, and central nervous systems, as well as somatomotor activity and behavioral pattern.
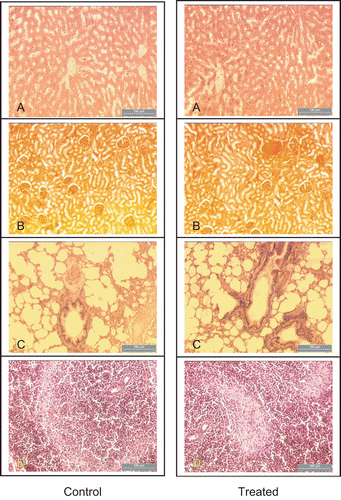
After this observation period of 14 days, seven mice of both sexes in each group were sacrificed to measure organ/body weight indices. Liver, lungs, spleen, and kidneys were removed from the remaining three mice of both sexes from each group after euthanizing and killing them by cervical dislocation. Tissues were fixed in 10% buffered formalin. After fixation, the tissues were dehydrated in a graded series of alcohol, cleared in xylene, and embedded in paraffin wax. Multiple 5 μm sections from each block were mounted on slides and stained with hematoxylin and eosin.
Toxicity testing against brine shrimp
Hatching shrimp
Brine shrimp eggs, Artemia salina L. (Artemiidae), were hatched in artificial seawater prepared by dissolving 38 g of sea salt in 1 L of distilled water. After a 24-h incubation period at room temperature (22–29°C), the larvae were attracted to one side of the vessel with a light source and collected with a pipette. The larvae were separated from the eggs by aliquoting them three times in small beakers containing seawater.
Brine shrimp assay
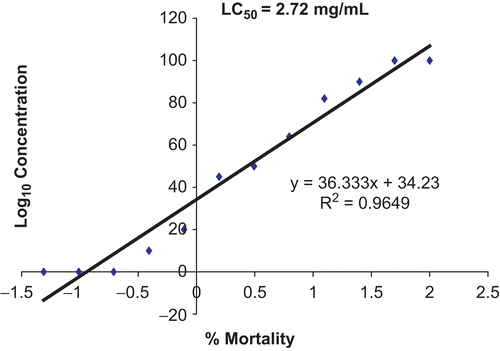
The bioactivity of the extract was monitored by the brine shrimp lethality test (CitationMeyer et al., 1982). Samples were dissolved in dimethylsulfoxide (DMSO) and diluted with artificial seawater. Seawater (2 mL) was placed in all bijoux bottles. A two-fold dilution was carried out to obtain concentrations from 100 to 0.195 mg/mL. The last bottle was filled with sea salt-water and DMSO only, serving as a drug-free or negative control. A suspension (0.1 mL) containing about 10–15 larvae was added to each bottle and incubated for 24 h. The bottles were then examined, and the number of dead larvae in each bottle was counted after 6 and 24 h. The total number of shrimp in each bottle was counted and recorded. The mean percentage mortality was plotted against the logarithm of concentration, and the concentration that could kill 50% of the larvae (LC50) was determined from the graph (CitationFinney, 1971; CitationGeran et al., 1972).
Calculations and statistics
The results are expressed as mean ± standard deviation (SD). The acute toxicity study data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s test for comparing the control and the various groups, using SPSS Version 12.0. The mean results of brine shrimp mortality against logarithm of concentration were plotted using the Microsoft Excel computer program, which also presents regression equations. The regression equations were used to calculate the LC50 value. Extracts giving LC50 values greater than 1.0 mg/mL were considered to be non-toxic (CitationSimionatto et al., 2005; CitationSasidharan et al., 2008). Statistical significance was assumed at the 0.05 level.
Results and discussion
Results for the toxicity of the crude extract of V. cinerea on mice and brine shrimp are shown in and . To establish the safety of the extract, 2000 mg/kg crude extract was administered to both male and female mice. No toxic symptoms or death was observed in any of the animals, and they lived up to 14 days. An autopsy at the end of the experimental period revealed no apparent changes in any organs (, ). Total body weight in both male and female extract-treated mice was similar to that of control mice. There were no significant differences in the organ-to-body weight indices of the heart, kidneys, liver, lungs, or spleen (). Gross examination at autopsy and histopathological evaluations of the various organs stained with hematoxylin and eosin revealed no significant differences (). The overall feed consumption of animals receiving V. cinerea extract was generally similar to that of the controls (data not shown). Similarly, the feed consumption in the recovery group was similar to the respective control. Food intake exhibited the same pattern of evolution in each sex and group. Therefore, the acute minimum fatal dose of the extract of V. cinerea for mice was higher than 2000 mg/kg body weight, which is the single high dose recommended by CitationOECD (2000) guidelines for testing oral acute toxicity. Thus, our test suggested that V. cinerea does not cause any apparent acute toxicity.
Table 1. Effect of Vernonia cinerea crude extracta on organ-to-body weight index (%) in mice.
The extract of V. cinerea showed no significant toxicity against brine shrimp, with LC50 values of 3.87 mg/mL (6 h) and 2.72 mg/mL (24 h). With respect to the effect of the time of exposure in the brine shrimp assay, no significant changes in toxicity were detected at 6 or 24 h of exposure ( and ). The results on brine shrimp assay indicate that the extract had an LC50 value greater than 1.0 mg/mL, which is the recommended cut-off point for detecting cytotoxic activity (CitationSimionatto et al., 2005). This suggests that this plant extract may not be toxic. A toxicity study was also reported by CitationKuo et al. (2003). They tested two novel sesquiterpene lactones, vernolide-A (1) and -B (2), isolated from the ethanol extract of stems of V. cinerea, against human KB (oral epidermoid carcinoma), DLD-1 (colon adenocarcinoma), NCI-661 (lung large cell carcinoma), and Hela (cervix epithelioid carcinoma) tumor cell lines. They reported that vernolide-A demonstrated potent cytotoxicity against human KB, DLD-1, NCI-661, and Hela tumor cell lines (ED50 = 0.02, 0.05, 0.53, 0.04 mg/mL for KB, DLD-1, NCI-661, and Hela, respectively); vernolide-B had marginal cytoxicity (ED50 = 3.78, 5.88, 6.42 mg/mL for KB, NCI-661, and Hela, respectively).
Figure 2. Toxic effects of Vernonia cinerea methanol extract after 6 h using brine shrimp lethality assay.
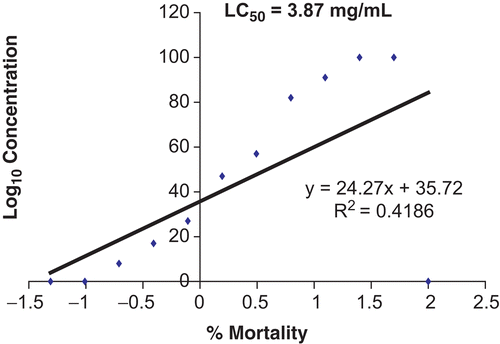
Figure 3. Toxic effects of Vernonia cinerea methanol extract after 24 h using brine shrimp lethality assay.
Thus, our test suggested that V. cinerea does not cause any apparent in vivo toxicity. The results of the current study concur with the use of this plant by traditional healers. A World Health Organization survey indicated that about 70–80% of the world’s population rely on non-conventional medicine, mainly of herbal source, in their primary healthcare (CitationAkerele, 1993). This is particularly the case in developing countries where the cost of consulting a Western style doctor and the price of medication are beyond the income of most people. Our present study showed that V. cinerea does not exhibit any apparent toxicity and may be used as an antimicrobial agent in known dosages, especially in rural communities where conventional drugs are unaffordable because of the high cost or are unavailable in developing countries. Studies of this type are needed before a phytotherapeutic agent can be generally recommended for use.
Declaration of interest: The authors report no conflicts of interest.
References
- Adeboye JO, Aije W, Awe SO (1997): Diuretic and antidiuretic activity of the leaf extracts of Vernonia cinerea. Phytother Res 11: 454–456.
- Akerele O (1993): Nature’s medicinal bounty: Don’t throw it away. World Health Forum 14: 390–395.
- Bajpai A, Ojha JK, Sant HR (1995): Medicobotany of the Varanasi district. Int J Pharmacog 33: 172–176.
- Bhattarai NK (1991): Folk herbal medicines of Makawanpur district, Nepal. Int J Pharmacog 29: 284–295.
- Chopra I, Hodgson J, Metcalf B, Poste G (1996): New approaches to the control of infections caused by antibiotic resistant bacteria. An industry perspective. JAMA 275: 401–403.
- Finney DJ (1971): Probit Analysis, 3rd ed. Cambridge, Cambridge University Press, pp. 26.
- Geran RI, Greenberg HM, McDonald M, Abbott BJ (1972): Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep 33: 1–17.
- Grainger CR (1996): Medicinal plants of Seychelles. R Soc Health J 116: 107–109.
- Iwalewa EO, Iwalewa OJ, Adeboye JO (2003): Analgesic, antipyretic, anti-inflammatory effects of methanol, chloroform and ether extracts of Vernonia cinerea less leaf. J Ethnopharmacol 86: 229–234.
- Jain SP, Puri HS (1984): Ethnomedicinal plants of Jaunsar-Bawar hills (Uttar Pradesh) India. J Ethnopharmacol 12: 213–222.
- John D (1984): One hundred useful raw drugs of the Kani tribes of Trivandrum. Int J Crude Drug Res 1: 17–39.
- Kuo YH, Kuo YJ, Yu AS, Wu MD, Ong CW, Yang Kuo LM, Huang JT, Chen CF, Li SY (2003): Two novel sesquiterpene lactones, cytotoxic vernolide-A and -B, from Vernonia cinerea. Chem Pharm Bull 51: 425–426.
- Latha RM, Geetha T, Varalakshmi P (1998): Effect of Vernonia cinerea less flower extract in adjuvant-induced arthritis. Gen Pharmacol 31: 601–606.
- Lee JN, Park CS, Kim HP, Hwang SY, Chung WG (2002): Single dose toxicity study of Hwangjaegongjinbo, an invigorator, in mice and rats. J Toxicol Public Health 18: 73–77.
- Mazumder UK, Gupta M, Manikandan L, Bhattacharya S, Haldar PK, Roy S (2003): Evaluation of antiinflammatory activity of Vernonia cinerea less extracts in rats. Phytomedicine 10: 185–188.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL (1982): Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 45: 31–34.
- Misra TN, Singh RS, Upadhyay J, Srivastava R (1984): Chemical constituents of Vernonia cinerea, Part I. Isolation and spectral studies of triterpenes. J Nat Prod 47: 368–372.
- Mukinda JT, Syce JA (2007): Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J Ethnopharmacol 112: 138–144.
- Nisha M, Xavier R, Sreeramanan S, Rajasekaran A, Sasidharan S, Yoga Latha L (2008): Antimicrobial activity and toxicity of Punica granatum L., peel.J Appl Biol Sci 2: 59–61.
- OECD (2000): Guidelines for the Testing of Chemicals, Revised Draft Guideline: Acute Oral Toxicity. Paris, Organization for Economic Co-operation and Development, p. 423.
- Rastogi RP, Mehrotra BN (1991): Compendium of Indian Medicinal Plants, Vol. II. New Delhi, Central Drug Research Institute, Lucknow and Publications and Information Directorate.
- Ryu SD, Park CS, Baek HM, Baek SH, Hwang SY, Chung WG (2004): Anti-diarrheal and spasmolytic activities and acute toxicity study of Soonkijangquebo, a herbal anti-diarrheal formula. J Ethnopharmacol 91: 75–80.
- Sasidharan S, Darah I, Jain K (2008): In vivo and in vitro toxicity study of Gracilaria changii. Pharm Biol 46: 413–417.
- Simionatto E, Porto C, da Silva UF, Squizani AMC, Dalcol II, Morel AF (2005): Composition and antimicrobial activity of the essential oil from Aloysia sellowii.J Braz Chem Soc 16: 1458–1462.
- Singh VK, Ali ZA (1989): Folk medicines of Aligarh (Uttar Pradesh) Indian. Fitoterapia LX: 483–490.
- Tomlinson TR, Akerele O (1998): Medicinal Plants: Their Role in Health and Biodiversity. Philadelphia, University of Pennsylvania Press, p. 208.
- Verma DM, Balakrishnan RR, Dixit RD (1993): Flora of Madhya Pradesh. Lucknow, India, Botanical Survey of India, pp. 90–191.
- Yoga Latha L, Darah I, Shaida FS (2005): Pharmacological screening of crude methanolic extract of Vernonia cinerea L. In: Proceedings of the 27th Symposium of the Malaysian Society for Microbiology. Penang, Malaysia, Malaysian Society for Microbiology, pp. 491–493.