Abstract
The water soluble matrix (WSM) of pearl powder [Hyriopsis cumingii Lea (Unionidae)] was extracted, and the insoluble residue was demineralized, size-fractionated, and named as MR14 (> 14 kDa), MR3–14 (3–14 kDa), and MR3 (< 3 kDa). The effects of WSM, MR14, MR3–14, and MR3 on primary mouse oral fibroblast proliferation, collagen accumulation, matrix metalloproteinase-2, -9 (MMP-2, -9) activities, and tissue inhibitor of metalloproteinase-1 (TIMP-1) production were tested by MTT assay, chloramine T method, gelatin zymography, and enzyme-linked immunosorbent assay (ELISA), respectively. The results showed that the WSM and MR14 could significantly (p < 0.05) promote fibroblast proliferation; all of the fractions could significantly promote collagen accumulation; MR14 significantly (p < 0.05) inhibited MMP-2 activity; and all of the fractions could significantly promote TIMP-1 production. This study has proved that the mechanism by which pearl powder promotes wound healing is partly due to its ability to stimulate fibroblast mitosis, collagen deposition, and TIMP-1 production, and the major active fraction may be MR14.
Introduction
Pearl powder, a traditional Chinese medicine, has been used to treat palpitations, convulsions, insomnia, epilepsy, and ulcers for thousands of years. According to the Pharmacopoeia of the People’s Republic of China, this medicine is processed from the pearl formed in Pterin martensii Dunker (Pteriidae), Hyriopsis cumingii Lea, Cristaria plicata Leach (Unionidae), etc. Modern research has shown that pearl powder has antioxidant (CitationXu et al., 2001), anti-aging, anti-radiative, and tonic activities (CitationCao et al., 1996). Clinical applications have also proved its obvious therapeutic efficacy in treating relapsed aphthous ulcer, gastric ulcer, and duodenal ulcer (CitationRuan et al., 2004). However, there are almost no reports about the mechanism by which pearl powder promotes wound healing at the cellular level.
Wound healing is a complicated biological process. During this process, fibroblasts play an important role. They can secrete collagens, fibronectin, and glycosaminoglycans to form new granulation tissue, secrete growth factors to stimulate proliferation, differentiation, and migration of other cells relevant to wound healing, and migrate and differentiate into myofibroblasts to accelerate contraction of the wound surface. It has been shown that increasing the number of fibroblasts in an artificial dermal substitute leads to improved healing in experimental wounds (CitationLamme et al., 2000). Any impediment to fibroblast function will prevent normal wound healing and result in chronic, non-healing wounds (CitationLerman et al., 2003). Fibroblasts and their functions are usually considered to be extremely important in the initial stages of tissue repair (CitationDong & Shi, 2006). Hence, we have cultured mouse oral fibroblasts and taken them as an in vitro model to investigate the effects of pearl powder on the growth, collagen accumulation, matrix metalloproteinase-2, -9 (MMP-2, -9) activities, and tissue inhibitor of metalloproteinase-1 (TIMP-1) production of fibroblasts, and finally attempted to determine the major active fractions of pearl powder.
Materials and methods
Material
Pearl powder [Hyriopsis cumingii Lea (Unionidae)] was purchased from Cunrentang Co. Ltd. (Zhenjiang, China) with the batch number of 060701003, and was identified by Dr. Han Bang-Xing (Institute of Food & Bioengineering, Jiangsu University, Zhenjiang, China). A voucher specimen has been deposited in our laboratory.
Extraction and size-fractionation
Pearl powder (500 g) was extracted by a non-decalcifying method (24 h, 4°C); the suspension was centrifuged (30 min, 12,000 rev/min), lyophilized, and named the water soluble matrix (WSM). The insoluble residue was treated with 2 L 10% acetic acid solution (12 h, 4°C). The suspension was centrifuged (30 min, 12,000 rev/min), and size-fractionated by 14 kDa cut-off dialysis bags against 5 L 10 mM Tris-HCl buffer (pH 7.0) at 4°C. After lyophilization, the retentate was named as MR14 (molecular weight > 14 kDa); the dialysate was further dialyzed by 3 kDa cut-off dialysis bags using the same method. Then, the retentate and dialysate were also lyophilized and named as MR3–14 (3 kDa < molecular weight < 14 kDa) and MR3 (molecular weight < 3 kDa), respectively. The protein content of the WSM, MR14, MR3–14, and MR3 were measured using a Coomassie Brilliant protein determination kit (Nanjing Jiancheng Bioengineering Institute, China).
Primary mouse oral fibroblast culture
BALB/c mice (20 ± 2 g) were purchased from the Experimental Animal Center of Jiangsu University (Zhenjiang, China) and housed under a 12-hour light/dark cycle with food and water ad libitum in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International. Mice were killed by cervical dislocation and the oral mucosa was stripped under sterile conditions; primary mouse oral fibroblasts were cultured according to a similar study (CitationO’Leary et al., 2004).
Cell proliferation assay
The effect of pearl powder on fibroblast proliferation was assayed by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] method (CitationO’Leary et al., 2004). Serial dilutions (15.625, 31.25, 62.5, 125, 250 μg/mL) of WSM, MR14, MR3–14, and MR3 were made in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 1% fetal calf serum (FCS); 1% FCS DMEM served as a blank control. After 48 and 72 h incubation, the absorbance was read at 570 nm. The minimum valid concentration was defined as the minimum concentration at which a statistically significant (p < 0.05) difference appeared when compared with the blank control after 72 h incubation. The results showed that the minimum valid concentrations of WSM, MR14, MR3–14, and MR3 were 15.625, 15.625, 250, and 125 μg/mL, respectively. In order to make the experiment more convenient, we selected 250 μg/mL as the minimum valid concentration of MR3.
Cell growth curve
Fibroblasts were seeded and exposed to WSM (15.625 μg/mL), MR14 (15.625 μg/mL), MR3–14 (250 μg/mL), and MR3 (250 μg/mL) for a further 8 days. Every day, the cells from five wells were detached and counted in a hemacytometer. The results were plotted and the population doubling times were estimated according to the formula: cell population doubling time = t·[lg2/(lgNt – lgN0)] (N0 and Nt represent the initial cell number and that at t hours after inoculation, respectively).
Hydroxyproline assay
The collagen production of fibroblasts was assessed in vitro by quantitating the hydroxyproline content. Fibroblasts were seeded in 24-well plates and exposed to WSM (15.625 μg/mL), MR14 (15.625 μg/mL), MR3–14 (250 μg/mL), and MR3 (250 μg/mL) for 72 h. 1% FCS DMEM and 10% FCS DMEM served as blank and positive controls, respectively. The hydroxyproline content was determined by the chloramine T method (CitationEdwards & O’Brien, 1980). Data are expressed as micrograms of collagen in 106 cells, assuming that collagen contains 13.5% hydroxyproline.
Gelatin zymography
Fibroblasts were exposed to WSM (15.625 μg/mL), MR14 (15.625 μg/mL), MR3–14 (250 μg/mL), and MR3 (250 μg/mL). After 72 h, the media were harvested and centrifuged for 30 min at 12,000 rev/min. The supernatants were stored at −70°C before use in gelatin zymography and the following TIMP-1 enzyme-linked immunosorbent assay (ELISA). The activities of MMP-2, -9 were determined by gelatin zymography assay and expressed as a multiplication product of the zone area and average gray value (CitationGibbs et al., 1999).
TIMP-1 enzyme-linked immunosorbent assay
The supernatants were collected as mentioned above. TIMP-1 levels in the supernatants were assayed using a commercially available mouse TIMP-1 ELISA kit (Wuhan Boster Biological Technology, Ltd., China) according to the manufacturer’s instructions. The standard curve of TIMP-1 was assessed, and the relative amounts of TIMP-1 in the samples were calculated by comparison with the standard curve and expressed as ng/mL. Each sample was analyzed in triplicate.
Statistical analysis
Statistical differences between experimental groups were determined by one-way analysis of variance (ANOVA). All the data given represent the mean ± standard error of the mean (SEM).
Results and discussion
Extraction
In this study, the yield of WSM was only 92 mg per 100 g pearl powder; further isolation and purification were difficult, so a demineralizing method was adopted and the intracrystalline matrix was also extracted. After size-fractionation, the yields of MR14 and MR3–14 were 88.2 and 67.8 mg per 100 g pearl powder, respectively; the yield of MR3 was not calculated because it contained too much inorganic salt. The protein contents of WSM, MR14, MR3–14, and MR3 were 12.16, 18.90, 15.16, and 2.92%, respectively (). The yield and protein content of WSM were a little less than that of a previous study (CitationBedouet et al., 2001).
Table 1. Yield and protein content of the extract and fractions.
Morphologic characteristics of primary cultured fibroblasts
After 15 days, an 80% area of the culture flask was occupied by fibroblasts; the cells were polygonal or long spindle-shaped in appearance (). The cells were then subcultured. When subcultured to the second generation, the cells had a more spindle-shaped or polygonal appearance (); subcultured to the third generation, the cells were mainly spindle-shaped and a few were sheet-shaped (). The cells used in this study were between the third and sixth generations.
Effects on fibroblast proliferation
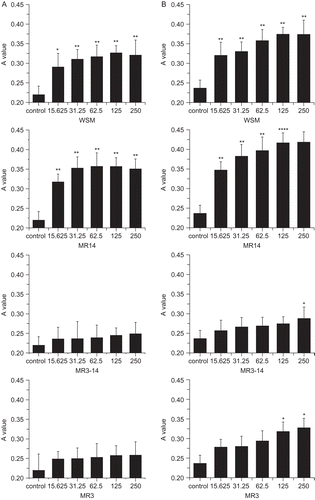
As seen in , from 15.625 to 250 μg/mL, WSM caused statistically significant (p < 0.05) increases in the growth of fibroblasts when compared with the control. Among the fractions, MR14 had a stronger activity than WSM. After 48 h incubation, MR14 (15.625 μg/mL) could significantly (p < 0.01) promote cell proliferation by 44.49% above the control, which was higher than the effect of WSM (32.29%). This result was similar to that of another study (CitationMouries et al., 2002). As for MR3–14 and MR3, although MR3–14 (250 μg/mL) and MR3 (125 μg/mL) could significantly (p < 0.05) stimulate fibroblast proliferation after 72 h incubation, no statistical significance could be seen after 48 h incubation. Thus, we deduce that MR14 is the major active fraction of pearl powder in the promotion of fibroblast proliferation.
Effects on cell growth curve
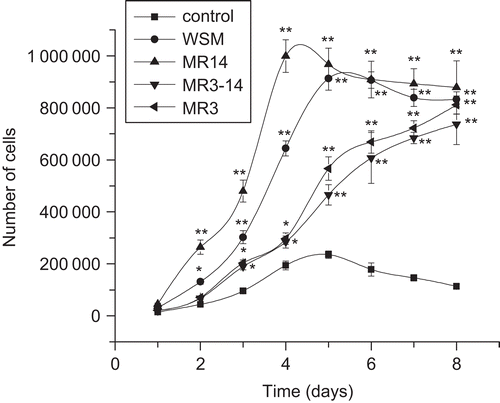
As shown in , the growth rate with MR14 treatment was the fastest of all treatments. The population doubling times of MR14 and WSM treatments were 16.13 and 19.71 h, respectively. Growth with MR3–14 and MR3 treatments was very slow. This experiment further proved that MR14 is the major active fraction of pearl powder to promote fibroblast proliferation.
Effects on collagen production
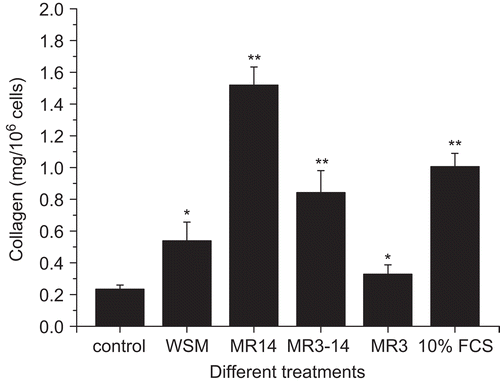
As shows, the collagen level with WSM (15.625 μg/mL) treatment was 0.5385 ± 0.11845 mg/106 cells, which was significantly (p < 0.05) higher than that of the control. The result was similar to that of a previous report (CitationRousseau et al., 2008). The highest collagen level (1.5199 ± 0.1134 mg/106 cells) was found with MR14 (15.625 μg/mL) treatment; this was even higher than that of the positive control (10% FCS medium). MR3–14 (250 μg/mL) and MR3 (250 μg/mL) also significantly (p < 0.05) promoted collagen accumulation. As the extracellular matrix is very important for wound healing (CitationLerman et al., 2003), we think that all of MR14, MR3–14, and MR3 are important for pearl powder promotion of wound repair, but especially MR14.
Effects on the enzymatic activities of MMP-2, -9
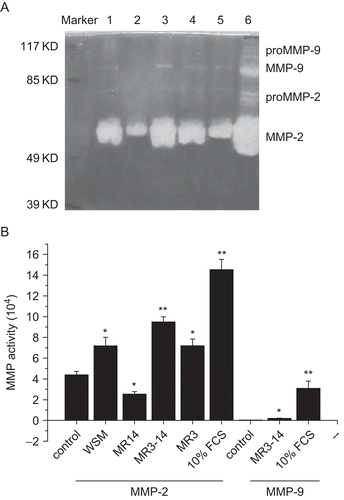
As shown in , WSM (15.625 μg/mL), MR3–14 (250 μg/mL), and MR3 (250 μg/mL) could significantly increase MMP-2 activity; MR3–14 (250 μg/mL) also could significantly (p < 0.05) stimulate MMP-9 activity when compared with the control, but MR14 (15.625 μg/mL) significantly (p < 0.05) inhibited MMP-2 activity and almost had no effect on MMP-9 activity. As MMP-2, -9 are two important metalloproteinases, we think that MR14 is important for collagen deposition.
Figure 5. Effects of WSM, MR14, MR3–14, and MR3 on MMP-2, -9 activities. (A) Results of gelatin zymography. Lane 1: WSM; lane 2: MR14; lane 3: MR3–14; lane 4: MR3; lane 5: 1% FCS control; lane 6: 10% FCS control. (B) Activities of MMP-2, -9 expressed as multiplication product of zone area and average gray value. Data represent the mean ± SEM (n = 5). *p < 0.05, **p < 0.01.
TIMP-1 ELISA
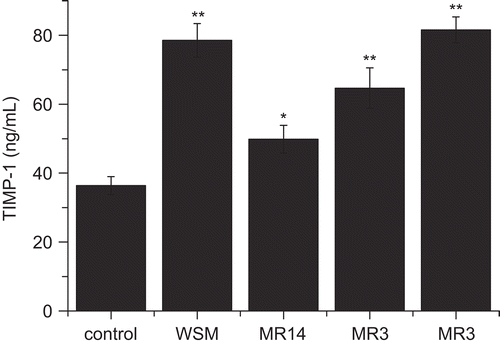
As shown in , an up to 2.6-fold increase in the production of TIMP-1 was found in WSM treatment when compared with the control. As for the fractions, the lowest level of TIMP-1 was found with MR14 treatment, but it was still significantly (p < 0.05) higher than that of the control. The highest level of TIMP-1 was found with MR3–14 treatment.
Figure 6. Effects of WSM, MR14, MR3–14, and MR3 on the production of TIMP-1, Data are expressed as the mean ± SEM (ng/mL). *p < 0.05, **p < 0.01.
As WSM, MR14, MR3–14, and MR3 could significantly promote TIMP-1 production, this might partly counteract the increase of MMP-2, -9 activities measured in the above gelatin zymography assay. This finding might also be helpful in explaining the mechanism of pearl powder to promote wound healing.
In conclusion, this study has established a simple in vitro model to screen active compounds from natural products related to wound healing. The findings obtained in this study indicate that pearl powder extract has a moderate wound healing activity, whose mechanism seems to activate fibroblast mitosis, promote extracellular matrix deposition, and increase the production of TIMP-1. Moreover, based on the findings that MR14 can significantly promote fibroblast proliferation, accelerate collagen accumulation, inhibit MMP-2 activity, and increase TIMP-1 production, we think that MR14 is the major active fraction of pearl powder, and deduce that MR14 may become an effective drug to treat wounds.
Acknowledgements
The technical assistance of Professor Gao Jing (School of Pharmacy, Jiangsu University, Zhenjiang, China) is gratefully acknowledged.
Declaration of interest: The authors report no conflicts of interest.
References
- Bedouet L, Schuller MJ, Marin F, Milet C, Lopez E, Giraud M (2001): Soluble proteins of the nacre of the giant oyster Pinctada maxima and of the abalone Haliotis tuberculata: Extraction and partial analysis of nacre proteins. Comp Biochem Physiol B Biochem Mol Biol 128: 389–400.
- Cao G, Xu Z, Wei H, Yao S, Liu Y (1996): Pearl and mother-of-pearl powder in health-care. Chin Pharm J 21: 635–638.
- Dong Y, Shi JR (2006): Application of primary culture technique to traditional Chinese medicine research. Chin J Integr Med 4: 90–93.
- Edwards CA, O’Brien WD (1980): Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta 104: 161–167.
- Gibbs DF, Warner RL, Weiss SJ, Johnson KJ, Varani J (1999): Characterization of matrix metalloproteinases produced by rat alveolar macrophages. Am J Respir Cell Mol Biol 20: 1136–1144.
- Lamme EN, Van LRT, Beandsma K, Van MJ, Middelkoop E (2000): Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J Pathol 190: 595–603.
- Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC (2003): Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 162: 303–312.
- Mouries LP, Almeida MJ, Milet C, Berland S, Lopez E (2002): Bioactivity of nacre water-soluble organic matrix from the bivalve mollusk Pinctada maxima in three mammalian cell types: fibroblasts, bone marrow stromal cells and osteoblasts. Comp Biochem Physiol B Biochem Mol Biol 132: 217–229.
- O’Leary R, Rerek M, Wood EJ (2004): Fucoidan modulates the effect of transforming growth factor (TGF)-beta1 on fibroblast proliferation and wound repopulation in in vitro models of dermal wound repair. Biol Pharm Bull 27: 266–270.
- Rousseau M, Boulzaguet H, Biagianti J, Duplat D, Milet C, Lopez E, Bedouet L (2008): Low molecular weight molecules of oyster nacre induce mineralization of the MC3T3-E1 cells. J Biomed Mater Res A 85: 487–497.
- Ruan SB, Wu FH, Wu SS (2004): Clinical observation on treatment of 320 patients with relapsed aphthous ulcer by pearl aphthous granule. Chin J Integr Trad West Med 24: 254–255.
- Xu H, Huang K, Gao Q, Gao Z, Han X (2001): A study on the prevention and treatment of myopia with nacre on chicks. Pharmacol Res 44: 1–6.