Abstract
The ability of Alchornea cordifolia (Schum. and Thonn.) Müll. Arg. (Euphorbiaceae) leaves to inhibit human neutrophil elastase (HNE) and superoxide anion (O2•–) activities was evaluated on aqueous and ethyl acetate extracts as they allow for a targeted extraction of polyphenols. The direct effect of A. cordifolia extracts on HNE and O2•– was assessed in an acellular system. Results showed that extracts scavenge HNE and O2•– in a dose-dependent manner. Better activity was exhibited by the ethyl acetate extract with lower IC50 (2.2 and 4. 1 mg/L for HNE and O2•–, respectively) than for the aqueous extract. Cellular systems including isolated human polymorphonuclear neutrophils (PMN) were investigated to assess the effect of extracts on PMN metabolism. PMN were stimulated with 4β-phorbol-12-myristate-13-acetate (PMA), calcium ionophore (CaI), or N-formyl-methionyl-leucine-phenylalanine (fMLP), each stimulant having its own stimulation pathway. From the IC50 obtained, it can be concluded that A. cordifolia reduces HNE and O2•– liberation. Furthermore it was demonstrated that A. cordifolia extracts have no cytotoxic activity on PMN by measuring release of the cytosolic enzyme lactate dehydrogenase. As the ethyl acetate extract offers a higher rate of total phenols than the aqueous extract as well as better scavenging activity, it can be supposed that polyphenols, which are well known for their potent antioxidant and antielastase activity, are implicated in the activity of the plant. Phenolic substances such as quercetin, myricetin-3-glucopyranoside, myricetin-3-rhamnopyranoside, and proanthocyanidin A2 were identified in the ethyl acetate extract. In conclusion, the study provides proof of ethnomedical claims and partly explains the mechanisms of the anti-inflammatory action of A. cordifolia leaves.
Introduction
From Senegal to Uganda the leaves of Alchornea cordifolia (Schum. and Thonn.) Müll. Arg. (Euphorbiaceae) are successfully used by healers practicing traditional African medicine. The plant is used internally in the treatment of gastrointestinal disorders, malaria, and respiratory and urinary tract infections and externally as an anti-inflammatory in numerous ailments such as toothache, piles, arthritis, and rheumatism (CitationAdjanohoun, 1994; CitationAdjanohoun & Aké Assi, 1979; CitationIwu, 1983).
Research was done on the antielastase and antioxidant actions of this plant to try to elucidate its anti-inflammatory mechanism. Human neutrophil elastase (HNE) has a high affinity for cartilage tissue and can deteriorate major cartilage tissue components (CitationSiedle et al., 2007). The production of superoxide anion (O2•–) has been implicated in the pathology of a number of inflammatory conditions, including inflamed arthritic joints (CitationAfonso et al., 2007; CitationHenrotin et al., 2005). HNE and O2•– have therefore been the subject of intensive research to find inhibitors that target their destructive and proinflammatory action.
Neutrophils have a central role in inducing and regulating the inflammatory process. Cells have been shown to liberate potent proteinases such as elastase and generate some reactive oxygen species (ROS) such as O2•–. HNE, which is a serine protease primarily stored within granules in polymorphonuclear leukocytes, has been shown to deteriorate all the components in the extracellular matrix and also cleave the protein elastin, the connective tissue which is widely distributed in vertebrate tissue, and is particulary abundant in the lung, arteries, skin, and ligaments (CitationBode et al., 1989; CitationNenan et al., 2005). O2•– is transformed into a reactive secondary oxidant and will then damage intracellular macromolecules, oxidizing lipids, DNA, or proteins (CitationRoos, 1991). These reactive substances (ROS and HNE) can be beneficial, acting as defense mechanisms such as phagocytosis and in eradicating microorganisms, and they have an important role in cell signaling and in controlling the inflammatory process (CitationBieth, 1998; CitationWiedow et al., 1992). But in the case of overload, the production and liberation of HNE and O2•– may damage cells. HNE and O2•– are implicated in many illnesses related to inflammation: rheumatoid arthritis, pulmonary emphysema, chronic bronchitis, respiratory distress syndrome of adults (CitationChabot et al., 1998), atherosclerosis, cardiovascular diseases (CitationDhalla et al., 2000), glomerulonephritis, psoriasis, tumoral invasion, and cerebral ischemia (CitationChan, 2001).
Besides synthetic HNE and O2•– inhibitors, other drugs or human proteins such as albumin (CitationImai et al., 2003; CitationKouoh et al., 2002; CitationObayashi, 2005; CitationQuilan et al., 2004) and various plant compounds, namely phenolic compounds such as flavonoids and procyanidins, have been shown to possess inhibitory activity against HNE and O2•–. The aim of this study was to investigate the capacity of A. cordifolia to inactivate HNE and O2•– activity. Two extracts, ethyl acetate and aqueous, were tested to assess the contribution of polyphenols to the activity of the plant.
First, inhibition of HNE and O2•– was tested in an acellular model. Second, the effect of A. cordifolia on HNE and O2•– release was assessed in a cellular model. The stimuli used [4β-phorbol-12-myristate-13-acetate (PMA), N-formyl-methionyl-leucine-phenylalanine (fMLP), or calcium ionophore (CaI)] possess differing transductional mechanisms. PMA directly activates protein kinase C (PKC) which induces NADPH (reduced nicotinamide adenine dinucleotide phosphate)-oxidase activation, and so produces elastase. Another stimulus is fMLP, which activates phospholipase C and leads to the production of inositol triphosphate and diacylglycerol. These two mediators are responsible for PKC activation (CitationBaggliolini et al., 1993). The third and last stimulant used, CaI, induces calcium translocation by creating new pores in the cell membrane, causing calcium influx, followed by HNE and O2•– release.
Materials and methods
Plant material
Fresh leaves of Alchornea cordifolia (Schum. and Thonn.) Müll. Arg. (Euphorbiaceae), Herbarium N° 183, were harvested at the Floristic National Center of Abidjan and authenticated by an expert botanist (Professor Aké Assi, Department of Botany, University of Abidjan) to be identical to the sample in the Herbarium at the Center. Voucher specimens were deposited in the Botanical Department of the Centre National de Floristique at the Cocody University of Abidjan (Côte d’Ivoire). The leaves were collected and dried in a 25°C air-conditioned room.
Chemicals and biochemicals
PMA, fMLP, CaI, superoxide dismutase, methoxy- Suc-(Ala)2-Pro-Val-pNa, phenylmethylsulfonyl fluoride, ferricytochrome C, hypoxanthine, ethylenediaminetetraacetic acid (EDTA), KOH, xanthine oxidase, solvents, and chemical reagents were obtained from Sigma and elastase from Biosys SA.
Instrumental analysis
Ultraviolet (UV) absorbance was measured with a Kontron Uvikon 860 spectrophotometer. One-dimensional (1D) and 2D nuclear magnetic resonance (NMR) spectra were recorded in the Laboratoire d’Application de RMN (LARMN), at the University of Lille 2, on a Bruker Avance 300 MHz spectrometer, using tetramethylsilane (TMS) as an internal standard. Electrospray ionization-mass spectrometry (ESI-MS) was carried out on an API 3000 instrument (PerkinElmer Sciex), in the Laboratoire d’Application de Spectrométrie de Masse, at the University of Lille 2.
Preparation of ethyl acetate and aqueous extracts
Ethyl acetate extract
The dried leaves (50 g) were powdered and macerated for 24 h at a temperature of 4°C in 500 mL methanol/acetone/water (70:70:30, v/v/v). The filtrates were then low-pressure concentrated at 30°C. After centrifugation the aqueous phase was washed with dichloromethane to remove lipophilic pigments and extracted three times in ethyl acetate. The final phase was evaporated to dryness and the dried extract maintained at 4°C. For further assays this powder was dissolved in the appropriate solvents.
Aqueous extract
Powdered dried leaves (50 g) were extracted by maceration for 48 h in 500 mL distilled water. After filtration, the aqueous solution was evaporated to dryness and the dried extract was maintained at 4°C. A stock solution was obtained by dissolving small samples of the extract in water.
Isolation of pure compounds
The ethyl acetate extract of A. cordifolia was dissolved in H2O and submitted to column chromatography, using Sephadex LH-20, Silicagel 60H, and Silicagel 60, under nitrogen pressure with a step gradient (H2O–CH3OH), yielding four pure compounds. Compounds were identified by means of 1D (1H, 13C) and 2D NMR (correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC), heteronuclear multiple bond correlation (HMBC)) experiments and mass spectrometry.
Isolation of human polymorphonuclear neutrophils
Human polymorphonuclear neutrophils (PMN) were purified from fresh heparinized venous blood from healthy human subjects. Briefly, PMN were isolated by a gradient density technique over Ficoll Histopaque, followed by isotonic ammonium chloride hemolysis (CitationCabanis et al., 1994). Cells isolated in this way were viable as confirmed by the Trypan blue exclusion test, and cells were suspended in Hank’s balanced salt solution-HEPES (HBSS-H) pH 7.4 buffer.
Lactate dehydrogenase assay
The potential toxicity of A. cordifolia extracts toward PMN was measured by releasing the cytosolic enzyme lactate dehydrogenase (LDH) (CitationSrinivas et al., 1993). PMN (2 × 106/mL) were stimulated for 15 min by 160 nM PMA after 30 min incubation at 37°C with A. cordifolia extracts at different concentrations (from 0.1 to 1.0 g/L). After centrifugation and lysis of the cells by isotonic shock, LDH activities released by PMN were determined in the supernatant using a conversion rate of NADH (reduced nicotinamide adenine dinucleotide) to NAD by UV spectrophotometry at 340 nm in the presence of pyruvate.
Control PMN were not incubated with A. cordifolia leaf extracts, and control LDH activity released in the reaction mixture was determined after centrifugation in the supernatant (SRef). After lyzing control pellets by hypotonic shock and centrifuging, LDH activity (SLyzRef) was also measured as above.
The results are expressed as the percentage of LDH release by the chemical compounds as follows:
%P = Y × 100/X
where X = SRef LDH activity + SLyzRef LDH activity and Y = S LDH activity – SRef LDH activity, as previously reported (CitationDaels-Rakotoarison et al., 2003).
Dosage of human neutrophil elastase
Cell-free system
Neutrophils were incubated for 30 min at a concentration of 2 × 106/mL in HBSS-H buffer in the presence of PMA (1 μM). The tubes were then centrifuged at 4°C for 5 min in order to collect elastase from the supernatant; 400 IU of supernatant was added to 100 IU of increasing concentrations of plant extracts at 37°C for 15 min. After incubation, 400 IU of specific substrate (methoxy- Suc-(Ala)2-Pro-Val-pNa) was added to reaction mixtures in a final volume of 1 mL for 5 min. The reaction was stopped with phenylmethylsulfonyl fluoride (PMSF) (0.1 U/mL) and absorbance was measured at 405 nm in a spectrophotometer. The concentration of HNE was calculated from a standard curve using various concentrations of HNE. The results are expressed as the percentage of elastase inhibition (CitationKhalfi et al., 1996).
Cellular system
Neutrophils were incubated for 15 min at a concentration of 2 × 106/mL in HBSS-H buffer, pH 7.4, in the presence of increasing concentrations of plant extracts at 37°C. Then PMA (160 nmol/L), fMLP (1 μmol/L), or CaI (4 μmol/L) was added and the reaction mixtures incubated for 30 min at 37°C. After incubation, the tubes were centrifuged for 5 min at 4°C. Cell-free supernatants were assayed for elastase activity. Briefly, 400 μL supernatant was added to 400 μL specific substrate methoxy- Suc-(Ala)2-Pro-Val-pNa (0.5 mmol/L in 0.1 mol/L HEPES–0.5 mol/L NaCl pH 7.5 buffer) in a final volume of 1 mL. After 5 min incubation at 37°C, the reaction was stopped with PMSF and absorbance was measured at 405 nm. The elastase concentration was obtained from a standard curve using various concentrations of reagent grade elastase. Results are expressed as the percentage of elastase activity inhibited by plant extracts (CitationDaels-Rakotoarison et al., 2003).
Dosage of superoxide anion
Acellular model
Superoxide anion (O2•–) was generated by the hypoxanthine–xanthine oxidase system (CitationGressier et al., 1995). Reaction mixtures contained 50 μL of EDTA (30 mmol/L) in KOH (50 mmol/L), 5 μL of hypoxanthine in KOH (30 mM), 50 μL of ferricytochrome C (0.5 mmol/L), and 150 μL of A. cordifolia extracts (in concentrations ranging from 0.5 to 20 mg/L) in a final volume of 1.5 mL buffered in PBS, pH 7.4. The reaction was started by adding 100 μL of xanthine oxidase (1 U/mL) and the rate of reduced ferricytochrome C was measured at 550 nm against a reference cuvette.
The amount of O2•– generated was calculated using the Beer–Lambert law with an extinction coefficient ϵ550 = 2.1 × 10−2 μM−1 cm−1. The percentage of inhibited O2•– production by A. cordifolia extracts was then calculated and results expressed as IC50 values, as previously reported (CitationHennebelle et al., 2007).
Cellular model
O2•– production by human PMN stimulated by PMA (160 nmol/L), fMLP (1 μmol/L), or CaI (4 μmol/L) was measured by reducing ferricytochrome C (CitationCohen & Chovianiec, 1978). 400 μL of 2.5 × 106 PMN/mL stimulated by one of these three agents was incubated for 5 min at 37°C in the presence of increasing concentrations of A. cordifolia extracts and 0. 3 mg ferricytochrome C in HBSS-H buffer, pH 7.4. After centrifugation at 4°C, the absorbance of the supernatants was measured by UV spectrophotometry at 550 nm against a reference cuvette containing 310 U superoxide dismutase. The amount of O2•– was calculated as described for the acellular system (CitationDaels-Rakotoarison et al., 2002).
Statistical analysis
Results are expressed as mean ± SEM, and comparison of fraction values was established using the Wilcoxon test. Differences were considered to be statistically significant when p < 0.05.
Results
Cellular viability
The potential cytotoxicity of leaf extracts of A. cordifolia was tested at concentrations from 0.1 to 1.0 g/L. PMN viability, assessed by LDH liberation, was not modified after 1 h of incubation in the presence of ethyl acetate or aqueous extract of A. cordifolia.
Effect of Alchornea cordifolia extracts on human neutrophil elastase
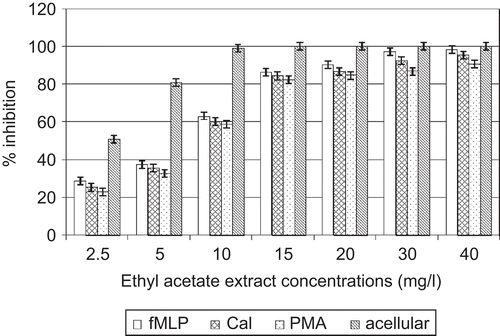
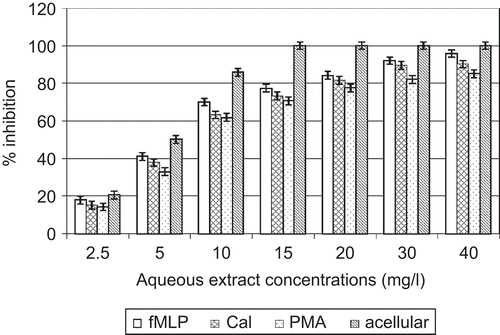
In the cell-free system, direct contact between neutrophil elastase and various concentrations of A. cordifolia extracts showed that they have the capacity to limit neutrophil elastase activity in a dose-dependent manner. IC50 values were 2.2 and 4. 7 mg/L for ethyl acetate extract and aqueous extract, respectively ( and ).
In the cellular experiment, A. cordifolia ethylacetate extracts decreased elastase activity with IC50 values of 8.6, 5.9, and 7. 4 mg/L when PMN were stimulated by PMA, fMLP, and CaI, respectively. A. cordifolia aqueous extracts decreased elastase activity with IC50 values of 12.1, 7.3, and 9. 2 mg/L when PMN were stimulated by PMA, fMLP, and CaI, respectively ( and ).
Effect of Alchornea cordifolia extracts on superoxide anion
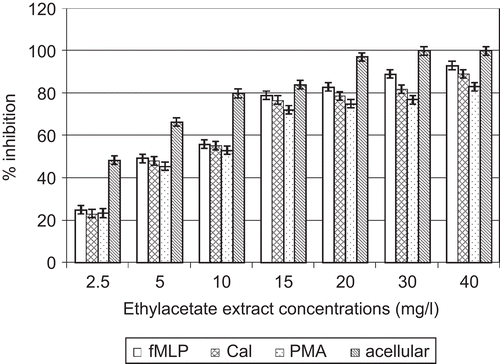
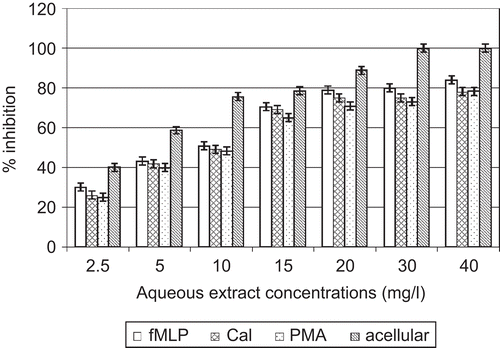
In acellular experiments, direct contact between O2•– and various concentrations of A. cordifolia extracts showed that each extract had the capacity to limit O2•– activity in a dose-dependent manner. IC50 values obtained were 4.1 and 5. 0 mg/L for ethyl acetate and aqueous extracts, respectively ( and ).
In the cellular experiment, A. cordifolia ethylacetate extracts decreased O2•– activity with IC50 values of 9.2, 6.5, and 6. 7 mg/L when PMN were stimulated by PMA, fMLP, and CaI, respectively. A. cordifolia aqueous extracts decreased O2•– activity with IC50 values of 13.4, 8.5, and 10. 3 mg/L when PMN were stimulated by PMA, fMLP, and CaI, respectively ( and ).
Structure of pure compounds
Four compounds were isolated from the A. cordifolia ethyl acetate extract. They were identified by MS and NMR spectroscopy and their spectral data compared with those of reference compounds. Three of them were flavonoids: one flavonol aglycone, quercetin (1), two myricetin glycosides, myricetin-3-glucopyranoside (2) and myricetin-3-rhamnopyranoside (3), and the fourth was a dimeric proanthocyanidin, proanthocyanidin A2 (4). These are all known compounds, but this is the first time that 2, 3, and 4 have been isolated from A. cordifolia leaves, 1 being previously identified in the leaves of this plant (CitationLamikanra et al., 1990; CitationOgungbamila & Samuelsson, 1990).
Discussion
The antioxidant and antielastase activities of A. cordifolia in acellular and cellular systems, with predominant activity in the acellular system, are of considerable interest. In the acellular system, A. cordifolia antagonizes HNE and O2•– added to the medium. It is therefore possible that A. cordifolia reacts directly with HNE and O2•–, either through a chemical effect (scavenging effect) or by an inhibition of HNE and O2•– activities.
In the cellular system, A. cordifolia extracts present no cytotoxic activity on PMN. Three stimuli were used to detect the ability of the plant to interfere with the liberation of HNE and other oxidants. These stimuli enhanced the exocytosis of oxidants and HNE by neutrophils through different pathways. But in the cellular system with PMA-, fMLP-, or CaI-stimulated human neutrophils, the activity was no better. On the contrary, these extracts were more active in the cell-free system. This action may be explained by a scavenging effect on the oxygen species (O2•–).
Comparing the aqueous and ethyl acetate extracts, the more active extract was the ethyl acetate extract, with higher contents of polyphenols and/or flavonoids. It is well known that there is a good correlation between antioxidant activity and total phenol level (CitationHatano et al., 1989).
Our results support the ability of A. cordifolia to reduce topical inflammation induced by croton oil on mouse ear (CitationMavar-Manga et al., 2004) and egg albumin on rat paw (CitationOsadebe & Okoye, 2003), and these studies showed a concentration of flavonoids related to anti-inflammatory activity.
Phenolics, such as flavonoids, have been reported to be direct HNE and oxidant inhibitors (CitationBlackburn et al., 1987; CitationJovanovic et al., 1994; CitationSartor et al., 2002). Compounds with a catecholic structural element, including two neighboring phenolic hydroxyl groups, showed remarkable activity which is significantly decreased by the methylation of one of the phenolic groups. Inhibitory activity may also be dependent on the double C-3/C-2 bond in the flavonoid C-ring, and on the additional presence of both 3- and 5-OH groups (CitationBombardelli & Morazzoni, 1993). The molecules identified in our study, quercetin, myricetin rhamnoside, and myricetin glucoside, are flavonoids with structures favorable to antioxidant and antielastase activities. Previous studies identified quercetin in the leaves of A. cordifolia (CitationLamikanra et al., 1990; CitationOgungbamila & Samuelsson, 1990), but this is the first to identify myricetin-3- glucopyranoside, myricetin-3-rhamnopyranoside, and proanthocyanidin A2 in A. cordifolia.
It is now considered worthwhile to screen plant extracts for elastase and oxidant inhibitors (CitationPerry et al., 1999; CitationSiedle et al., 2007). With such data, A. cordifolia leaves can be expected to have a protective effect against oxidants and elastase, acting through scavenging activity rather than inhibition of release.
These antioxidant and antielastase activities confirm traditional applications in topical inflammatory diseases, in the treatment of rheumatism, arthritis, chronic bronchitis, and other respiratory ailments, and in the effective healing of wounds and ulcers. The presence of flavonoids and proanthocyanidin surely testifies to the antioxidant and antielastase properties of A. cordifolia.
Acknowledgement
The authors wish to thank Professor Aké Assi for identifying the plant species.
Declaration of interest: The authors report no conflicts of interest.
References
- Adjanohoun EJ (1994): Fiche espèce: Alchornea cordifolia. Bull Med Trad Pharm 8: 203–213.
- Adjanohoun EJ, Aké Assi L (1979): Contribution au recensement des plantes médicinales en Côte d’Ivoire. Abidjan, Centre National de Floristique, pp. 41, 66, 56, 118, 205, 218.
- Afonso V, Champy R, Mitrovic D, Collin P (2007): Radicaux libres dérivés de l’oxygène et superoxydes dismutases: rôle dans les maladies rhumatismales. Rev Rhum Mal Osteoartic 74: 636–643.
- Baggliolini M, Boulay F, Badwey JA, Curnutte JT (1993): Activation of neutrophil leukocytes: chemoattractant receptors and respiratory burst. FASEB J 7: 1004–1010.
- Bieth JG (1998): Leucocyte elastase. In: Barret AJ, Rawlings ND, Woessner JF, eds., Handbook of Proteolytic Enzymes. London, Academic Press, pp. 54–60.
- Blackburn WD, Heck LW, Wallace RW (1987): The bioflavonoid quercetin inhibits neutrophil degranulation, superoxide production, and the phosphorylation of specific neutrophil proteins. Biochem Biophys Res Commun 144: 1229–1236.
- Bode W, Meyer E, Powers JC (1989): Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry 28: 1951–1963.
- Bombardelli E, Morazzoni P (1993): The flavonoids: new perspectives in biological activities and therapeutics. Chim Oggi 11: 25–27.
- Cabanis A, Gressier B, Lebegue S, Dine T, Luyckx M, Cazin M, Cazin JC (1994): A rapid density gradient technique for separating polymorphonuclear granulocytes. APMIS 102: 109–121.
- Chabot F, Mitchell JA, Guteridge CMM, Evans TW (1998): Reactive oxygen species in acute lung injury. Eur Respir J 11: 745–757.
- Chan PH (2001): Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cerebr Blood Flow Metab 21: 2–14.
- Cohen HJ, Chovianiec ME (1978): Superoxide generation by digitonin stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest 61: 1081–1087.
- Daels-Rakotoarison DA, Gressier B, Trotin F, Brunet C, Luyckx M, Dine T, Bailleul F, Cazin M, Cazin JC (2002): Effects of Rosa canina fruit extract on neutrophil respiratory burst. Phytother Res 16: 157–161.
- Daels-Rakotoarison DA, Kouakou G, Gressier B, Dine T, Brunet C, Luyckx M, Bailleul F, Trotin F (2003): Effects of caffeine-free Cola nitida nuts extract on elastase/α1 PI balance. J Ethnopharmacol 89: 143–150.
- Dhalla NS, Temsah RM, Netticadam T (2000): Role of oxidative stress in cardiovascular diseases. J Hypertens 18: 655–673.
- Gressier B, Cabanis A, Lebegue S, Brunet C, Dine T, Luyckx M, Cazin M, Cazin JC (1995): Scavenging of reactive oxygen species by letosteine, a molecule with two blocked-SH groups. Comparison with free-SH drugs. Pharm World Sci 17: 76–80.
- Hatano T, Edmatsu R, Hiramatsu M, Moti A, Fujita Y, Yasuhara T, Yoshida T, Okuda T (1989): Effects of the interaction of tannins and related polyphenols on superoxide anion radical and on 1,1 diphenyl 2 picrylhydrazyl radical. Chem Pharm Bull 37: 2016–2021.
- Hennebelle T, Sahpaz S, Gressier B, Joseph H, Bailleul F (2007): Antioxidant and neurosedative properties of polyphenols and iridoids from Lippia alba. Phytother Res 22: 256–258.
- Henrotin Y, Kurz B, Aigner T (2005): Oxygen and reactive oxygen species in cartilage degradation: Friends or foes? Osteoarthr Cartilage 13: 643–654.
- Imai H, Graham DI, Masayasu H, Macrae JM (2003): Antioxidant ebselen reduces oxidative damage in focal cerebral ischemia. Free Rad Biol Med 34: 56–63.
- Iwu MM (1983): Traditional Igbo Medicine. Nsukka, Institute of African Studies, University of Nigeria, p. 75.
- Jovanovic SV, Steenken S, Tosic M, Marjanovic B, Simic MG (1994): Flavonoids as antioxidants. J Am Chem Soc 116: 4846–4851.
- Khalfi F, Gressier B, Brunet C, Dine T, Luyckx M, Cazin M, Cazin JC (1996): Involvement of the extracellular calcium in the release of elastase and the human neutrophils oxidative burst. Cell Mol Biol 42: 1211–1218.
- Kouoh F, Gressier B, Dine T, Luyckx M, Brunet C, Ballester L, Cazin JC (2002): Antioxidant effects and antielastase activity of the calcium antagonist nicardipine on activated human and rabbit neutrophils. A potential antiatherosclerotic property of calcium antagonists? Cardiovasc Drug Ther 16: 515–520.
- Lamikanra A, Ogundani AO, Ogungbamila FO (1990): Antibacterial constituents of Alchornea cordifolia leaves. Phytother Res 4: 198–200.
- Mavar-Manga MH, Brkic D, Marie DEP, Quetin-Leclercq J (2004): In vivo anti-inflammatory activity of Alchornea cordifolia (Schmach. & Thonn.) Müll. Arg. (Euphorbiaceae). J Ethnopharmacol 92: 209–214.
- Nenan S, Boichot E, Lagente V, Bertrand CP (2005): Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem Inst Oswaldo Cruz 100 (Suppl. 1): 167–172.
- Obayashi H (2005): Current synthetic inhibitors of human neutrophil elastase in 2005. Expert Opin Ther Pat 15: 759–771.
- Ogungbamila FO, Samuelsson G (1990): Smooth muscle relaxing flavonoids from Alchornea cordifolia. Acta Pharm Nord 2: 421–422.
- Osadebe PO, Okoye EC (2003): Anti-inflammatory effects of crude methanolic extract and fractions of Alchornea cordifolia leaves. J Ethnopharmacol 89: 19–24.
- Perry EK, Pickering AT, Wang WW, Houghton PJ, Perry NS (1999): Medicinal plants and Alzheimer’s disease: from ethnobotany to phytotherapy. J Pharm Pharmacol 51: 527–534.
- Quilan GJ, Mumby S, Martin GS, Bernard GR, Gutteridge JM, Evans TW (2004): Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Crit Care Med 32: 755–759.
- Roos D (1991): The respiratory burst of phagocytic leukocytes. Drug Invest 3: 48–58.
- Sartor L, Pezzato E, Dell’Aica I, Caniato R, Biggin S, Garbisa S (2002): Inhibition of matrix-proteases by polyphenols: chemical insights for anti-inflammatory and anti-invasion drug design. Biochem Pharmacol 64: 229–237.
- Siedle B, Hrenn A, Merfort I (2007): Natural compounds as inhibitors of human neutrophil elastase. Planta Med 73: 401–420.
- Srinivas VC, Habibullah CM, Ayesha Q, Hassan SI, Khaleel VR, Rehman K, Moshins S (1993): Lactate dehydrogenase: a marker of cellular integrity. Meth Find Exp Clin Pharmacol 15: 709–713.
- Wiedow O, Wiese F, Streit V, Kalm C, Christophers E (1992): Lesional elastase activity in psoriasis, contact dermatitis and atopic dermatitis. J Invest Dermatol 99: 306–309.