Abstract
Physalis alkekengi L. (Solanaceae) is a popular plant in traditional European and Chinese folk medicine, and it has been reported to have many ethnopharmacological properties including antifungal, anti-cough, anti-inflammatory, analgesic, and febricide activities. Some active components from Physalis species have been investigated. However, no antimicrobial activity studies on extracts and physalins of P. alkekengi have been carried out. In this study, we attempted to identify the possible antimicrobial activities of the methanol extract from aerial parts of P. alkekengi and the dichloromethane extract from calyces of the plant. The extracts were tested against five Gram-positive and five Gram-negative bacteria and five Candida species by using disk diffusion and broth microdilution methods. The extracts were fractionated to isolate physalins using chromatographic techniques, and physalin D was isolated from the extracts. The structure of the compound was elucidated on the basis of 1H-NMR spectroscopic study, and confirmed by comparison with a reference sample and literature data. Results indicated that all the extracts and physalin D were characterized by antibacterial action, especially against Gram-positive bacteria, with MIC values between 32 and 128 μg/mL. The methanol extract had moderate activity against fungi at MICs ranging from 128 to 512 μg/mL, but the dichloromethane extract and physalin D had low activity against fungi at MICs ranging from 256 to 512 μg/mL. Additionally, the antioxidant activity of physalin D was evaluated by qualitative DPPH (1,1-diphenyl-2-picrylhydrazyl) radical and TBA (thiobarbituric acid) assays. Physalin D showed low antioxidant activity with an IC50 value of ≥ 10 ± 2.1.
Introduction
The genus Physalis, which belongs to the family Solanaceae, includes about 120 species mainly distributed in Mexico, Europe, and Southeast and Central Asia (CitationAhmad et al., 1999; CitationPerez-Castorena et al., 2004). Some of them (i.e. P. peruviana L., P. philadelphica Lam., P. angulata L., and P. alkekengi L.) are used as food as well as in popular medicine. P. peruviana, commonly known as “cape gooseberry” or “goldenberry,” is a tropical plant bearing orange edible fruits. The plant has been used in East and Southeast Asian folk medicine for treating cancer, leukemia, hepatitis, rheumatism, and other diseases (CitationAhmad et al., 1999; CitationRamadan & Mörsel, 2003; CitationWu et al., 2004). P. philadelphica has been used for the treatment of gastrointestinal disorders in Guatemala, and for treating leprosy, purifying the blood, and as a poison antidote in Mexico. The fruits of P. philadelphica, known as tomatillos, are used as an ingredient in foods such as sauces and relishes in place of tomatoes in North America (CitationSu et al., 2002). P. angulata is an annual herb indigenous to many parts of the tropics and subtropics, including Taiwan. Extracts from this plant have been used in traditional medicine in Taiwan to treat malaria, hepatitis, rheumatism, liver problems, and tumors (CitationBastos et al., 2006; CitationHsieh et al., 2006; CitationHe et al., 2007). P. alkekengi, known as “Winter cherry,” is used in Chinese medicine as an expectorant, antitussive, diuretic, and oxytocic agent. Use of the fruits of P. alkekengi as an analgesic, antibacterial, and contraceptive drug in folk medicine has been reported (CitationVessal et al., 1991; CitationBasey et al., 1992). The Physalis genus is represented by one species in Turkey. This species is P. alkekengi L., which grows in South, North, and Central Anatolia (CitationBaytop, 1978). The plant has been used as a diuretic, antipyretic, and sedative in Turkish folk medicine (CitationBaytop, 1999).
Physalis species are known to contain 16,24-cyclo-13, 14-secosteroids called physalins. CitationMatsuura et al. (1970) reported the isolation and structural determination of physalin A as a bitter principle of P. alkekengi var. francheti. The presence of physalins B and C in the same plant was also reported (CitationKawai & Matsuura, 1970). P. alkekengi var. francheti has led to the identification of physalins A–C, physalins L–S, and physalin T (CitationKawai & Matsuura, 1970; CitationMatsuura et al., 1970; CitationKawai et al., 1987, Citation1992, Citation2001; Makino et al., Citation1995a). More than a dozen physalins (from physalin C to physalin T) have been isolated from Japanese and Indian Physalis species (CitationRow et al., 1978, Citation1980; CitationKawai et al., 1987, Citation1992, Citation1995, Citation2001; Makino et al., Citation1995a, Citation1995b), but P. alkekengi originating from Turkey has not been previously investigated. Additionally, alkaloids, flavonoids, volatile compounds, carotenoids and inorganic elements have also been determined in different parts of the plant as minor metabolites (CitationBasey et al., 1992; CitationRamadan & Mörsel, 2003; CitationPerez-Castorena et al., 2004).
Previous studies have focused on the isolation and characterization of secondary metabolites, whereas it has been demonstrated that some of the extracts or active principles obtained from Physalis species have a broad spectrum of biological activities, including antileukemic, antitumor, immunomodulator, cytotoxic, antimycobacterial, antimicrobial, molluscicidal, antihepatoma, and antinociceptive activities in recent studies (Chiang et al., Citation1992a, Citation1992b; CitationJanuário et al., 2002; CitationShim et al., 2002; CitationSu et al., 2002; CitationDos Santos et al., 2003; CitationSoares et al., 2003; CitationWu et al., 2004; CitationChoudhary et al., 2005; CitationLee & Houghton, 2005; CitationSilva et al., 2005; CitationBastos et al., 2006; CitationMagalhaes et al., 2006; CitationDamu et al., 2007; CitationCastro et al., 2008). It has been previously reported that the crude extract (i.e. methanol/water, ethanol, or aqueous extract) or physalin-containing fractions obtained from P. angulata aerial parts possess antibacterial, antimycobacterial, and antigonorrheal activities, which can be related to the presence of physalin compounds of the extracts (CitationCáceres et al., 1995; CitationJanuário et al., 2002; CitationSilva et al., 2005). Nevertheless, there is no information about antibacterial and antifungal properties of other Physalis species, including P. alkekengi. Therefore, the crude methanol extract from P. alkekengi aerial parts and the dichloromethane fraction of the aqueous extract from calyces of the plant were prepared. The extracts have been bioassayed for antibacterial and antifungal activities. A compound, physalin D, was isolated from the extracts, and then physalin D was evaluated to correlate possible antimicrobial activity of this plant. In addition, the antioxidant activity of physalin D was examined.
Materials and methods
General experimental procedures
All 1H-nuclear magnetic resonance (NMR) spectra of the isolated compounds were recorded in dimethylsulfoxide (DMSO)-d6 using trimethylsilane (TMS) as internal standard at 600 MHz using a Varian Unity-plus 600 spectrometer. Vacuum liquid chromatography was performed on silica gel 60 (0.040–0.063 mm). Thin layer chromatography (TLC) was carried out on precoated silica gel 60 F254 (0.2 mm thick plates). The solvent systems were hexane–EtOAc (8:2; 6:4; 3:7; 4:6; 2:8). The detection reagents added were cerium sulfate–sulfuric acid or 10% H2SO4 in methanol followed by heating at 105°C. The sample isolated from P. alkekengi var. francheti was used as the authentic compound for physalin D. For the thiobarbituric acid (TBA) test, absorbance at 532 nm was measured by a Shimadzu UV-1601 UV/VIS (ultraviolet-visible) spectrophotometer.
Plant material
Aerial parts of P. alkekengi were collected from southern Turkey (C5: Içel, Çamlıyayla, near Tarsus) in August, 2003. The plant was identified by Dr. A. Everest (Department of Biology, Faculty of Arts and Sciences, University of Mersin, Mersin, Turkey) and a voucher specimen (AEF 23175) was deposited at the Herbarium of the Faculty of Pharmacy, Ankara University, Ankara, Turkey.
Extraction and isolation procedures
Air-dried leaves and stems of P. alkekengi (1300 g) were ground into a powder and extracted three times with methanol for 3 days at 40°C and filtered. The combined filtrates were concentrated to dryness by rotary evaporation at 45°C, yielding 7.6% (w/w). The extract was dissolved in a mixture of MeOH and H2O (1:1) and then partitioned successively with hexane and diethyl ether. The diethyl ether fraction was concentrated to dryness (58 g) by rotary evaporation at 40°C. Fractionation of the diethyl ether extract using vacuum liquid chromatography (VLC) (silica gel; Merck), eluting with mixtures of CH2Cl2–MeOH (from 100:1 to 1:1), gave 15 fractions (F01–F15). After monitoring with TLC, F05–F09 were combined. These fractions were rechromatographed on silica gel by VLC and eluted with gradient mixtures of petroleum ether–acetone (from 3:1 to 1:1) to give 11 subfractions (F050901–F050911). Subfractions F050903–F050906 were combined and chromatographed over a silica gel column, eluting with gradient mixtures of petroleum ether–EtOAc–MeOH (from 10:10:1 to 10:10:3), to give 13 subfractions. Compound 1 (53 mg) was obtained as a white amorphous solid from the subfractions F1–F4 (eluted with petroleum ether–EtOAc–MeOH, 10:10:1).
Powdered calyces of P. alkekengi (210 g) were extracted three times with water by heating from ambient temperature to 90°C and filtered. The combined filtrates were extracted with CH2Cl2 (4 × 400 mL). The CH2Cl2 extract was concentrated using a rotary evaporator at a maximum temperature of 45°C, yielding 3.7% (w/w). This extraction procedure from the calyces of the plant was carried out according to the method previously described by CitationDinan et al. (1997). The dichloromethane extract was subjected to VLC using silica gel, eluting with hexane–EtOAc and EtOAc–MeOH in increasing polarity to give 64 subfractions (C01–C64). C35 and C36 subfractions were purified by preperative TLC, and developed with hexane–EtOAc (2:8) to afford compund 2 (21.2 mg).
Antimicrobial assays
Test microorganisms
Staphylococcus aureus ATCC 25923, S. epidermidis ATCC 12228, Enterococcus faecalis ATCC 29212, Bacillus subtilis ATCC 6633, B. cereus ATCC 14579, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Enterobacter cloacae ATCC 13047, Klebsiella pneumoniae ATCC 10031, Proteus vulgaris ATCC 13315, Candida albicans ATCC 90028, C. krusei ATCC 6258, C. glabrata ATCC 32554, C. parapsilosis ATCC 22019, and C. tropicalis ATCC 20336 were used as test microorganisms. These microorganisms were obtained from the Refik Saydam Hifzisihha Institute, Ankara, Turkey, and the Microbiology Culture Collection of Inonu University, Malatya, Turkey.
Antibacterial activity was determined by using Mueller–Hinton agar and Mueller–Hinton broth, obtained from Difco, Detroit, USA. Antifungal activity was determined by using Sabouraud dextrose agar and Sabouraud dextrose broth, also obtained from Difco.
Antibacterial and antifungal assays
Antimicrobial activity was determined using the disk diffusion assay (CitationAndrews, 2001). Petri plates were prepared with Mueller–Hinton agar for bacteria and Sabouraud dextrose agar for fungi. Colonies of the test organisms were suspended directly in a small volume of 0.9% saline and further diluted until turbidity matched the McFarland standard No. 0.5. These microbial suspensions were inoculated onto Mueller–Hinton agar and Sabouraud dextrose agar plates. Sterile filter paper disks soaked in known concentrations of extracts in DMSO were applied over each of the culture plates previously seeded with the 0.5 McFarland cultures of microorganisms. The tests were conducted at three different concentrations of the methanol extract of the aerial parts of P. alkekengi (100, 250, and 500 μg/mL per disk), two different concentrations of the dichloromethane fraction of the aqueous extract of the calyces (100 and 250 μg/mL per disk), and one concentration of physalin D (100 μg/mL per disk). Blank disks (6 mm, sterile blank; Difco) impregnated with DMSO were used as negative controls, and disks of amikacin (Oxoid, England) (30 μg/mL) and fluconazole (Oxoid, England) (100 μg of fluconazole in a paper disk) were used. A total of 100 μg of fluconazole, by applying 20 μL of a 5 mg/mL fluconazole solution in sterile water in a paper disk, was used as positive control in the antimicrobial assay. The plates were incubated overnight at 37°C for bacteria and 25°C for yeasts. The inhibition zones (mm) were measured. Each experiment was repeated at least three times and the mean of the diameter of the inhibition zones was calculated.
Determination of minimal inhibitory concentration
The minimal inhibitory concentrations (MICs) of the extracts and physalin D were determined by the broth microdilution method following procedures recommended by the CitationClinical Laboratory Standards Institute (2008). MICs of the extracts were determined in Mueller–Hinton broth for bacteria and in Sabouraud dextrose broth for fungi, at pH 7.4. The microorganisms were grown overnight in Mueller–Hinton broth at 37 ± 1°C, and the final inoculum size was 105 cfu/mL for the antibacterial assay. The yeasts were maintained in Sabouraud dextrose broth after incubation for 24 h at 25 ± 1°C. The final inoculum size was 104 cfu/mL for the antifungal assay. A set of tubes containing only inoculated broth were kept as controls. The two-fold serial dilution technique was applied.
The extracts were dissolved in DMSO to obtain stock solutions and then diluted in Mueller–Hinton broth for bacteria and Sabouraud dextrose broth for fungi, to give an initial concentration of 8 mg/mL. Further dilutions of the extracts and standard drugs in the test medium were prepared at the required quantities of 800, 400, 200, 100, 75, 50, 25, 12.5, and 6.25 μg/mL concentrations. In order to ensure that the solvents had no effect on microbial growth, a control test was also performed containing inoculated broth supplemented with DMSO at the same dilutions used in the experiments and found to be inactive in culture medium.
The culture tubes were incubated at 37 ± 1°C for 24 h (bacteria), and 25 ± 1°C for 48 h (yeasts). The MIC (μg/mL) was determined as the lowest concentration of the extract and the compound that did not show any visible growth when compared with control tubes. Each MIC experiment was repeated twice in order to define the MIC value.
Cytotoxicity assay
In this study, the HEp-2 continuous cell line (HEp-2 cell line no: ATCC CCL23) was used to evaluate the effects of P. alkekengi. The HEp-2 cells were obtained from the Department of Virology, Veterinary Faculty, Ankara University. In preparation of the cell cultures, EMEM (Eagle’s minimum essential medium) was used as the medium, with fetal bovine serum (Seromed) at a ratio of 10% as the growth factor. Incubation of the cells was performed in an atmosphere of 5% carbon dioxide at 37°C.
To evaluate the effects of the extracts of P. alkekengi on HEp-2 cells, they were infected with the extracts, and control cells (HEp-2 cells not infected with plant extracts) were also observed. In order to test the effects of the extracts on HEp-2 cells, 5 × 104 cells were seeded into each well of 12-well plates, cultured for 6 h at 28°C, and allowed to grow for an additional 48 h in the presence of increasing amounts of the extracts at 0.5, 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1024, and 2048 μg/mL. The cytotoxicity of the extracts was determined by a conventional hemocytometer using the trypan blue exclusion method (CitationBorenfreund & Puerner, 1984). The highest non- cytocidal (on HEp-2 cells) concentration of the extracts was determined to be 1024 μg/mL. The non-toxic concentration was determined to be up to 1024 μg/mL. We used these concentrations in all of the experiments to test the effects of the extracts on bacterial growth; therefore, a concentration of up to 1024 μg/mL of the extracts was used for the determination of antimicrobial activities (CitationBorenfreund & Puerner, 1984).
Antioxidant activity assays
The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical assay was used as a rapid TLC screening method to evaluate the antioxidant activity of physalin D isolated from the extracts due to free-radical scavenging. The method has been fully described previously (CitationGüvenç et al., 2005). In brief, using Wiretrol II micropipettes, 2 μL of a 1 mg/mL MeOH solution of physalin D was applied to a silica gel TLC plate (Merck, Darmstadt, Germany), and sprayed with 0.2% DPPH• solution in EtOH, left at 20°C, and examined 30 min after spraying.
The in vitro antioxidant activity test was carried out by lipid peroxidation of liposomes, where TBA (thiobarbituric acid) was used to assess the efficacy of the compounds to protect liposomes from lipid peroxidation. The method has also been described previously (CitationHalliwell & Chirico, 1993; CitationGüvenç et al., 2005). Physalin D was prepared in MeOH at seven different concentrations (1; 0.02; 0.04; 0.008; 0.0016; 0.00032; 0.000064 mg/mL). For the test reaction of the physalin D, a mixture of liposomes (brain extract, Sigma B 3635), FeCl3 (Sigma F 1513), ascorbic acid (Aldrich 255564), and PBS (phosphate buffered saline; Sigma P 4417) were used. All of the tubes were incubated at 37°C for 20 min. After that, the TBA test was performed by adding butylated hydroxytoluene (Sigma B 1378) in ethanol followed by TBA (Sigma T 5500) in NaOH (Merck 6462) and HCl (Merck 314). The tubes were heated to 90°C for 30 min and then allowed to cool completely. The chromogen was extracted by n-butanol (Merck 00988). The mixture was vortexed to ensure complete extraction of the chromogen and then centrifuged at 3500 rpm for 15 min at room temperature in order to separate the two layers. The absorbance of the upper layer, which contained the chromogen, was determined by a Shimadzu UV-1601 UV/VIS spectrophotometer at 532 nm. The calculation of percentage inhibition of lipid peroxidation was done by comparing the absorbance of the full reaction mixture with that of the extract test reaction mixtures where the substance to be assessed was included. Propyl gallate was used as the reference compound in the same concentration range (1–6.4 × 10−5 mg/mL). Four replicate experiments were performed for the compound. The half-maximal inhibitory concentrations (IC50) of physalin D and propyl gallate were calculated by linear regression analysis.
Results and discussion
The methanol extract from the aerial parts of P. alkekengi was fractionated with hexane, and diethyl ether, respectively. The diethyl ether fraction was fractionated by silica gel VLC and the fractions were monitored by TLC. The fractions F05–F09 were rechromatographed to produce subfractions. Subfractions F050903–F050906 were refractionated using silica gel VLC and compound 1 was isolated as a pure substance from the fractions F1–F4. Compound 1 was identified as physalin D by comparison of its 1H-NMR spectral data () with those reported in the literature (CitationKawai et al., 2001). Fractionation using silica gel VLC of the dichloromethane phase obtained from the aqueous extract of P. alkekengi calyces yielded compound 2. The 1H-NMR spectrum of compound 2 confirmed the structure as physalin D. Compound 1 and compound 2 were also identified by their identical chromatographic behavior (the solvent system was hexane–EtOAc, 3:7) with that of authentic physalin D.
Table 1. Comparison between experimental data and authentic physalin D: 600 MHz 1H-NMR spectral data for physalin D in DMSO-d6 (chemical shift δ ppm, spin multiplicity, and coupling constant/Hz in parentheses).
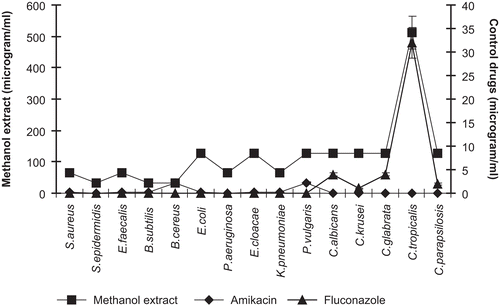
In this study, the antimicrobial activity of the extracts of P. alkekengi aerial parts and calyces and physalin D has been evaluated in vitro against Gram-positive and Gram-negative bacteria and yeast-like fungi. The study showed that the methanol extract of the aerial parts of P. alkekengi had an antibacterial effect against tested bacteria at different rates (). This extract showed a significant zone of inhibition against Gram-positive bacteria, especially B. cereus, B. subtilis, and E. faecalis with inhibition zones ranging from 28.2 ± 1.8 to 31.8 ± 1.9 at 500 μg/mL/disk. The inhibition zones against B. cereus and E. faecalis were larger than those of amikasin. The methanol extract inhibited the growth of Gram-negative bacteria, especially P. aeruginosa, K. pneumoniae, and E. coli with inhibition zones ranging from 14.6 ± 1.1 to 16.6 ± 3.2 at 500 μg/mL/disk. The extract exhibited a moderate effect on the tested fungi, producing a zone diameter of inhibition from 9.6 ± 2.2 to 14.2 ± 0.8 mm, depending on the susceptibility of the fungi. The inhibition zones were smaller than those of fluconazole.
Table 2. Antimicrobial activity of methanol extract and physalin D obtained from aerial parts of P. alkekengi.
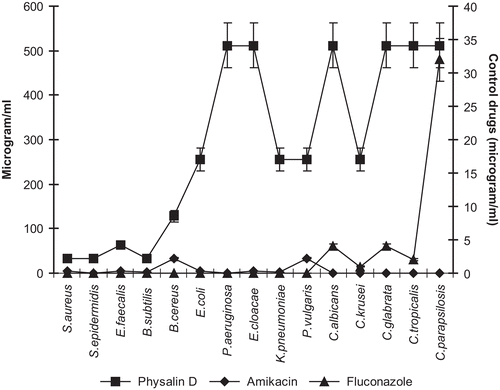
Physalin D isolated from the methanol extract was found to be active against Gram-positive bacteria as well as the methanol extract with the same concentration (100 μg/mL/disk) (). In addition, physalin D was not effective on Gram-negative bacteria and fungi.
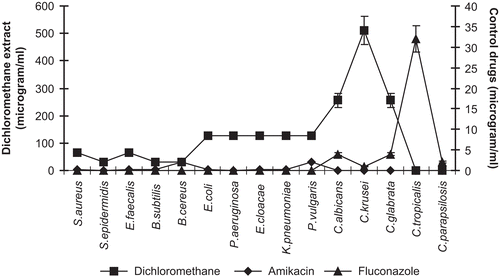
All the microorganisms were found to be less susceptible to the dichloromethane extract from the calyces of P. alkekengi than the methanol extract of the plant. As can be seen from , the dichloromethane extract showed a moderate effect on Gram-positive bacteria such as B. cereus, B. subtilis, S. epidermidis, S. aureus, and E. faecalis and Gram-negative bacteria such as P. aeruginosa and E. cloacae. This extract did not show any significant activity against all tested fungi.
Table 3. Antimicrobial activity of dichloromethane fraction of aqueous extract of calyces of P. alkekengi.
Comparing the two extracts used for the present study, the methanol extract showed a higher antibacterial activity than that of the dichloromethane extract. The tested extracts showed MIC values of 32–128 μg/mL in tests with bacteria while the values recorded were 128–512 μg/mL in the antifungal assay ( and and ). On the other hand, the MIC values obtained in antibacterial assays using physalin D were 32–128 μg/mL against Gram-positive bacteria, while the values recorded in the antifungal assays were 256–512 μg/mL ( and ).
Table 4. Minimum inhibitory concentration (μg/mL) of dichloromethane and methanol extracts of P. alkekengi, and physalin D obtained from aerial parts of P. alkekengi.
Results of the DPPH• test demonstrated that physalin D had free radical-quenching activity. In the TBA test, the antioxidant activity of physalin D was determined. The result showed that physalin D had a low antioxidant activity, with an IC50 value of (μg/mL) ≥ 10 ± 2.1, as compared with the positive control propyl gallate (). In the literature, there is no report about the antioxidant activity of physalins or the extracts of Physalis species. Our report is the first for physalin D.
Table 5. Antioxidant activity of physalin D obtained from aerial parts of P. alkekengi.
Previous phytochemical studies on the aerial parts of Physalis plants have led to the identification of physalins, which are believed to be the bioactive compounds of the genus Physalis (CitationJanuário et al., 2002; CitationDos Santos et al., 2003; CitationWu et al., 2004; CitationChoudhary et al., 2005; CitationLee & Houghton, 2005; CitationMagalhaes et al., 2006; CitationDamu et al., 2007; CitationCastro et al., 2008). However, there is relatively little information in the literature regarding the steroid content of the calyces of Physalis species (CitationDinan et al., 1997; CitationQiu et al., 2008). In the present study, physalin D was isolated from the extracts of P. alkekengi. The presence of this compound in the aerial part of P. alkekengi is being reported here for the first time, whereas physalin D has been previously isolated from the aerial parts of P. minima, P. angulata, and P. lancifolia (CitationRow et al., 1978, Citation1980; CitationKawai et al., 1987, Citation2001; Makino et al., Citation1995a). The isolation of physalin D from the calyces of the plant is consistent with the results reported by CitationQiu et al. (2008).
According to our results, the methanol extract of P. alkekengi has strong antibacterial activity against Gram-positive bacteria. Also, physalin D (100 μg/mL) inhibited S. epidermidis, E. faecalis, S. aureus, and B. subtilis, as well as the methanol extract with the same concentration. Therefore, physalin D may be considered mainly responsible for the antibacterial activity. Similar to our results, CitationSilva et al. (2005) reported that physalin B and the fraction of physalins containing physalins B, D, F, and G inhibited different S. aureus strains and N. gonorrhoeae. Additionally, antimicrobial activity was described by several authors for methanol/ethanol or aqueous extracts of P. angulata and P. philadelphica against some Gram-positive and Gram-negative bacteria (CitationCáceres et al., 1991; CitationShim et al., 2002). The present antimicrobial activity study on P. alkekengi verified that physalins are the main bioactive compounds of Physalis species.
Concerning the present antioxidant activity study, physalin D has low activity as compared with the positive control propyl gallate. As far as we know, there is a lack of studies on the antioxidant activity of physalin D and other physalins.
Conclusion
We can conclude that the methanol extract presents a good action potential against Gram-positive bacteria. Also, physalin D isolated from the extracts of P. alkekengi has remarkable antibacterial activity against especially Gram-positive bacteria. The presence of physalin D in the extracts suggests that it could play an important role in antibacterial activity. Physalin D, an ergostane type polyoxyfunctional lipophilic structure, could be associated with the mechanisms of antibacterial activity. Further comparative antimicrobial activity studies of different physalins as well as physalin D will help to elucidate the roles played by the functional groups of physalins in the activity.
Acknowledgements
The financial support of the research fund of the University of Mersin (project number: BAP-SBE EMB (SH) 2003-2YL) is gratefully acknowledged. The authors thank Assoc. Prof. Ayşe Everest (Department of Biology, Faculty of Arts and Sciences, University of Mersin, Mersin, Turkey) for identification of the plant material. The authors are grateful to Prof. H. Yamamura, Graduate School of Engineering, Nagoya Institute of Technology, for 1H-NMR measurements.
Declaration of interest: The authors report no conflicts of interest.
References
- Ahmad S, Malik A, Yasmin R, Ulah N, Gul W, Khan PM, Nawaz HR, Afza N (1999): Withanolides from Physalis peruviana. Phytochemistry 50: 647–651.
- Andrews JM (2001): BSAC standardized disc susceptibility testing method. J Antimicrob Chemother 48: 43–57.
- Basey K, McGaw BA, Woolley JG (1992): Phygrine, an alkaloid from Physalis species. Phytochemistry 31: 4173–4176.
- Bastos GNT, Santos ARS, Ferreira VMM, Costa AMR, Bispo CI, Silveira AJA, Do Nascimento JLM (2006): Antinociceptive effect of the aqueous extract obtained from roots of Physalis angulata L. on mice. J Ethnopharmacol 103: 241–245.
- Baytop A (1978): Physalis L. In: Davis PH, ed., Flora of Turkey and the East Aegean Islands, Vol. 6. Edinburgh, The Edinburgh University Press, pp. 444.
- Baytop T (1999): Therapy with Medicinal Plants in Turkey (Past and Present). Istanbul, Nobel Medical Publishers, pp. 216.
- Borenfreund E, Puerner, JA (1984): A simple quantitative procedure using monolayer cultures for cytotoxicity assays. J Tissue Cult Methods 9: 7–9.
- Cáceres A, Alvarez AV, Ovando AE, Samayoa BE (1991): Plants used in Guatemala for the treatment of respiratory diseases. 1. Screening of 68 plants against gram-positive bacteria. J Ethnopharmacol 31: 193–208.
- Cáceres A, Ménendez H, Méndez E, Cohobón E, Samayoa BE, Jauregui E, Peralta E, Carrillo G (1995): Antigonorrheal activity of plants used in Guatemala for the treatment of sexually transmitted diseases. J Etnopharmacol 48: 85–88.
- Castro DP, Figueiredo MB, Ribeiro IM, Tomassini TCB, Azambuja P, Garcia ES (2008): Immune depression in Rhodnius prolixus by secosteroids, physalins. J Insect Physiol 54: 552–562.
- Chiang HC, Jaw SM, Chen CF, Kan WS (1992a): Antitumor agent, Physalin F from Physalis angulata L. Anticancer Res 12: 837–844.
- Chiang HC, Jaw SM, Chen PM (1992b): Inhibitory effects of physalin B and physalin F on various human leukemia cells in vitro. Anticancer Res 12: 1155–1162.
- Choudhary MI, Yousaf S, Ahmed S, Yasmeen K, Rahman A (2005): Antileishmanial physalins from Physalis minima. Chem Biodivers 2: 1164–1173.
- Clinical Laboratory Standards Institute (2008): Performance Standards for Antimicrobial Susceptibility Testing; Ninth Informational Supplement. NCCLS document M100-S9. Wayne, PA, NCCLS.
- Damu AG, Kuo PC, Su CR, Kuo TH, Chen TH, Bastow KF, Lee KH, Wu TS (2007): Isolation, structures, and structure-cytotoxic activity relationships of withanolides and physalins from Physalis angulata. J Nat Prod 70: 1146–1152.
- Dinan LN, Sarker SD, Sik V (1997): 28-Hydroxywithanolide E from Physalis peruviana. Phytochemistry 44: 509–512.
- Dos Santos JAA, Tomassini TCB, Xavier DCD, Ribeiro IM, Da Silva MTG, De Morais Filho ZB (2003): Molluscusidal activity of Physalis angulata L. extracts and fractions on Biomphalaria tenagophila under laboratory conditions. Mem Inst Oswaldo Cruz 98: 425–428.
- Güvenç A, Houghton PJ, Duman H, Coşkun M, şahin P (2005): Antioxidant activity studies on selected Sideritis species native to Turkey. Pharm Biol 43: 173–177.
- Halliwell B, Chirico S (1993): Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57: 715–725.
- He QP, Ma L, Luo JY, He FY, Lou LG, Hu LH (2007): Cytotoxic withanolides from Physalis angulata L. Chem Biodivers 4: 443–449.
- Hsieh WT, Huang KY, Lin HY, Chung JG (2006): Physalis angulata induced G2/M phase arrest in human breast cancer cells. Food Chem Toxicol 44: 974–983.
- Januário AH, Rodrigues FE, Pietro RCLR, Kashima S, Sato DN, Franca SC (2002): Antimycobacterial Physalins from Physalis angulata L. Phytother Res 16: 445–448.
- Kawai M, Matsuura T (1970): The structure of physalin C, a bitter principle of Physalis alkekengi var. francheti. Tetrahedron 26: 1743–1745.
- Kawai M, Matsuura T, Kyuno S, Matsuki H, Takenaka M, Katsuoka T, Butsugan Y, Saito K (1987): A new physalin from Physalis alkekengi. Structure of physalin L. Phytochemistry 26: 3313–3317.
- Kawai M, Ogura T, Makino B, Matsumoto A, Yamamura H, Butsugan Y, Hayashi M (1992): Physalins N and O from Physalis alkekengi. Phytochemistry 31: 4299–4302.
- Kawai M, Makino B, Yamamuro H, Butsugan Y, Koji K, Hatsuo Y (1995): New physalins possessing an additional carbon- carbon bond from P. alkekengi var. francheti. Tetrahedron 51: 12529–12538.
- Kawai M, Yamamoto T, Makino B, Yamamura H, Araki S, Butsugan Y, Saito K (2001): The structure of physalin T from Physalis alkekengi var. francheti. J Asian Nat Prod Res 3: 199–205.
- Lee CC, Houghton P (2005): Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol 100: 237–243.
- Magalhaes HIF, Veras ML, Torres MR, Alves APNN, Pessoa ODL, Silveira ER, Costa-Lotufo LV, de Moraes MO, Pessoa C (2006): In-vitro and in-vivo antitumour activity of physalins B and D from Physalis angulata. J Pharm Pharmacol 58: 235–241.
- Makino B, Kawai M, Iwata Y, Yamamuro H, Butsugan Y, Ogawa K, Hayahsi M (1995a): Physalins possessing an endoperoxy structure from Physalis alkekengi var. francheti. Structural revision of physalin K. Bull Chem Soc Jpn 68: 219–226.
- Makino B, Kawai M, Ogura T, Nakanishi M, Yamamura H, Butsugan Y (1995b): Structural revision of physalin H isolated from Physalis angulata. J Nat Prod 58: 1668–1674.
- Matsuura T, Kawai M, Nakashima R, Butsugan Y (1970): Structures of physalin A and physalin B, 13,14-seco-16,24-cyclosteroids from P. alkekengi var. francheti. J Chem Soc (C): 664–670.
- Perez-Castorena AL, Garcia M, Martinez M, Maldonado E (2004): Physalins from Physalis solanaceus. Biochem Syst Ecol 32: 1231–1234.
- Qiu L, Zhao F, Jiang ZH, Chen LX, Zhao Q, Lin HX, Yao XS, Qiu F (2008): Steroids and flavonoids from P. alkekengi var. francheti and their inhibitory effects on nitric oxide production. J Nat Prod 71: 642–646.
- Ramadan MF, Mörsel JT (2003): Oil goldenberry. J Agric Food Chem 51: 969–974.
- Row LR, Sarma NS, Reddy KS, Matsuura T, Nakashima R (1978): The structures of physalins F and J from Physalis angulata and P. lancifolia. Phytochemistry 17: 1647–1650.
- Row LR, Reddy KS, Sarma NS, Matsuura T, Nakashima R (1980): New physalins from Physalis angulata and Physalis lancifolia. Structure and reactions of physalins D, I, G and K. Phytochemistry 19: 1175–1181.
- Shim JS, Kyung-Min P, Chung JY, Hwang JK (2002): Antibacterial activity of oleanolic acid from Physalis angulata against oral pathogens. Nutr Food 7: 215–218.
- Silva MTG, Simas SM, Batista TGFM, Cardarelli P, Tomassini TCB (2005): Studies on antimicrobial activity, in vitro, of Physalis angulata L. (Solanaceae) fraction and physalin B bringing out the importance of assay determination. Mem Inst Oswaldo Cruz 100: 779–782.
- Soares MBP, Bellintani MC, Riberio IM, Tomassino TCB, Riberio dos Santos R (2003): Inhibition of macrophage activation and lipopolysaccride induced death by seco-steroids purified from Physalis peruviana L. Eur J Pharmacol 459: 107–112.
- Su BN, Misico R, Park EJ, Santarsiero BD, Mesecar AD, Fong HHS, Pezzuto JM, Kinghorn AD (2002): Isolation and characterization of bioactive principles of the leaves and stems of Physalis philadelphica. Tetrahedron 58: 3453–3466.
- Vessal M, Mehrani HA, Omrani GH (1991): Effects of an aqueous extract of Physalis alkekengi fruit on estrus cycle, reproduction and uterine creatine kinase BB-isozyme in rats. J Ethnopharmacol 34: 69–78.
- Wu SJ, Ng LT, Lin DL, Huang SN, Wang SS, Lin CC (2004): Physalis peruviana extract induces apoptosis in human HepG2 cells through CD95/CD95L system and the mitochondrial signaling transduction pathway. Cancer Lett 215: 199–208.