Abstract
This study investigated some pharmacological properties of virgin coconut oil (VCO), the natural pure oil from coconut [Cocos nucifera Linn (Palmae)] milk, which was prepared without using chemical or high-heat treatment. The anti-inflammatory, analgesic, and antipyretic effects of VCO were assessed. In acute inflammatory models, VCO showed moderate anti-inflammatory effects on ethyl phenylpropiolate-induced ear edema in rats, and carrageenin- and arachidonic acid-induced paw edema. VCO exhibited an inhibitory effect on chronic inflammation by reducing the transudative weight, granuloma formation, and serum alkaline phosphatase activity. VCO also showed a moderate analgesic effect on the acetic acid-induced writhing response as well as an antipyretic effect in yeast-induced hyperthermia. The results obtained suggest anti-inflammatory, analgesic, and antipyretic properties of VCO.
Introduction
Coconut (Cocos nucifera Linn) is a plant in the Palmae family. In Thai traditional medicine, the coconut has been used as a medicinal plant for centuries. Coconut milk helps solve urinary problems, gallstones, and hematemesis. Coconut oil has been used as a burn wound remedy (CitationChaichit, 2004). Coconut oil also appears to promote the immune system response. Feeding coconut oil completely abolishes the expected immune factor responses to endotoxin and diminishes the production of proinflammatory cytokines in vivo (CitationWan & Grimble, 1987; CitationSadeghi et al., 1999). Recently, the biological properties of virgin coconut oil (VCO) have been widely investigated. It has been found that lauric acid is an effective compound in VCO. Lauric acid is the precursor of monolaurin (CitationPereira et al., 2004), which has been shown to modulate immune cell proliferation (CitationWitcher et al., 1996) and possess antimicrobial activity (CitationBergsson et al., 2002). Inflammation involves many other processes of the immune system; for example, during both acute and chronic inflammatory response, the immunological component cells are activated in response to foreign organisms or antigenic substances (CitationWagner et al., 2004). Recent studies point to the important role of inflammation in a wide variety of human diseases that are not primarily disorders of the immune system. These include cancer, atherosclerosis, ischemic heart disease, and some neurodegenerative diseases such as Alzheimer’s disease (CitationKumar et al., 2005). Therefore, it is of interest to investigate the anti-inflammatory action of virgin coconut oil in both acute and chronic phases of inflammation as well as the analgesic and antipyretic activities.
Materials and methods
Animals
Male Sprague-Dawley rats weighing 40–60, 100–120, and 200–220 g and male Swiss albino mice weighing 30–40 g, purchased from the National Laboratory Animal Center, Nakorn Pathom, were used. All animals were kept in a room maintained under environmentally controlled conditions of 24 ± 1°C and a 12 h light–12 h dark cycle. All animals had free access to water and a standard diet (Pokphan Animal Feed Co., Ltd., Bangkok, Thailand). They were acclimatized at least 1 week before starting the experiments. All animal experiments were approved by the Animal Ethics Committee, Faculty of Medicine, Chiang Mai University.
Preparation of virgin coconut oil
Coconuts were collected from the Chiang Rai province, Northern Thailand. A voucher specimen, QBG 29961, was deposited at Queen Sirikit Botanical Garden, Chiang Mai, Thailand. Virgin coconut oil (VCO) was separated from the coconut as follows. The fresh coconut meat was shredded and then cold-pressed to make coconut milk. The coconut milk was fermented for 48 h, whereupon the oil naturally separated out from the milk, producing crystal clear oil. This unique process means that the pure oil does not have a coconut taste or odor. The virgin coconut oil was filtered and kept in a refrigerator.
Chromatography conditions
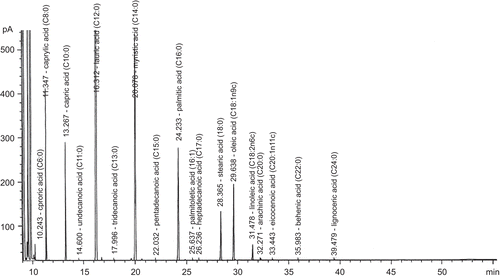
Gas chromatography (GC) separation was performed on a capillary column: 100 m × 25 mm ID × 0.20 μm thick film (Supelco SP-2560); oven temperature: 140°C (hold 5 min) to 250°C at 3°C min−1, hold for 17 min; flame ionization detector (FID): at temperature of 250°C with nitrogen (CitationNielsen, 1998). Test VCO consisted of caprylic acid (C 8:0) 6.54%, capric acid (C 10:0) 5.28%, lauric acid (C 12:0) 44.45%, myristic acid (C 14:0) 18.64%, palmitic acid (C 16:0) 8.71%, stearic acid (C 18:0) 3.17%, oleic acid (C 18:1) 5.21%, and linoleic acid (C 18:2) 0.87%; saturated fatty acids 87.54%, unsaturated fatty acids 6.13%, and polyunsaturated acids 0.87% (). The quality characteristics of the VCO were 242.61 mg KOH/g oil saponification value, 1.35 mg KOH/g oil acid value, 0.48 g/100 g oil free fatty acid value, 7.05 g I2/100 g oil iodine value, 2.22 mEq peroxide/kg oil peroxide value, and 0.91 specific gravity (40°C/water at 20°C).
Preparation of test drugs and drug administration
All test drugs were suspended in 5% Tween 80. They were orally administered in an equivalent volume of 0.5 mL/100 g body weight of the rats and in a volume of 0.1 mL/10 g body weight of the mice. In the ear edema model, all test drugs were dissolved in acetone and applied locally to outer and inner surfaces of the ear. Control groups received vehicle only in the same volume and by the same route of administration.
Ethyl phenylpropiolate-induced ear edema in rats
The anti-inflammatory activity of VCO was tested using the method described by CitationBrattsand et al. (1982). Ethyl phenylpropiolate (EPP; dissolved in acetone) at a dose of 1 mg/20 μL/ear was applied locally using an automatic microliter pipet to the inner and outer surfaces of both ears of each rat (40–60 g). Test drugs were applied in the same manner and volume just before EPP. Before and at 15, 30, 60, and 120 min after edema induction, the thickness of each ear was measured with vernier calipers.
Carrageenin- and arachidonic acid-induced hind paw edema in rats
Male rats weighing 100–120 g were used. Paw edema was induced by an intradermal injection of either carrageenin (1% in normal saline solution) (CitationWinter et al., 1962) or arachidonic acid (0.5% in 0.2 M carbonate buffer, pH 8.4) (CitationDiMartino et al., 1987) into the plantar surface of the right hind paw of the rats at a volume of 0.05 or 0.1 mL, respectively. The edema volume was determined using a plethysmometer (model 7150; Ugo Basile, Italy) prior to and 1, 3, and 5 h after carrageenin injection or 1 h after arachidonic acid injection (CitationWinter et al., 1962). Test drugs were given 1 h prior to carrageenin or 2 h prior to arachidonic acid injection (CitationDiMartino et al., 1987). The control group received vehicle only.
Cotton pellet-induced granuloma formation in rats
The method of CitationSwingle and Shideman (1972) was used. Test drugs were administered orally to male rats weighing 200–220 g in a once-daily dosage regimen for 7 days; the control group received vehicle only. Two sterilized pellets of cotton wool were implanted subcutaneously, one on each side of the abdomen of the animal, under light ether anesthesia and sterile technique. The rats were sacrificed on the 8th day. The body weight gain of the rats was recorded, then they were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneally). Blood was collected by cardiac puncture for determination of the amount of alkaline phosphatase (ALP) and total protein. The implanted pellets and the thymus were dissected out and the wet weights of the pellets were recorded. Both pellets and thymus were dried at 60°C for 18 h and the dry weights were recorded.
Acetic acid-induced writhing response in mice
The hyperalgesic response was induced by injection of an aqueous solution of 0.75% acetic acid in a volume of 0.1 mL/10 g body weight into the peritoneal cavity. Test drugs in a volume of 0.1 mL/10 g body weight were administered orally 1 h before the acetic acid injection. The mice were then placed in a transparent plastic box for 5 min and the number of writhes, a response consisting of contraction of the abdominal wall and pelvic rotation followed by hind limb extension, was counted during continuous observation for 15 min (CitationKoster et al., 1959).
Yeast-induced hyperthermia
The method of CitationTeotino et al. (1963) was used. The animals were first restrained in plastic cages, and the initial rectal temperature was recorded using a 10-channel electric thermometer (model MC 8940; Exacon Scientific Instruments Aps, Denmark) connected to a probe (model H-RRA; Exacon Instruments Aps) which was inserted into the rat rectum to about 5 cm depth. To adapt a rat to the handling procedure for probe insertion, the basal rectal temperature was taken 1 h after probe insertion. Thereafter hyperthermia was induced by subcutaneous injection of 1 mL/100 g body weight of 25% yeast in normal saline solution (NSS). When the temperature was at a peak, 18 h after yeast injection, the rectal temperature was again recorded. Those rats that showed a rise in rectal temperature of more than 1°C were used. Test drugs were then administered orally, and the rectal temperatures of animals were recorded at 30 min intervals for 2 h following drug treatment.
Statistical analysis
The results are presented as mean ± SEM. Statistical comparison between groups was made by using one-way analysis of variance (ANOVA) and post hoc least-significant difference (LSD) test. A p value less than 0.05 was considered to be significant.
Results and discussion
Effects of VCO and reference drugs on EPP-induced ear edema in rats
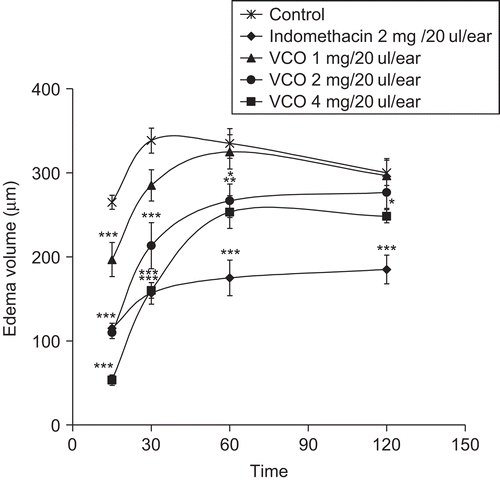
The effect of VCO on EPP-induced ear edema in rats is shown in . At 15 and 30 min after EPP, VCO at the doses of 1, 2, and 4 mg/20 μL/ear significantly reduced ear edema formation in a dose-related manner (r = −0.83, p < 0.001 and −0.69, p < 0.01, respectively). The inhibitory effect of VCO at the doses of 2 and 4 mg/20 μL/ear gradually decreased at 60 and 120 min after EPP application, while VCO at the dose of 1 mg/20 μL/ear did not show any effect at these assessment times. This result suggests the short action of topical VCO. Indomethacin at the dose of 2 mg/20 μL/ear significantly reduced ear edema formation at all assessment times. Topical application of EPP induces the release of inflammatory mediators such as histamine, serotonin, and prostaglandins (PGs), which synergistically increase the vascular permeability and promote vasodilatation as well as edema formation (CitationCarlson et al., 1985). Prostaglandins act directly on blood vessels to cause vasodilatation and indirectly to increase vascular permeability by potentiating the actions of histamine and bradykinin (CitationGoodman, 2003). Indomethacin, a nonsteroidal anti-inflammatory drug (NSAID), elicits its antiedematous property through inhibition of the biosynthesis of PGs, and decreases the sensitivity of vessels to bradykinin and histamine (CitationWagner et al., 2004). The inhibitory effect of VCO might influence the release and/or synthesis of these inflammatory mediators responsible for edema formation.
Figure 2. Effects of VCO and indomethacin on ethyl phenylpropiolate (EPP)-induced ear edema in rats. Test drugs were applied on the outer and inner ear before EPP application; control received acetone only. Values are expressed as mean ± SEM (n = 6). Significantly different from control: *p < 0.05, **p < 0.01, and ***p < 0.001.
Effects of VCO and reference drugs on carrageenin- and arachidonic acid-induced hind paw edema in rats
VCO at doses of 1000, 2000, and 4000 mg/kg significantly reduced the edema formation induced by carrageenin at all assessment time points. Indomethacin, a cyclooxygenase (COX) inhibitor, at a dose of 10 mg/kg markedly reduced the paw edema in this model (). The development of rat paw edema after carrageenin injection has been described by CitationVinegar et al. (1969) as a biphasic event. The initial phase is mediated by histamine and serotonin (5-HT), which increase vascular permeability during the first hour. In the second phase, around the third hour, swelling is caused by the release of PGs, and the continuity between the two phases is provided by kinins (CitationTeotino et al., 1963; CitationCrunkhorn & Meacock, 1971; CitationDi Rosa & Willoughby, 1971; CitationMoncada et al., 1973). A moderate anti-inflammatory effect of VCO in this model was found at the doses of 1000, 2000, and 4000 mg/kg. The significant suppression by VCO on the first phase of rat paw edema formation is probably due to the inhibitory effect on the release and/or synthesis of the early released mediators such as histamine, 5-HT, and kinins. The action of VCO on the second phase of paw edema may be explained by the inhibitory effect on the release and/or synthesis of PGs.
Figure 3. Effects of VCO and reference drugs on (A) carrageenin- and (B) arachidonic acid-induced hind paw edema in rats. Test drugs were orally administered 1 h before carrageenin injection; control received 5% Tween 80 only. Values are expressed as mean ± SEM (n = 6). Significantly different from control: *p < 0.05, **p < 0.01, and ***p < 0.001.
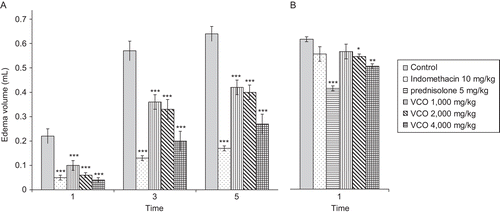
The effect of VCO, prednisolone, and indomethacin on hind paw edema induced by arachidonic acid injection is shown in . At the doses of 2000 and 4000 mg/kg orally, VCO slightly but significantly reduced the paw edema formation. Prednisolone, at an oral dose of 5 mg/kg, exhibited a significant inhibitory effect on rat paw edema while indomethacin did not show an inhibitory effect on the edema formation in this model. Arachidonic acid-induced rat paw edema has been reported to be sensitive for detecting the anti-inflammatory activity of lipoxygenase inhibitors and resistance to selective COX inhibitors (CitationDiMartino et al., 1987). The antiedematous effect of the VCO in this model suggests that its inflammatory effect also in part influences the lipoxygenase pathway.
Effects of VCO, indomethacin, and prednisolone on cotton pellet-induced granuloma formation in rats
The daily oral dose of VCO at 4000 mg/kg for 7 days exhibited a significant inhibitory effect on the transudative weight, granuloma formation, and alkaline phosphatase activity, as presented in . Prednisolone at a dose of 5 mg/kg significantly reduced all those parameters assessed, as well as body weight gain and dry thymus weight. As indomethacin had to be given orally for 7 days, the dose of indomethacin used in this model was reduced to 2.5 mg/kg in order to prevent gastrointestinal effects. It was found that indomethacin also demonstrated significant suppressive effects on all parameters except body weight gain. Transudate was seen in the earliest phase of inflammation due to increased vascular permeability (CitationMichell & Cotran, 2003; CitationKumar et al., 2005). The decrease of transudative weight with VCO in this model suggests its inhibitory effect on the permeability of the vascular endothelium. Granulomatous inflammation is a distinctive pattern of chronic inflammatory reaction (CitationKumar et al., 2005). The inhibition of granuloma formation by anti-inflammatory drugs is probably via their ability to interfere with the proliferative phase of inflammation (CitationSwingle & Shideman, 1972). The thymus contains germinal centers for lymphocytes, and large numbers of T lymphocytes are formed and mature within it (CitationGoodman, 2003). Steroidal drugs produce a pronounced decrease in the number of circulating thymus-derived lymphocytes as well as a catabolic effect on lymphoid and connective tissue, thereby reducing the dry thymus weight and body weight gain (CitationBerne & Levy, 1998). Indomethacin, which is a potent nonselective COX inhibitor, may also inhibit phospholipase A, reduce neutrophil migration, and decrease T cell and B cell proliferation (CitationWagner et al., 2004). The results obtained suggest that VCO probably inhibits the proliferative phase of chronic inflammation but avoids the steroidal-like effect, as it had no effect on body weight gain and dry thymus weight. During inflammation, phagocytic cells release lysosomal enzymes which damage the nearby cells (CitationGould, 2002). Alkaline phosphatase is a lysosomal enzyme found in all tissues. During inflammation, this enzyme is markedly increased and released into the blood circulation (CitationFirkin et al., 1989). The normal group, which had neither implantation nor drug administration, showed a serum alkaline phosphatase activity of 31.20 ± 0.95, which is significantly different from that of the control group. The activity of prednisolone and indomethacin on the serum alkaline phosphatase level is mainly due to stabilization of the lysosomal membrane (CitationGoodman, 2003; CitationWagner et al., 2004). The activity of VCO on the alkaline phosphatase level in serum may be due to its inhibitory effect on inflammatory cell activity and/or stabilization of the lysosomal membrane.
Table 1. Effects of virgin coconut oil (VCO), indomethacin, and prednisolone on transudative weight, granuloma formation, body weight gain, dry thymus weight, and serum alkaline phosphatase (ALP) activity of cotton pellet-induced granuloma in rats.
Effects of VCO and indomethacin on acetic acid-induced writhing response in mice
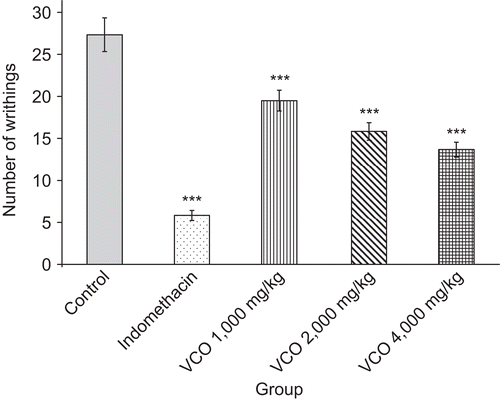
The inhibitory effect of VCO on the acetic acid-induced writhing response in mice is demonstrated in . VCO at the doses of 1000, 2000, and 4000 mg/kg significantly inhibited the number of writhes induced by acetic acid. Indomethacin at a dose of 10 mg/kg showed a marked inhibitory effect on the writhing response, with a percentage of 78.67%. Indomethacin has analgesic properties distinct from its anti-inflammatory effects, and there is evidence for both a central and a peripheral action (CitationBurke et al., 2006). Acetic acid-induced algesia is supposed to act indirectly, possibly by liberating endogenous substances such as H+, K+, serotonin, histamine, bradykinin, PGs, and substance P that excite pain-nerve endings (CitationRaj, 1996). In a previous study, it was found that PGs lower the pain threshold by increasing the sensitivity of the receptors to stimulus (CitationKadar, 1998). Prostaglandins also sensitize nerve endings of fibers to other mediators of inflammation such as histamine, serotonin, bradykinin, and substance P, thereby producing hyperalgesia (CitationGoodman, 2003). The inhibitory effect of VCO on the writhing response could be both due to a peripheral effect by blocking the synthesis and/or release of the endogenous substances responsible for pain and in part via a central effect.
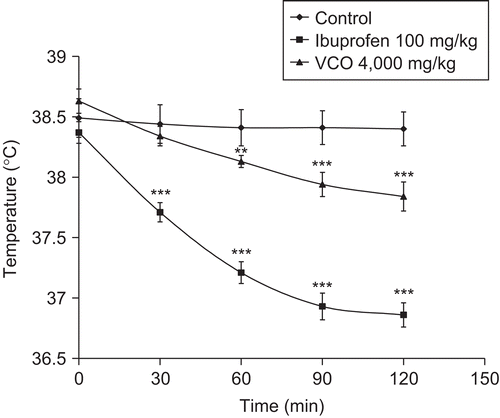
Effects of VCO and ibuprofen on yeast-induced hyperthermia in rats
The antipyretic effect of VCO and ibuprofen is demonstrated in . In the control group, the high rectal temperature was stable at all assessment times. The oral administration of VCO at a dose of 4000 mg/kg slightly decreased the rectal temperature, with activity being significant from 60 min after drug administration. Ibuprofen at an oral dose of 100 mg/kg significantly reduced the high rectal temperature back to normal at all assessment times. Fever is a clinical hallmark of inflammation (CitationMurphy & Ward, 2005). Yeasts, exogenous pyrogens, are capable of stimulating the release of endogenous pyrogens (interleukin-1 and tumor necrosis factor) from polymorphonuclear leukocytes, monocytes, and other cells. These pyrogens act on the thermoreceptive region in the preoptic anterior hypothalamus to release arachidonic acid, stimulate PG synthesis, and raise the set point of the temperature-regulating center, which leads an to increase of body temperature (CitationBernheim et al., 1980; CitationKadar, 1998; CitationKumar et al., 2005). Ibuprofen, a non-steroidal anti-inflammatory agent, possesses antipyretic activity by inhibiting cyclooxygenase and thus blocking the synthesis or release of PGs in the thermoregulatory center (CitationWagner et al., 2004; CitationKumar et al., 2005). The antipyretic activity of VCO is probably due to a similar mechanism, by inhibition of the synthesis and/or release of PGs in the central nervous system (CNS).
Figure 5. Effects of VCO and ibuprofen on yeast-induced hyperthermia in rats. Test drugs were orally administered 18 h after yeast injection; control received 5% Tween 80 only. Values are expressed as mean ± SEM (n = 6). Significantly different from rectal temperature after yeast injection 18 h: **p < 0.01 and ***p < 0.001.
In conclusion, VCO caused significant reductions of ear edema, paw edema, and granuloma formation. The results obtained suggest anti-inflammatory activities of VCO on both acute and chronic phases of inflammation when it is used in high doses. In addition, VCO showed moderate inhibitory effects on the writhing response induced by acetic acid and yeast-induced hyperthermia, which reflect its analgesic and antipyretic activities.
Further study on the safety of VCO, i.e., acute and subchronic toxicity, will be performed to confirm the effect of VCO on complete blood count, blood chemistry, and gross histopathology.
Acknowledgements
The authors thank the Thailand Research Fund (TRF) and Commission on Higher Education (CHE) for providing financial support; the Department of Pharmacology, Faculty of Medicine, Chiang Mai University, for providing laboratory facilities; and the McCormick Faculty of Nursing, Payap University, for the support of this project.
Declaration of interest: The authors report no conflicts of interest.
References
- Bergsson G, Steingrímsson ó, Thormar H (2002): Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int J Antimicrob Agents 20: 258–262.
- Bernheim HA, Gilbert TM, Stitt JT (1980): Prostaglandin E levels in third ventricular cerebrospinal fluid of rabbits during fever and changes in body temperature. J Physiol 301: 69–78.
- Berne RM, Levy MN (1998): The adrenal glands. In: Berne RM, Levy MN, eds., Physiology, 4th ed. St. Louis, MO, Mosby-Year Book, pp. 930–950.
- Brattsand R, Thalen A, Roempke K, Kallstrom L, Gruvstad E (1982): Influence of 16α,17α-acetal substitution and steriod nucleus fluorination on the topical to systemic activity ratio of glucocorticoids. J Steroid Biochem 16: 779–786.
- Burke A, Smyth EM, FitzGerald GA (2006) In: Hardman JG, Limbrid LE, eds., Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 11th ed. New York, McGraw-Hill, pp. 671–716.
- Carlson RP, O’Neill-Davis L, Chang J, Lewis AJ (1985): Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions 17: 197–204.
- Chaichit C (2004): Thai Herbs and Herbal Products. Bangkok, Srimuang Printing Co., pp. 155–156.
- Crunkhorn P, Meacock SCR (1971): Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol 42: 392–402.
- DiMartino MJ, Campbell GK, Wolff CE, Hanna N (1987): The pharmacology of arachidonic acid-induced rat paw edema. Agents Actions 21: 303–305.
- Di Rosa M, Willoughby DA (1971): Screens for anti-inflammatory drugs. J Pharm Pharmacol 23: 297–298.
- Firkin F, Chesterman C, Penington D, Rush B (1989): White cells: neutrophilia and eosinophilia; neutropenia and agranulocytosis; infectious mononucleosis. In: Firkin F, Chesterman C, Penington D, Rush B, eds., De Gruchy’s Clinical Haematology in Medical Practice, 5th ed. Melbourne, Blackwell Scientific Publications, pp. 216–235.
- Goodman HM (2003): Adrenal glands. In: Johnson LR, ed., Essential Medical Physiology, 3rd ed. Los Angeles, Elsevier, pp. 607–635.
- Gould BE (2002): Inflammation and Healing. Pathophysiology for the Health Professions, 2nd ed. Philadelphia, WB Saunders Company, pp. 12–33.
- Kadar D (1998): Anti-inflammatory analgesics. In: Kalant H, Roschlau WH, eds., Principles of Medical Pharmacology, 6th ed. New York, Oxford University Press, pp. 411–412.
- Koster R, Anderson M, De Beer EJ (1959): Acetic acid for analgesic screening. Fed Proc 18: 412.
- Kumar V, Abbas AK, Fausto N (2005): Acute and chronic inflammation. In: Kumar V, Abbas AK, Fausto N, ed., Robbins and Cotran Pathologic Basis of Disease, 7th ed. Philadelphia, Elsevier Saunders, pp. 47–86.
- Michell RN, Cotran RS (2003): Acute and chronic inflammation. In: Kumar V, Cotran RS, Robbins SL, ed., Robbins Basic Pathology, 7th ed. Philadelphia, WB Saunders Company, pp. 33–59.
- Moncada S, Ferreira SH, Vane JR (1973): Prostaglandins, indomethacin-like drugs and the oedema of inflammation. Nature 246: 217–218.
- Murphy HS, Ward PA (2005): Inflammation. In: Rubin E, Gorstein F, Rubin R, Schwarting R, Strayer D, eds., Rubin’s Pathology: Clinicopathologic Foundations of Medicine, 4th ed. Baltimore, MD, Lippincott Williams & Wilkins, pp. 40–83.
- Nielsen SS (1998): Food Analysis, 2nd ed. Gaithersburg, MD, Aspen Publishers, pp. 203–233.
- Pereira CC, Da Silva MA, Langone MA. (2004): Enzymatic synthesis of monolaurin. Appl Biochem Biotechnol 113–116: 433–445.
- Raj PP (1996): Pain Medicine: A Comprehensive Review. St. Louis, MO, Mosby-Year Book, pp. 12–23.
- Sadeghi S, Wallace FA, Calder PC (1999): Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology 96: 404–410.
- Swingle KF, Shideman FE (1972): Phases of the inflammatory response to subcutaneous implant-action of cotton pellet and their modification by certain anti-inflammatory agents. J Pharmacol Exp Ther 183: 226–234.
- Teotino UM, Friz LP, Gandini A, Dellabella D (1963): Thio derivatives of 2,3-dihydro-4H-1,3-benzoxazin-4-one synthesis and pharmacological properties. J Med Chem 55: 248–250.
- Vinegar R, Schreiber W, Hugo R (1969): Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther 166: 96–103.
- Wagner W, Khanna P, Furst DE (2004): Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, and drugs used in gout. In: Katzung BG, ed., Basic & Clinical Pharmacology, 9th ed. Singapore, McGraw-Hill, pp. 576–603.
- Wan JM, Grimble RF (1987): Effect of dietary linoleate content on the metabolic response of rats to Escherichia coli endotoxin. Clin Sci 72: 383–385.
- Winter CA, Risley EA, Nuss GW (1962): Carrageenin-induced edema in hind paw of the rat as assay for anti-inflammatory drug. Proc Soc Exp Biol Med 111: 544–547.
- Witcher KJ, Novick RP, Schlievert PM. (1996): Modulation of immune cell proliferation by glycerol monolaurate. Clin Diagn Lab Immunol 3: 10–13.