Abstract
Four quaternary isoquinoline alkaloids, dehydrocorydalmine, jatrorrhizine, columbamine, and oxyberberine, have been isolated from the whole plant of Argemone mexicana Linn. (Papaveraceae) and their structures established by spectral evidence. This is the first report of these alkaloids (dehydrocorydalmine, jatrorrhizine, columbamine, and oxyberberine) from Argemone mexicana and the Argemone genus.
Introduction
Argemone mexicana L. (Papaveraceae) is an erect, prickly annual herb with bright yellow flowers, up to 1.2 m in height. This plant is naturalized throughout India up to an altitude of 1500 m, and is also distributed in Nepal, Bangladesh, Fiji Island, Mauritius, Mexico, and America. It is a very common weed in both agricultural and non-cultivated land, and grows during the summer season. The plant prefers light sandy soil and can grow in nutritionally poor soil. It is commonly known as Mexican Poppy (CitationAnonymous, 1985; CitationDas & Khanna, 1997). The plant is bitter, diuretic, and purgative; it is reported to destroy worms, cures itching, and is used in the treatment of leprosy, various skin diseases, inflammations, bilious fevers, diarrhea, and dysentery (CitationAnonymous, 1985). The seeds are highly toxic due to the presence of the alkaloids sanguinarine and dihydrosanguinarine. Consumption of edible oils contaminated with A. mexicana seed oil causes various toxic manifestations leading to epidemic dropsy, which poses a serious threat to human health (CitationDas & Khanna, 1997).
A review of the literature revealed the presence of a number of protopine, berberine, benzo-phenanthridine, and benzylisoquinoline type alkaloids (CitationSlavikova & Slavik, 1955; CitationMishra et al., 1961; CitationHaisova & Slavik, 1975; CitationDoepke et al., 1976; CitationHussain et al., 1983; CitationNakkady & Shamma, 1988; CitationChang et al., 2003a, Citation2003b). In view of our interest in alkaloid constituents, we report here the isolation of four quaternary isoquinoline alkaloids from the whole plant of A. mexicana, not previously reported from this plant or the Argemone genus.
Materials and methods
Plant material
The whole plant of Argemone mexicana was collected in the month of May, 2007, from Varanasi district, India. The plant was identified by Professor N. K. Dubey, Department of Botany, Banaras Hindu University, Varanasi, India. A voucher specimen, No. 221, has been deposited in the herbarium of the department.
Extraction and isolation
Melting points were taken on Toshniwal apparatus and are uncorrected. Ultraviolet (UV) spectra were recorded on a PerkinElmer Carry-14 spectrophotometer and infrared (IR) spectra on a PerkinElmer 221 spectrophotometer. 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were recorded on a Bruker spectrophotometer using tetramethylsilane as internal reference. Mass spectra (MS) were recorded using a Kratos MS 50 spectrophotometer operating at 70 eV, with evaporation of the sample in the ion source at about 200°C. BDH (India) grade solvents and chemicals were used throughout the experiment. Column chromatography was carried out over SiO2 gel (60–120 mesh; BDH, India). Thin layer chromatography (TLC) plates were prepared with SiO2 G (Centron Research Laboratories, India), and spots on TLC plates were visualized by spraying with Dragendorff’s reagent.
The whole plant was collected, dried in sunlight, and powdered. The powdered plant material (3 kg) was extracted with MeOH (15 L) in a Soxhlet extractor. The extract was evaporated to dryness on a water bath, which furnished a brown, gummy mass (320 g). The MeOH extract was stirred with aqueous citric acid (7%) using a mechanical stirrer for 10 h. The acidic solution obtained after filtration was basified with NH4OH (pH ~9) and exhaustively extracted with CHCl3 (1.5 L) in a separating funnel. The aqueous alkaline solution left after CHCl3 extraction was acidified with dilute HCl and treated with Mayer’s reagent (HgCl2 + KI) until complete precipitation of alkaloids. The precipitate was filtered and washed with water until free from excess reagent. The precipitate was suspended in ion-free water and stirred with IRA 410 (Cl−) resin repeatedly until all the alkaloid constituents came into aqueous solution (CitationPandey et al., 1976). The aqueous solution was separated from the resin and concentrated under reduced pressure to a light brown, gummy mass (3.75 g). It was chromatographed over a SiO2 gel column and eluted with a mixture of CHCl3 and MeOH in 100 mL flasks. Each eluent was monitored by thin layer chromatography for its homogeneity. The eluates from CHCl3–MeOH (50:1, 30:1, 20:1, and 8:1) were evaporated to dryness and crystallized from MeOH, which furnished, respectively, alkaloid 1 as light yellow granules (23 mg), m.p. 250–252°C; alkaloid 2 as yellow granules (17 mg), m.p. 205–206°C; alkaloid 3 as yellow shiny granules (21 mg), m.p. 209–211°C; and alkaloid 4 as golden yellow needles (24 mg), m.p. 198–200°C.
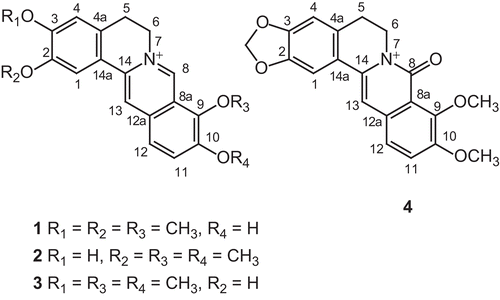
Dehydrocorydalmine (1)
C20H20NO4, UV (MeOH) λmax (log ϵ) nm: 226 (4.4), 265 (4.3), 350 (4.2), 430 (3.5); IR (KBr) νmax, cm−1: 3400 (OH); high resolution electron impact mass spectrometry (HREIMS), m/z (relative intensity, %): 338.1405 (M+, 48) [calcd: 338.1392], 323 (25), 322 (10), 294 (28).
Jatrorrhizine (2)
C20H20NO4, UV (MeOH) λmax (log ϵ) nm: 229 (4.6), 266 (4.4), 349 (3.75), 429 (3.75); IR (KBr) νmax, cm−1: 3350 (OH); HREIMS, m/z (relative intensity, %): 338.1400 (M+, 35) [calcd: 338.1392], 323 (35), 308 (20), 280 (16).
Columbamine (3)
C20H20NO4, UV (MeOH) λmax (log ϵ) nm: 230 (4.5), 266 (4.6), 355 (4.0), 432 (3.55); IR (KBr) νmax, cm−1: 3345 (OH); HREIMS, m/z (relative intensity, %): 338.1410 (M+, 42) [calcd: 338.1392], 323 (35), 308 (20), 280 (16).
Oxyberberine (4)
C20H17NO5, UV (MeOH) λmax (log ϵ) nm: 222 (4.5), 290 (4.1), 340 (4.4); IR (KBr) νmax, cm−1: 1630, 1610, 1570, 1540; HREIMS, m/z (relative intensity, %): 351.110 (M+, 38) [calcd: 351.1106], 337 (15), 322 (25), 294 (28).
Results and discussion
Chromatographic resolution of the quaternary alkaloid fraction of the MeOH extract of the whole plant of A. mexicana resulted in the isolation of alkaloids 1–4 (). The 1H NMR, and 13C NMR, data ( and ) in comparison with the reported data fully supported the structure of compounds 1, 2, 3, and 4, respectively, as dehydrocorydalmine (CitationDoskotch et al., 1967), jatrorrhizine (CitationWa et al., 1967), columbamine (CitationShamma & Rothenberg, 1978), and oxyberberine (CitationChatterjee & Maiti, 1955). The structures of 1–3 were further proved by borohydride reduction. The sodium borohydride reduction of alkaloids 1, 2, and 3 gave, respectively, the corresponding tetrahydro-derivatives as corydalmine (CitationBhakuni & Gupta, 1982), C20H23NO4 (HRMS, m/z 341.1633 [M]+), m.p. 170–172°C; corypalmine (CitationPreininger et al., 1978), C20H23NO4 (HRMS, m/z 341.1630 [M]+), m.p. 225–227°C; and isocorypalmine (CitationPreininger et al., 1978), C20H23NO4 (HRMS, m/z 341.1633 [M]+), m.p. 338–340°C. The structure of compound 4 was proved by heating berberine with NaOH (CitationShamma, 1972), which furnished a compound identical to compound 4. The structures of compounds 1–4 were also substantiated by 1H-1H correlation spectroscopy (COSY) NMR in which C-5 and C-11 protons coupled, respectively, with protons of C-6 and C-12.
Table 1. 500 MHz 1H NMR (CDCl3 + 20% CD3OD) spectroscopic data of alkaloids 1–4.
Table 2. 125 MHz 13C NMR (CDCl3 + 20% CD3OD) spectroscopic data of alkaloids 1–4.
Although these quaternary alkaloids have been reported previously from other plant families (CitationGrycová et al., 2007), this is the first report of these alkaloids from Argemone mexicana and the Argemone genus.
Acknowledgement
One of the authors (S.S.) is thankful to UGC, New Delhi, for financial assistance.
Declaration of interest: The authors report no conflicts of interest.
References
- Anonymous (1985): The Wealth of India, Raw Materials, Vol I: A (Revised). New Delhi, Publication & Information Directorate, Council of Scientific & Industrial Research, pp. 415–417.
- Bhakuni DS, Gupta S (1982): The alkaloids of Stephania glabra. J Nat Prod 45: 407–411.
- Chang YC, Chang FR, Khalil AT, Hsieh PW, Wu YC (2003a): Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Z Naturforsch 58: 521–526.
- Chang YC, Hsieh PW, Chang FR, Wu RR, Liaw CC, Lee KH, Wu YC (2003b): Two new protopines argemexicaines A and B and the anti-HIV alkaloid 6-acetonyldihydrochelerythrine from Formosan Argemone mexicana. Planta Med 69: 148–152.
- Chatterjee R, Maiti PC (1955): Plant alkaloids. Part VII. Lambertine and berlambine. J Indian Chem Soc 32: 609–610.
- Das M, Khanna SK (1997): Clinicoepidemiological, toxicological and safety evaluation studies on argemone oil. Crit Rev Toxicol 27: 273–297.
- Doepke W, Hess U, Jimenez V (1976): On the structure of a new alkaloid from Argemone mexicana. Z Chem 16: 54–55.
- Doskotch RW, Malik MY, Beal JL (1967): The identification of dehydrocorydalmine and new protoberberine alkaloid, stepharanine in Stephania glabra tubers. J Org Chem 32: 3253–3254.
- Grycová L, Dostál J, Marek R (2007): Quaternary protoberberine alkaloids. Phytochemistry 68: 150–175.
- Haisova K, Slavik J (1975): Minor alkaloids from Argemone mexicana. Collect Czech Chem Commun 40: 1576–1578.
- Hussain SF, Nakkady S, Khan L, Shamma M (1983): Oxyhydrastinine, an isoquinolone alkaloid from the Papaveraceae. Phytochemistry 22: 319–320.
- Mishra PS, Bhakuni DS, Sharma VN, Kaul KN (1961): Chemical constituents of Argemone mexicana. J Sci Ind Res 20: 186.
- Nakkady S, Shamma M (1988): Studies on the chemical constituents of Argemone mexicana. Egypt J Pharm Sci 29: 53–61.
- Pandey VB, Ray AB, Dasgupta B (1976): Quaternary alkaloids of Fumaria indica. Phytochemistry 15:545–546.
- Preininger V, Novák J, Śimánek V, Śantávy F (1978): Isolation and chemistry of the alkaloids from plants of the Papaveraceae LXXIII. Planta Med 33: 396–402.
- Shamma M (1972): The Isoquinoline Alkaloid. New York, Academic Press, pp. 285.
- Shamma M, Rothenberg AS (1978): The alkaloids of Thalictrum dioicum. Lloydia 41: 169–178.
- Slavikova L, Slavik J (1955): Alkaloids of Papaveraceae VII Argemone mexicana. Chemicke Listy Pro Vedu Prumysl 49: 1546–1549.
- Wa MT, Beal JL, Doskotch RW (1967): A study of the quaternary alkaloids of Stephania glabra. Lloydia 30: 245–249.