Abstract
The aim of the study was to explore the role of basic fibroblast growth factor (bFGF) in ovarian cancer progression. This was done by investigating the effects of bFGF on both the secretion of urokinase-type plasminogen activator (uPA) and the invasion of tumor cells in SKOV3 ovarian cancer cells. Human ovarian cancer cell line SKOV3 was cultured in vitro. The expression of uPA gene and protein was induced in SKOV3 cells; the impact of bFGF on the expression of uPA gene in SKOV3 cells was studied by RT-PCR, and the impact of bFGF on the expression of uPA protein was tested by ELISA. Ets-1 antisense oligonucleotides were transfected into SKOV3 cells by liposome protocol. The effects of bFGF on Ets-1 expression and the invasion ability of SKOV3 cells were determined both before and after exposure to different concentrations of bFGF for 24 h. The expression of both uPA gene and protein was induced in SKOV3 cells, p < 0.05. The expression of uPA was suppressed by Ets-1 antisense oligonucleotides in SKOV3 cells, p < 0.05. The invasion ability of SKOV3 cells was increased by 2.3-fold, and this effect was also suppressed by Ets-1 antisense oligonucleotides. bFGF can enhance the invasion ability of ovarian cancer cells in vitro by inducing the expression of uPA, and this effect is also regulated by the transcription factor Ets-1.
Introduction
Basic fibroblast growth factor (bFGF), a 146 amino-acid polypeptide, is a member of a family of molecules encoded by seven genes; it is involved in embryogenesis, angiogenesis, and wound healing (CitationPötgens et al., 1995). As a potent angiogenesis factor, bFGF is frequently found in malignant tumor and tumor-adjacent tissue, related to the microvessel density of a tumor.
In ovarian cancer, bFGF expression has been found in tumor tissues, cancer cell lines, serum, and ascetic and malignant effusions, related to tumor grading and staging (CitationDi Blasio et al., 1995; CitationBarton et al., 1997; CitationYoneda et al., 1998; CitationUeda et al., 2001; Davidson et al., Citation2002a). Investigations by Davidson and colleagues (Citation2002a) showed that bFGF was the major angiogenic factor in ovarian carcinoma. Also, bFGF has a prognostic value. In fact, the level of serum bFGF combined with CA125 as an ovarian tumor marker has a higher specificity than CA125 alone (Davidson et al., Citation2002b; CitationLe Page et al., 2006).
The way in which bFGF promotes the progression and metastasis of malignant tumors through regulating angiogenesis has been well elucidated (CitationPötgens et al., 1995). CitationSako et al. (2003) found that bFGF could increase the expression of vascular endothelial growth factor (VEGF) of mesothelial cells and promote peritoneal metastasis. More recently, bFGF was found to be related to paclitaxel resistance (CitationGan et al., 2006). All these findings have thrown light on the mechanism by which bFGF participates in tumor progression and metastasis, but the underlying mechanisms are not yet well understood. In endothelial cells, bFGF can promote cell invasion and migration by stimulating the expression of proteases such as urokinase-type plasminogen activator (uPA) and metalloproteinases (MMPs) (CitationIwasaka et al., 1996). In cervical carcinoma, glioma tumor, and gastric cancer, etc., bFGF has been found to be related to protease expression (CitationMori et al., 2000; CitationForbes et al., 2003; CitationKanda et al., 2005; CitationZhao et al., 2005). Among the large number of proteases involved in cellular invasion, uPA plays an important role as it initiates the activation of metalloproteinases and the conversion of plasminogen to plasmin (CitationCollen, 1999; CitationDanø et al., 2005). The overexpression of uPA is found in nearly all kinds of malignant tumors, including advanced ovarian cancer. CitationCai et al. (2007) found that uPA was involved in metastasis and had a prognostic value related to epithelial ovarian cancer (CitationSchmalfeldt et al., 2001). Based on all these findings, we hypothesize here that bFGF can stimulate uPA expression in ovarian cancer cells, and promote the invasion of cancer cells. Also, as uPA is one of the target genes regulated by transcription factor Ets-1 (CitationWatabe et al., 1998), we hypothesize that the effect of bFGF is regulated by Ets-1.
In this study, we observed the effects of bFGF on the expression of uPA and Ets-1, and on cell invasion in SKOV3 ovarian cancer cell lines, before and after the inhibition of Ets-1 expression using Ets-1 antisense oligonucleotides.
Materials and methods
Cell culture
SKOV3 cells were cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% heat inactivated fetal bovine serum (FBS), 100 units/mL of penicillin, and 100 μg/mL of streptomycin, and the cells were incubated at 37°C in a humidified 95% air and 5% CO2 atmosphere. When the cells reached subconfluence, basal medium (Invitrogen) containing 0.5% FBS with different concentrations of bFGF (0.1, 1, 10, 100 ng/mL) were incubated for 24 h. Recombinant human bFGF was purchased from Invitrogen, and solutions were prepared in Tris with 0.5% bovine serum albumin (Sigma).
Reverse transcription polymerase chain reaction (RT-PCR) analysis
The cell total RNA was extracted using a Total RNA Isolation Kit (Takara) and treated with RNase-free DNase. The reverse transcription (RT) reaction was carried out using the following: 4 μg of total RNA, 1 μL of oligo primer, 1 μL of 10 mM dNTP, 4 μL of 25 mM MgCl2, 2 μL of 0.1 mM dithiothreitol (DTT), and 2 μL of SuperScript II Reverse Transcriptase (Invitrogen) at 42°C for 50 min.
For the polymerase chain reaction (PCR), 2 μL of cDNA was incubated with 2 μL of 5 × buffer, 1.6 μL of dNTP, 0.1 μL of DNA polymerase (Invitrogen), 2 μL of 25 mM MgCl2, 1 μL of primer pairs, and 10.3 μL of water. PCR was performed for 33 cycles of 1 min at 94°C, 1 min at 58°C, 1 min at 72°C, and a final elongation step of 7 min at 72°C.
The human Ets-1 PCR primers were 5′-TCACAGAGTCCTATCAGACGC-3′ (sense) and 5′-GTCCTTATTGAGGTCAGCACG-3′ (antisense); the human uPA primers were 5′-AGAATTCTACCGACTATCTC-3′ (sense) and 5′-ATTCTCTTCCTTGGTGTGAC-3′ (antisense); and the human β-actin primers were 5′-GTGGGGCGCCCCAGGCACCA-3′ (sense) and 5′-CTCCTTAATGTCACGCACGATTTC-3′ (antisense).
Amplified products were separated on 2% agarose gel and visualized by means of ethidium bromide ultraviolet fluorescence. The expression of a respective target gene was calculated by the density of the corresponding PCR product divided by that of β-actin.
Measurement of uPA levels by ELISA
The supernatant was obtained from cultures of SKOV3 cells treated with different concentrations of bFGF using a uPA enzyme linked immunosorbent assay (ELISA) kit (Adlitteram Diagnostic). Samples were assayed in triplicate. All data were within the linear range of the standard curve generated by use of 0.0, 0.10, 0.25, 0.50, 0.75, and 1.0 ng/mL uPA, which was provided with the kit.
Antisense oligonucleotide treatment of cell monolayers
A 20-mer antisense oligonucleotide was used to knock down Ets-1 expression. The sequences were: antisense 5′-AGATCGACGGCCGCCTTCAT-3′, and sense control 5′-ATGAAGGCGGCCGTCGATCT-3′. The oligonucleotides were purified by high-pressure liquid chromatography. Cells were treated with oligonucleotides by the method described in the manufacturer’s instructions for DOTAP (liposomal transfection reagent; Roche). Briefly, cells were grown to be about 60–70% confluent and rinsed with serum-free RPMI 1640, and then incubated in serum-free RPMI 1640 with 6 μg/mL DOTAP (Roche) and 16 μg/mL oligonucleotides for 6 h.
Invasion assay
The invasion ability of cells treated with or without Ets-1 was assessed by the following method. Cells were washed, and resuspended at 105 cells/200 μL in serum-free medium with 10 ng/mL bFGF and Ets-1 oligonucletides or bFGF only. The cells were distributed to upper transwells coated with Matrigel (BD Bioscience). The bottom chambers were filled with 10 μg/mL fibronectin. The cells were then incubated at 37°C in a humidified 95% air and 5% CO2 atmosphere for 72 h. After that, the lower surface of the filter was fixed and stained. The number of invading cells was calculated as the sum of cells counted in five fields at a ×40 magnification.
Statistical analysis
Results were assessed by Student’s t-test. The SPSS computer package was used for all analyses, with p < 0.05 considered as significant.
Results
Effects of bFGF on mRNA expression of uPA and Ets-1 in SKOV3 cells
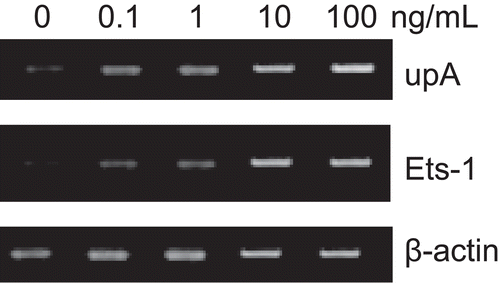
The expressions of uPA and Ets-1 after incubation in serum-free medium for 24 h were used as basal levels. After incubation with different concentrations of bFGF (0.1, 1, 10, 100 ng/mL) for 24 h, we observed a significant increase of both uPA and Ets-1 expression. The mRNA levels of uPA and Ets-1 were increased by 3.2- and 2.5-fold, respectively, when treated with bFGF (10 ng/mL), compared with control. The up-regulation of these two genes showed a dose-dependent response ().
Figure 1. RT-PCR analysis of uPA and Ets-1 genes. After SKOV3 cells were cultured in basal medium and different concentrations of bFGF (0.1, 1, 10, 100 ng/mL) for 24 h, a significant change of expression of uPA and Ets-1 mRNA of SKOV3 cells was observed. The increase of both genes presented a dose-dependent response. β-actin was functioning here as a housekeeping gene.
Effect of bFGF on protein secretion of uPA
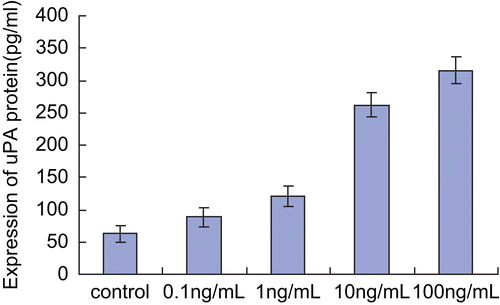
Using ELISA, we found that uPA protein expression was increased with bFGF treatment and was highest with the treatment of 100 ng/mL bFGF. This effect showed a dose-dependent response ().
Figure 2. Effect of bFGF on secretion of uPA protein. Each bar represents mean ± SEM. After treatment with different concentrations of bFGF (0.1, 1, 10, 100 ng/mL), supernantant was obtained from cultures of SKOV3 cells, and tested by ELISA. The expression of uPA protein increased due to stimulation with bFGF in a dose-dependent manner.
Effects of antisense Ets-1 oligonucleotides on Ets-1 and uPA expression
The use of antisense oligonucleotides blocked the induction effect of bFGF on Ets-1 and uPA expression. Sense oligonucleotides, as control, showed no change of effect. uPA mRNA expression was increased by 3.2-fold with 10 ng/mL bFGF treatment and decreased to 1.4-fold when cells were transfected with antisense Ets-1 oligonucleotides. Similar results were observed with uPA protein expression: a 2.5-fold increase reduced to 1.4-fold upon transfection with antisense oligonucleotides. These results indicate that Ets-1, a bFGF-regulated transcription factor, plays an important role in regulation of uPA expression in SKOV3 cells.
Effect of bFGF on cell invasion of SKOV3 cells
Finally, as uPA plays key roles in tumor invasion, we wanted to know whether knock-down of Ets-1 expression altered tumor invasion induced by bFGF. After induction by bFGF, we observed a 2.3- and 2.4-fold increase of cell invasion in the control and sense Ets-1 oligonucleotide groups, respectively, but only a 1.5-fold increase in the antisense oligonucleotide group (). This shows that Ets-1 plays an essential role in tumor invasion induced by bFGF.
Table 1. Effect of bFGF on cell invasion of SKOV3 cells and the effect of antisense oligonucleotides on that procedure.
Discussion
Among the angiogenic factors, bFGF is best characterized. It stimulates endothelial cells to secrete proteases such as MMPs and uPA, which results in degradation of the vessel basement membrane, allows cells to invade the surrounding matrix, and contributes to the neovascularization of tumors (CitationMandriota & Pepper, 1997). However, little was known about the mechanism of the effect of bFGF on protease secretion or cell invasion in cancer cells.
In this study, we tested the effect of bFGF on the expression of uPA in ovarian cancer cell line SKOV3. As we hypothesized, bFGF stimulated the expression of uPA in ovarian cancer cells in a dose-dependent manner. A similar finding was reported for glioma cells in a previous study (CitationMori et al., 2000).
Urokinase-type plasminogen activator is one of the most important proteases in tumor progression, not only because it participates in angiogenesis through degradation of the vessel basement membrane, but also because it contributes to tumor invasion and metastasis by degradation of the extracellular matrix, thus allowing cells to overcome the constraints of cell–cell and cell–matrix interactions (CitationCollen, 1999; CitationDanø et al., 2005). In this study, we found that bFGF increased the invasion ability of SKOV3 cells and this effect was accompanied by increased uPA expression. This finding showed a clear correlation of uPA expression and tumor cell invasion. This possibility was also supported by the fact that Ets-1-inhibited expression significantly diminished the invasion ability of SKOV3 cells induced by bFGF, and decreased uPA expression. Together with previous findings in glioma, our studies support a novel mechanism of the effect of bFGF on tumor progression: in addition to contributing to angiogenesis, bFGF promotes tumor invasion through the up-regulation of uPA expression (CitationKitange et al., 1999).
Finally, we tested the possible pathway of bFGF in uPA regulation. As uPA is one of the target genes of Ets-1 in breast cancer cells (CitationWatabe et al., 1998), we hypothesized that Ets-1 participates in this regulation. Using an antisense oligonucleotide of Ets-1, we found that the expression of uPA gene and protein was significantly decreased by suppressing the Ets-1 gene, and so was the cell invasion ability. Our studies indicated that bFGF regulation of uPA expression and cell invasion is mediated by Ets-1. CitationKitange et al. (1999) described a similar phenomenon in a study of glioma cells. A recent study by CitationFujimoto et al. (2004) also showed a significant relationship between Ets-1 and invasive ovarian cancer (CitationDavidson et al., 2003).
The serum concentration of bFGF was reported to range from 4 to 33 ng/mL in ovarian cancer patients (CitationDi Blasio et al., 1995). However, the concentration of bFGF in the peritoneal cavity is much higher, since cellular injury or death may release more bFGF. Hence, we chose the following concentration gradient of bFGF: 0.1, 1, 10, 100 ng/mL. In this range, we found a dose-dependent response in the regulation of uPA expression.
Our experiments were performed in vitro, and the effects may not be the same in vivo, but the results enable us to reach a better understanding of the various mechanisms that regulate the metastatic process; furthermore, they can help to provide reasonable targets for the development of new antimetastatic therapies.
Declaration of interest: The authors report no conflicts of interest.
References
- Barton DP, Cai A, Wendt K, Young M, Gamero A, De Cesare S (1997): Angiogenic protein expression in advanced epithelial ovarian cancer. Clin Cancer Res 3: 1579–1586.
- Cai Z, Li YF, Liu FY (2007): Expression and clinical significance of uPA and PAI-1 in epithelial ovarian cancer. Ai Zheng 26: 312–317.
- Collen D (1999): The plasminogen (fibrinolytic) system. Thromb Haemost 82: 259–270.
- Danø K, Behrendt N, Høyer-Hansen G (2005): Plasminogen activation and cancer. Thromb Haemost 93: 676–681.
- Davidson B, Reich R, Kopolovic J, Berner A, Nesland JM, Kristensen GB, Tropé CG, Bryne M, Risberg B, van de Putte G, Goldberg I (2002a): Interleukin-8 and vascular endothelial growth factor mRNA and protein levels are down-regulated in ovarian carcinoma cells in serous effusions. Clin Exp Metastasis 19: 135–144.
- Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Ben-Baruch G, Nesland JM, Reich R (2002b): The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Mol Cell Endocrinol 187: 39–45.
- Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Risberg B, Ben-Baruch G, Reich R (2003): Coordinated expression of integrin subunits, matrix metalloproteinases (MMP), angiogenic genes and Ets transcription factors in advanced-stage ovarian carcinoma: a possible activation pathway? Cancer Metastasis Rev 22: 103–115.
- Di Blasio AM, Carniti C, Viganò P, Vignali M (1995): Basic fibroblast growth factor and ovarian cancer. J Steroid Biochem Mol Biol 53: 375–379.
- Forbes K, Webb MA, Sehgal I (2003): Growth factor regulation of secreted matrix metalloproteinase and plasminogen activators in prostate cancer cells, normal prostate fibroblasts and normal osteoblasts. Prostate Cancer Prostatic Dis 6: 148–153.
- Fujimoto J, Aoki I, Toyoki H, Khatun S, Sato E, Sakaguchi H, Tamaya T (2004): Clinical implications of expression of ETS-1 related to angiogenesis in metastatic lesions of ovarian cancers. Oncology 66: 420–428.
- Gan Y, Wientjes MG, Au JL (2006): Expression of basic fibroblast growth factor correlates with resistance to paclitaxel in human patient tumors. Pharm Res 23: 1324–1331.
- Iwasaka C, Tanaka K, Abe M, Sato Y (1996): Ets-1 regulates angiogenesis by inducing the expression of urokinase-type plasminogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J Cell Physiol 169: 522–531.
- Kanda K, Ueda M, Futakuchi H, Yamaguchi H, Mori K, Terai Y, Ueki M (2005): Transcriptional expression of the genes implicated in angiogenesis and tumor invasion in cervical carcinomas. Gynecol Oncol 98: 453–461.
- Kitange G, Shibata S, Tokunaga Y, Yagi N, Yasunaga A, Kishikawa M, Naito S (1999): Ets-1 transcription factor-mediated urokinase-type plasminogen activator expression and invasion in glioma cells stimulated by serum and basic fibroblast growth factors. Lab Invest 79: 407–416.
- Le Page C, Ouellet V, Madore J, Hudson TJ, Tonin PN, Provencher DM, Mes-Masson AM (2006): From gene profiling to diagnostic markers: IL-18 and FGF-2 complement CA125 as serum-based markers in epithelial ovarian cancer. Int J Cancer 118: 1750–1758.
- Mandriota SJ, Pepper MS (1997): Vascular endothelial growth factor-induced in vitro angiogenesis and plasminogen activator expression are dependent on endogenous basic fibroblast growth factor. J Cell Sci 110: 2293–2302.
- Mori T, Abe T, Wakabayashi Y, Hikawa T, Matsuo K, Yamada Y, Kuwano M, Hori S (2000): Up-regulation of urokinase-type plasminogen activator and its receptor correlates with enhanced invasion activity of human glioma cells mediated by transforming growth factor-alpha or basic fibroblast growth factor. J Neurooncol 46: 115–123.
- Pötgens AJ, Westphal HR, de Waal RM, Ruiter DJ (1995): The role of vascular permeability factor and basic fibroblast growth factor in tumor angiogenesis. Biol Chem Hoppe Seyler 376: 57–70.
- Sako A, Kitayama J, Yamaguchi H, Kaisaki S, Suzuki H, Fukatsu K, Fujii S, Nagawa H (2003): Vascular endothelial growth factor synthesis by human omental mesothelial cells is augmented by fibroblast growth factor-2: possible role of mesothelial cell on the development of peritoneal metastasis. J Surg Res 115: 113–120.
- Schmalfeldt B, Prechtel D, Härting K (2001): Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res 7: 2396–2404.
- Ueda M, Terai Y, Kumagai K, Ueki K, Yamaguchi H, Akise D, Ueki M (2001): Vascular endothelial growth factor C gene expression is closely related to invasion phenotype in gynecological tumor cells. Gynecol Oncol 82: 162–166.
- Watabe T, Yoshida K, Shindoh M, Kaya M, Fujikawa K, Sato H, Seiki M, Ishii S, Fujinaga K (1998): The Ets-1 and Ets-2 transcription factors activate the promoters for invasion-associated urokinase and collagenase genes in response to epidermal growth factor. Int J Cancer 77: 128–137.
- Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ (1998): Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst 90: 447–454.
- Zhao ZS, Zhou JL, Yao GY, Ru GQ, Ma J, Ruan J (2005): Correlative studies on bFGF mRNA and MMP-9 mRNA expressions with microvascular density, progression, and prognosis of gastric carcinomas. World J Gastroenterol 11: 3227–3233.
