Abstract
The naringinase-treated methanol extract of Sophora japonica L. (Fabaceae) seeds showed potent estrogen agonist activity. Through bioassay-guided isolation of the main active constituents from the naringinase-treated methanol extract of S. japonica, the aglycones genistein and kaempferol were found to be the main phytoestrogens in the naringinase-treated extract. In addition, kaempferol was nearly equipotent to genistein as an estrogen agonist. Concerning the compounds isolated from the untreated methanol extract, sophoricoside showed weak estrogenic activity on ERβ only.
Introduction
Estrogens are the key regulators of the cellular processes involved in development and maintenance of the reproductive function. There are two subtypes of estrogen receptor and several isoforms of each subtype. The first subtype is estrogen receptor α (ERα) (CitationGreen et al., 1986), and the second subtype is estrogen receptor β (ERβ) (CitationKuiper et al., 1996).
Phytoestrogens are plant-derived compounds that structurally or functionally mimic mammalian estrogens, and therefore are considered to play an important role in the prevention of cancers, heart disease, menopausal symptoms, and osteoporosis. There are several classes of phytoestrogens, such as steroidal estrogens found in a few plants; the more common phenolic estrogens are isoflavones, coumestans, and lignans (CitationOsoski & Kenelly, 2003). Other classes of phytoestrogens that have been reported include: anthraquinones (CitationMatsuda et al., 2001), chalcones (CitationRafi et al., 2000), flavones (CitationMilligan et al., 1999), prenylated flavonoids (CitationKitaoka et al., 1998; CitationAhn et al., 2004b), naphthalenes, naphthopyrones, sesquiterpenoidal naphthoquinones (CitationEl-Halawany et al., 2007a, Citation2007b), and saponins (CitationChan et al., 2002).
Sophora japonica L. (Fabaceae) is a tree native to China and Korea. It is also named the Japanese pagoda tree or Chinese scholar tree. Flavones from the buds and pericarps were reported as hemostatic constituents (CitationTang et al., 2001). Triterpenes, phospholipids, alkaloids, polysaccharides, and fatty acids have been reported as the main chemical constituents of the seeds (CitationGorbachova et al., 1995; CitationMukhamedova & Glushenkova, 1997). Despite these discoveries, there is no previous report about the use of S. japonica as an estrogen replacement therapy (ERT). S. japonica was selected for this investigation due to its high flavonoid content.
The current study reports the biologically guided isolation of the major phytoestrogens from the methanol extract of S. japonica seeds before and after naringinase treatment.
Materials and methods
Chemicals
Naringinase was purchased from Sigma Co. (St. Louis, MO, USA). O-Nitrophenyl β-d-galactoside (ONPG) was purchased from Nacalai Tesque Co. (Kyoto, Japan). 17β-Estradiol was obtained from Calbiochem Co. (Darmstadt, Germany). Tamoxifen was purchased from Wako Chemical Co. (Osaka, Japan). Zymolyase 20T was obtained from Seikagaku Kogyo Co. (Tokyo, Japan).
General experimental procedures
Thin layer chromatography (TLC) was carried out on pre-coated silica gel 60 F254 (0.25 mm; Merck) and RP-18 F254S (0.25 mm; Merck, Darmstadt). Column chromatography (CC) was carried out on BW-820MH silica gel and ODS DM 1020T (Fuji Silysia, Nagoya, Japan). Medium pressure liquid chromatography (MPLC) was performed on LiChroprep RP-18 (size A and B; Merck). 1H- and 13C-nuclear magnetic resonance (NMR) spectra were measured with a JHA-LAA 400 WB-FT (1H, 400 MHz; 13C, 100 MHz; Jeol, Tokyo) spectrometer, the chemical shifts being represented as ppm with tetramethylsilane as internal standard. Electrospray ionization mass spectrometry (ESI-MS) was carried out on an Esquire 3000 mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) system with ESI source.
Plant material
Seeds of S. japonica were collected from ripe fruits cultivated in the Medicinal Plant Station of the Faculty of Pharmacy, Cairo University, during December 2005. Authentication of the plant was established by Ass. Prof. Dr. Sherif El-Khanagry, Agriculture Museum, El-Dokki, Cairo, Egypt. A voucher specimen (No. S-1) is kept in the Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Egypt.
Yeast strains
The genetically modified yeast strains were provided by Professor Tsutomu Nishihara, Faculty of Pharmaceutical Sciences, Hyogo College of Medicine, Kobe, Japan.
Extraction and isolation
The pulverized seeds of S. japonica (1.3 kg) were extracted with MeOH (1 L × 3) at room temperature and the combined extracts were evaporated in vacuo. The methanol extract (104 g) was suspended in 50% aqueous MeOH (600 mL) and partitioned with chloroform (500 mL × 3) to produce a chloroform-soluble fraction (4 g). The remaining solution was applied on a column of Diaion HP-20 (60 cm × 6 cm). Washing with H2O (1 L) was followed by elution with 25% MeOH in H2O (2 L), 50% MeOH in H2O (2 L), and finally 100% MeOH (2 L). The eluates were evaporated under vacuum to give 8.5 g (fraction A), 14 g (fraction B), and 5.8 g (fraction C) of dry residue, respectively. All the fractions were screened for their estrogenic activity, before and after naringinase treatment, using yeast two-hybrid screen.
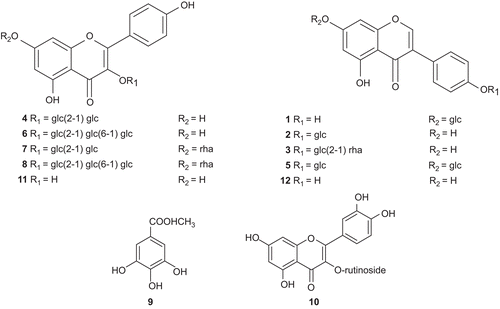
Most of fraction B (12 g) was applied to a silica gel column (300 g). Elution with CHCl3–MeOH–H2O (9:1:0.1, v/v/v) afforded eight fractions. Fraction 1 (735 mg) was purified on a silica gel column (25 cm × 2 cm) and eluted with CHCl3–MeOH (9:1, v/v) to obtain compound 9 (13 mg). Fraction 3 (2 g) gave a yellow precipitate upon concentration. After filtration, the precipitate was washed several times with a mixture of chloroform–methanol (1:1) to give compound 3 (193 mg). The filtrate was evaporated under vacuum and the residue was purified on a MPLC RP-18 column (size A) using MeOH–H2O (4:6, v/v) to afford compounds 7 (40 mg), 1 (2 mg), and 2 (5 mg). Fraction 4 gave compound 4 (358 mg) upon crystallization from MeOH, and the remaining supernatant was applied to a MPLC RP-18 column (size B) eluted with MeOH–H2O (3:7, v/v) to give compound 10 (5 mg). Fraction 5 gave compound 5 (180 mg) upon crystallization from a chloroform–methanol mixture (1:1, v/v). Fraction 6 (1.5 g) was purified on a Sephadex LH-20 column (30 cm × 3 cm) and eluted with MeOH–H2O (1:1, v/v), and sub-fractions (10 mL each) were collected. Sub-fractions 17–30 (150 mg) of this column were combined together and applied to a MPLC RP-18 column (size A) and eluted with MeOH–H2O (3:7, v/v) to give compound 6 (37.4 mg). Fraction 7 (545 mg) was purified on a Sephadex LH-20 column (20 cm × 3 cm) and eluted with MeOH, followed by a MPLC column (size A) using MeOH–H2O (1:4, v/v) to afford compound 8 (21 mg).
Naringinase treatment of 50% methanol fraction (fraction B) of S. japonica and isolation of aglycones
Part of fraction B (2 g) was dissolved in H2O and incubated with naringinase enzyme (1 g) in 100 mL of 0.2 M acetate buffer (pH 4.7) at 37°C for 24 h. The resulting hydrolysate was extracted with EtOAc (250 mL × 3), and the combined extracts were evaporated under reduced pressure to give 650 mg of dry residue. The residue was applied to a silica gel column (25 cm × 2 cm) and eluted with CHCl3–MeOH (9.5:0.5, v/v) to give 11 (25 mg) and 12 (10 mg) as the two major aglycones.
Yeast two-hybrid assay
The yeast two-hybrid assay was carried out according to the method of CitationNishikawa et al. (1999) and CitationKanayama et al. (2003). Briefly, yeast cells expressing ERα and ERβ were separately grown overnight at 30°C with shaking in a synthetic defined medium (SD) lacking tryptophan and leucine. Yeast cells were treated with 17β-estradiol and the test materials separately for 4 h at 30°C, and β-galactosidase activity was determined as follows. The growth of the yeast cells was monitored by measuring the turbidity at 600 nm. The treated yeast cells were collected by centrifugation (8000 × g, 5 min) and re-suspended in 200 μL of Z-buffer (0.1 M sodium phosphate, pH 7.0, 10 mM KCl, and 1 mM MgSO4) containing 1 mg/mL of zymolyase at 37°C for 15 min. The reaction was started by the addition of 40 μL of 4 mg/mL ONPG as substrate. When a yellow color developed (incubation time: t), 100 μL of 1 M Na2CO3 was added to stop the reaction. The absorbance of the solution (150 μL) was measured at 420 and 550 nm. The β-galactosidase activity (U) was determined using the following formula:
Anti-estrogenic assay
The antagonistic activity of various concentrations of test compounds was determined by measuring the inhibition of 17β-estradiol-induced β-galactosidase activity in the yeasts expressing ERα and ERβ.
Statistical analysis
Each set of experiments was repeated at least three times. Values are expressed as mean ± SEM. One-way analysis of variance followed by Dunnett’s test was used for statistical analysis.
Results
The yeast two-hybrid assay expressing ERα and ERβ was used to investigate the estrogenic activity of the methanol extract and fractions of S. japonica before and after naringinase treatment. Naringinase enzyme is a mixed enzyme of β-glucosidase and α-rhamnosidase activities. This treatment was developed in our laboratory (CitationAhn et al., 2004a) as a partial mimic to the metabolism process (de-glucosylation) which takes place in the gastrointestinal tract (GIT). The naringinase-treated extract and fractions showed more activity than the original compounds.
Further fractionation of the methanol extract of S. japonica afforded a CHCl3 soluble fraction, and 25% (fraction A), 50% (fraction B), and 100% methanol (fraction C) extracts. All the fractions were tested for their estrogenic activity before and after naringinase treatment (). The naringinase-treated 50% methanol fraction (fraction B-NT) exhibited the most potent estrogenic activity (). Both fraction B and fraction B-NT were chemically investigated and the isolated compounds were tested for their estrogenic activity.
Table 1. Induction of β-galactosidase in the yeast two-hybrid assay expressing ERβ by the methanol extract and fractions from S. japonica seeds before and after naringinase treatment (NT).
Fraction B was purified several times over silica gel, ODS, and Sephadex LH-20 columns to produce 10 compounds. After comparing their NMR data with those reported in the literature, the compounds were identified as genistin (1) (CitationKhalid et al., 2003), sophoricoside (2) (CitationMin et al., 1999), sophorabioside (3), sophoraflavonoloside (4), genistein 7,4’-di-O-β-d-glucopyransoide (5) (CitationWatanabe et al., 1993), kaempferol 3-O-α-l-rhamnopyranosyl (1→6) β-d-glucopyranosyl (1→2) β-d-glucopyranoside (6), kaempferol 3-O-β-d-sophoroside-7-O-α-l-rhamnopyranoside (7), kaempferol 3-O-α-l-rhamnopyranosyl (1→6) β-d-glucopyranosyl (1→2) β-d-glucopyranoside 7-O-α-l-rhamnopyranoside (8) (CitationTang et al., 2002), methylgallate (9), and rutin (10) ().
Fraction B-NT afforded kaempferol (11) and genistein (12) as the major components in the extract ().
Estrogenic activity of isolated compounds
In the yeast expressing ERβ (), an appreciable induction of β-galactosidase was found with sophoricoside (2) at a concentration of 10−4 M. Kaempferol (11) and genistein (12) showed estrogenic activity in a concentration-dependent manner.
Table 2. Induction of β-galactosidase in the yeast two-hybrid assay expressing ERα and ERβ by the isolated compounds from S. japonica.
In the yeast expressing ERα (), kaempferol (11) and genistein (12) showed a significant estrogenic activity at 10−4 M, while none of the tested glycosides showed any significant estrogenic activity.
Anti-estrogenic activity of isolated compounds
In the yeast expressing ERβ (), none of the compounds showed any anti-estrogenic activity. In the yeast expressing ERα (), sophoricoside (2), rutin (10), kaempferol (11), and genistein (12) exhibited weak anti-estrogenic activity at a concentration of 10−4 M.
Table 3. Inhibitory effects of the isolated compounds from S. japonica on induction of β-galactosidase activity by 17β-estradiol in yeast two-hybrid assay (ERα and ERβ).
Discussion
Through biologically guided isolation, kaempferol (11) and genistein (12) were found to be the major phytoestrogens of the naringinase-treated 50% MeOH fraction of S. japonica seeds, and are therefore responsible for the estrogenic activity. Chemical investigation of the 50% MeOH extract indicated that kaempferol and genistein are products of enzymatic hydrolysis of their corresponding glycosides (1–10).
Genistein (12) is known for its high activity as an ER agonist. Kaempferol (11) showed a nearly equipotent estrogenic effect to that of genistein on both ER subtypes. The abundance of kaempferol (11) in most medicinal herbs could make it a valuable source of phytoestrogens.
Among the tested glycosides, only sophoricoside (2) showed a weak estrogenic activity on ERβ. The activity of (2) and the inactivity of genistin suggest the importance of a free phenolic group at position 7, in genistein, for estrogenic activity.
It is also noted that the absence of quercetin in the 50% MeOH fraction can be attributed to the presence of rutin in a very low amount in the seeds, which is in accordance with the reported data (CitationBalbaa et al., 1974).
Finally, the marked estrogenic activity of the S. japonica extract and compounds after naringinase treatment indicates the possible activation of this plant and compounds after its oral administration through the action of GIT bacterial enzymes.
Declaration of interest: The authors report no conflicts of interest.
References
- Ahn E-M, Akao T, Nakamura N, Komatsu K, Nishihara T, Hattori M (2004a): Screening of medicinal plant extracts for estrogenic activity in combination with a glycosidase treatment. J Trad Med 21: 81–86.
- Ahn E-M, Nakamura N, Akao T, Nishihara T, Hattori M (2004b): Estrogenic and anti-estrogenic activities of the roots of Moghania philippinensis and their constituents.Biol Pharm Bull 27: 548–553.
- Balbaa SI, Zaki AY, El-Shamy AM (1974): Quantitative and qualitative study of the falvonoid content of the different organs of Sophora japonica at different stages of development. Planta Med 25: 325–30.
- Chan RYK, Chen W-F, Dong A, Guo D, Wong MS (2002): Estrogen-like activity of ginsenoside Rg1 derived from Panax notoginseng. J Clin Endocrinol Metab 87: 3691–3695.
- El-Halawany AM, Chung M, Ma C-M, Komatsu K, Nishihara T, Hattori M (2007a): Anti-estrogenic activity of mansorins and mansonones from the heartwood of Mansonia gagei DRUMM. Chem Pharm Bull 55: 1332–1337.
- El-Halawany AM, Chung M, Nakamura N, Ma C-M, Nishihara T, Hattori M (2007b): Estrogenic and anti-estrogenic activities of Cassia tora phenolic constituents. Chem Pharm Bull 55: 1476–1482.
- Gorbacheva LA, Grishkovets VI, Drozed GA, Chirva VYA (1995): Isolation and characterization of the polysaccharides of Sophora japonica fruits. Chem Nat Comp 31: 596–599.
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P (1986): Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320: 134–139.
- Kanayama T, Mamiya S, Nishihara T, Nishikawa J (2003): Basis of a high-throughput method for nuclear receptor ligands. J Biochem 133: 791–797.
- Khalid M, Ahmed A, Ali A (2003): Chemical constituents and anti-diabetic activity of Trifolium alexandrinum L. Bull Fac Pharm Cairo Univ 41: 253–263.
- Kitaoka M, Kadokawa H, Sugano M, Ichikawa K, Taki M, Takaishi S, Iijima Y, Tsutsumi S, Boriboon M, Akiyama T (1998):Prenylflavonoids: a new class of non-steroidal phytoestrogen (Part 1). Isolation of 8-isopentenylnaringenin and an initial study on its structure-activity relationship. Planta Med 64: 511–515.
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996): Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93: 5925–5930.
- Matsuda H, Shimoda H, Morikawa T, Yoshikawa M (2001): Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): structure-requirement of hydroxyanthraquinones for estrogenic activity. Bioorg Med Chem Lett 11: 1839–1842.
- Milligan S, Kalita J, Heyerick A, Rong H, Coolman L, Keukeleire D (1999): Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J Clin Endocrinol Metab 83: 2249–2252.
- Min B, Oh SR, Lee H-K, Takatsu K, Chang I-M, Min KP, Kim Y (1999): Sophoricoside analogs as the IL-5 inhibitors from Sophora japonica. Planta Med 65: 408–412.
- Mukhamedova KHS, Glushenkova AI (1997): Phospholipids of ripe Sophora japonica seeds. Chem Nat Comp 33: 445–448.
- Nishikawa JM, Saito K, Goto J, Dakeyama F, Matsuo M, Nishihara T (1999): New screening methods for chemicals with hormonal activities using interaction of nuclear hormone receptor with coactivator. Toxicol Appl Pharmacol 154: 76–83.
- Ososki AL, Kenelly EJ (2003): Phytoestrogens: a review of the present state of research. Phytother Res 17: 845–869.
- Rafi MM, Rosen RT, Vassil A, Ho C-T, Zhang H, Ghai G, Lambert G, Dipaola RS (2000): Modulation of bcl-2 and cytotoxicity by licochalcone-A, a novel estrogenic flavonoid. Anticancer Res 20: 2653–2658.
- Tang Y-P, Li Y-F, Hu J, Lou F-C (2002): Isolation and identification of antioxidants from Sophora japonica. J Asian Nat Prod Res 4: 123–128.
- Tang Y, Lou F, Wang J, Zhuang S (2001): Four new isoflavone triglycosides from Sophora japonica. J Nat Prod 64: 1107–1110.
- Watanabe K, Kinjo J, Nohara T (1993): Leguminous plants. XXXIX. Three new isoflavonoid glycosides from Lupinus luteus L. and Polyphyllus arboreus. Chem Pharm Bull 41: 394–396.
- Witkowska HE, Carlquist M, Engstrom O, Carlson B, Bonn T, Gustafsson J-A, Scackleton CHL (1997): Characterization of bacterially expressed rat estrogen receptor β ligand binding domain by mass spectrometry: structural comparison with estrogen receptor α. Steroids 62: 621–631.