Abstract
Conflicting reports are found in the literature about the efficacy of Mirazid® (MZ), which is a special formulation of myrrh obtained from the stem of Commiphora molmol (Nees), Engl. tree (Burseraceae), as an antischistosomal drug. This initiated the present study to further assess this drug in experimental schistosomiasis hematobium. The drug was administered orally to hamsters infected with Schistosoma hematobium (Citation) using 500 mg/kg body weight for six successive days on an empty stomach. The drug effect was examined after three periods: 4, 8 and 12 weeks post-treatment. Emphasis was given to certain parameters such as change in worm load, number of ova/mg tissue, oogram pattern and number of ova/g stool, and tegumental changes in the worms by electron microscopy after prolonged observation periods. The results showed very slight 3.4% worm reduction by MZ after the longest evaluation period (12 weeks), versus very high reduction (100%) by the reference drug praziquantel (PZQ). In comparison with the untreated control no change was found in the number of ova/mg tissue in MZ-treated hamsters regardless of the date of observation (4-12 weeks), versus significantly high reduction (99.6%) observed in the case of PZQ treatment. However, a significant decrease (22%) in the ratio of immature and increase in dead ova in tissues of MZ-treated hamsters was obvious at 12 weeks post treatment. In MZ-treated animals, a slight reduction (18.3%) in the number of stool eggs versus absence of eggs in PZQ-treated animals 12 weeks after treatment. Scanning electron microscopic examination of S. hematobium worms revealed intact tubercles, spines and sensory bulbs and no effect of the ventral side after MZ treatment. Meanwhile, PZQ treatment revealed extensive disruption of the tegument worm. Therefore, this experimental study gives extra support to previously reported negative evaluation about the effectiveness of this drug in the treatment of schistosomiasis against many other published positive results. This controversy about the efficacy of MZ may be attributed to inconsistency of its material which is obtained from natural origin.
Introduction
MZ is a purified extract of myrrh which is an oleo-gum-resin obtained from the stem of the tree Commiphora molmol (Nees) Engl. (Burseraceae) (CitationBadria et al., 2001). Since 2001 this compound has been marketed and claimed a safe effective natural product (CitationMassoud, 1999a, Citation1999b, Citation1999c; CitationBadria et al., 2001; CitationSheir et al., 2001; CitationEl Baz et al., 2003; CitationAbo-Madyan et al., 2004; CitationMassoud et al., 2004, Citation2005), possessing antischistosomal activity against Schistosoma mansoni (CitationSambon, 1907) and S. hematobium with high therapeutic index. CitationBotros et al. (2004) reported that LD50 value of acute toxicity for this drug was 3,139 mg/kg. CitationMassoud et al. (2004) reported that myrrh, which is the main constituent of MZ, has a valuable schistosomicidal effect against different maturation stages of S. mansoni when the drug was given in a daily dose 500 mg/kg body weight for five days. CitationAbo-Madyan et al. (2004) studied the effect of MZ in the treatment of patients with schistosomiasis hematobium and mansoni and found parasitological cure 97.4% and 96.2% after three months, respectively.
On the other hand, CitationBotros et al. (2005) found that MZ has a much lower cure rate, 9.1% and 8.9% in school children and household members, compared with 62.5% and 79.6%, in those treated with praziquantel respectively. Similarly, CitationBarakat et al. (2005) studied the effect of this drug on human schistosomiasis mansoni and found that the cure rate of MZ was 15.6% after one treatment and 8.9% after a second one.
In a trial for more evaluation of such a new drug and better understanding of such reported controversy on its efficacy, MZ was retested using hamsters infected with S. hematobium and its effect was followed for an expanded period of 12 weeks. Moreover, for verification of effect, scanning electron microscopic observations were taken on worms recovered from the treated animals with MZ.
Material and methods
Experimental animals
Fifty-four Syrian hamsters (Mesocricetus auratus) (Waterhouse, 1839), 100-120 g each, were obtained from the Schistosome Biological Supply Center (SBSC), Theodor Bilharz Research Institute, Giza. They were fed on a standard pellet diet containing 24% protein, 4% fat and about 4-5% fiber and water ad libitum. Schistosoma hematobium (Egyptian strain) cercariae were also obtained from SBSC and used to infect the hamsters with 200 cercariae each by abdominal skin exposure. The cercariae were shed from infected Bulinus truncatus (CitationAudouin, 1827) snails and used within one hour from shedding. The maintenance and care during experimentation of animals was compliant with international guidelines for the humane use of laboratory animals.
The drugs
MZ was obtained from the producing company Pharco Pharmaceuticals, Alexandria, Egypt. It was in the form of soft gelatin capsules each containing 300 mg purified Commiphora extract. It was used in a freshly prepared 2% Cremophore El suspension. Praziquantel (PZQ) powder (Sigma, St. Louis, MI, U.S.A.) was also suspended in the same carrier and used as a reference drug (positive control).
Experimental design
Infected hamsters were divided into three main groups, 18 hamsters each. One group was given orally 2% Cremophore El with distilled water and left as an untreated control, a second group was given by gavage 500 mg/kg body weight of MZ for 6 successive days on an empty stomach after overnight fasting and eating allowed after one hour (CitationMassoud, 1999a), and a third group was administrated 250 mg/kg body weight of PZQ for two consecutive days. Each treated group was divided into three subgroups (6 animals each). These were sacrificed after 4, 8, and 12 weeks post treatment in each case.
The efficacy of the drug was assessed by studying the following.
Parasitological parameters
The worms were collected by perfusion from the portal and mesenteric veins of infected untreated and treated animals after decapitation and their number was determined (CitationDuvall & Dewitt, 1967)
The oogram pattern was performed by microscopic examination of press preparations from three intestinal fragments (1 cm each) of infected animals (CitationPellegrino & Faria, 1965). One hundred eggs were systematically counted per fragment and classified into immature, mature and dead eggs.
The egg count in the liver, intestine and urinary bladder tissues was determined by taking a weighed portion, blotted between two filter papers and each placed in a test tube containing 5 mL 5% KOH solution (CitationCheever, 1970). Ova of homogenous emulsions were counted after being spread on slides and number of ova/mg tissues was calculated.
The egg count in the stool was determined by taking fecal samples (1 g) from hamsters in a small test tube and examining microscopically by merthiolate iodine formaldehyde concentration method (MIFC) (CitationBlagg et al., 1955).
Scanning electron microscopy
Immediately after perfusion of hamsters, worms were fixed in 3% glutaraldehyde. The samples were then washed in phosphate buffer and passed into rising concentrations of acetone. They were then mounted on stainless steel holders and subjected to a sputter coat of gold (CitationBricker et al., 1983) and were examined using a Joel JTM-1200 EXII scanning electron microscope at the Faculty of Science, Ain Shams University.
Statistical analysis
Comparison was carried out between the percentage change of treated and untreated (control) groups. Difference between the mean scores of any of the two groups was tested for significance, using an unpaired 2-tailed Student’s t-test. The data were considered significant if p value was less than 0.05.
Results
MZ in 500 mg/kg body weight/day given to S. hematobium infected hamsters on empty stomach for six consecutive days showed no significant change in the recovery of adult worms at all dates of sacrificing (4, 8, and 12 weeks post-treatment) (). The mean number of recovered worms in groups treated with MZ was almost similar to those recovered in the untreated control group while in the hamsters groups treated with PZQ almost no worms were recovered. The reduction of worms in the hamster group treated with MZ remained insignificant after 12 weeks post treatment. Similarly, no significant reduction was obtained in the number of ova/g stool in groups of hamsters treated with MZ and sacrificed 4 and 8 weeks post-treatment. At 12 weeks post treatment, the schistosome ova became somewhat reduced, the reduction in ova/g stool being 18.3%. Meanwhile, highly significant reduction in stool ova was observed in all groups of hamsters treated with PZQ.
Table 1. Worm burden in hamsters infected with Schistosoma hematobium and treated with Mirazid compared to Praziquantel.
Regarding the oogram pattern, no significant difference was observed in immature eggs in groups of hamsters treated with MZ and sacrificed after 4 weeks post-treatment, but the immature ova were found to be significantly lower in groups sacrificed 8 and 12 weeks after treatment. Meanwhile, the ratio of mature ova slightly increased in the group sacrificed 12 weeks post-treatment, being 52% compared with 45% in the control group. Dead ova showed slight increase in tissues of hamsters treated with MZ versus complete disappearance of immature and mature ova in PZQ-treated hamsters. All ova were dead in groups sacrificed 4, 8, and 12 weeks post-treatment. Change with time post-treatment in ratio of immature, mature and dead eggs in animal tissues as well as number of released eggs in stools showed the same patterns in untreated and MZ treated animals (). The mean number of ova/mg tissue of urinary bladder, liver and intestine showed insignificant change in treated hamsters with MZ at 4, 8, and 12 weeks post treatment compared with untreated control groups. Meanwhile, highly significant (P > 0.001) reduction was observed in groups of hamsters treated with PZQ in groups of animals sacrificed 4, 8, and 12 weeks post treatment, respectively ().
Table 2. Oogram pattern and number of ova/g stool in hamsters infected with S. hematobium and treated with Mirazid compared to Praziquantel.
Table 3. The number of ova /mg tissues in hamsters infected with S. hematoubium and treated with Mirazid compared to Praziquantel.
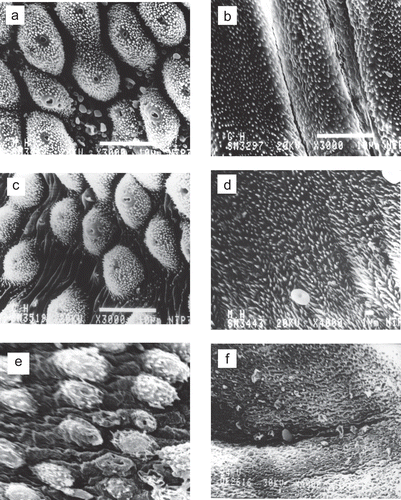
Scanning electron microscopic examination of the tegument of S. hematobium worms () recovered from hamsters sacrificed 12 weeks post treatment with MZ showed intact tubercles, spines and sensory bulbs and no change on the ventral side of the worms. Four weeks post treatment with PZQ, the worm tegument appeared extensively disrupted with shrinkage of tubercles.
Figure 1. Photomicrographs of control untreated and treated male Schistosoma haematobium worms as observed by a scanning electron microscope. (a) Dorsal surface of (control) untreated worms shows intact tubercles, spines and sensory bulbs. (b) Intact ventral surface of untreated control worms. (c) Dorsal surface of worms treated with Mirazid still shows intact tubercles, spines and sensory bulbs at 3 months post-treatment. (d) Intact ventral surface at 3 months Mirazid treatment time. (e) Extensive disruption of the tegument at one month PZQ treatment time. (f) Shrinkage ventral surface of worms at one month PZQ treatment time.
Discussion
The present results show that treatment of hamsters infected with S. hematobium with MZ, using 500 mg/kg body weight on an empty stomach for six consecutive days, showed no curative effect against the parasite after periods as long as 12 weeks from treatment. The present authors (CitationGuirguis & Mahmoud, 2003) previously reported on the same ineffectiveness of this drug in hamsters infected with S. mansoni and S. hematobium after treating with the same dose for 3 days. They found insignificant reduction in worm load, no change in oogram pattern, number of ova/g tissues and mean diameter of liver granuloma were observed in treated animals. The same ineffectiveness was later on claimed by CitationBotros et al. (2004) using mice and hamsters infected with different strains of S. mansoni. These authors claimed that they cannot recommend the use of MZ for treatment of human schistosomiasis. MZ was also used for the treatment of S. mansoni infected Egyptian patients by CitationBotros et al. (2005) and very low cure rates were found (9.1% and 8.9% in S. mansoni infected school children and household members), compared with 62.5% and 79.6% cure rate in those treated with praziquantel, respectively. CitationBarakat et al. (2005) studied the effect of MZ on human schistosomiasis mansoni and found that the cure rate was very low after one treatment (15.6%) and much less (8.9%) by another treatment.
On the other hand, several other authors reported on the efficacy of MZ or its natural form (Myrrh), alone or in the form of combined therapy of its constituents (resin and volatile oil), in the treatment of schistosomiasis using different doses ranging from 5-600 mg/kg body weight. These studies dealt with experimental animals and patients and showed that the cure rate ranged from 91.7% to 97.4% (CitationMassoud, 1999a, Citation1999b, Citation1999c; CitationBadria et al., 2001; CitationSheir et al., 2001; CitationEl Baz et al., 2003; CitationAbo-Madyan et al., 2004; CitationMassoud et al., 2004, Citation2005; CitationSoliman et al., 2004).
In the present work, scanning electron microscopy was utilized to confirm the parasitological results showing no effect on the tubercles, spines and sensory bulbs which appeared still intact as well as the ventral side of the worms. This technique was reported to show tegumental changes in schistosome worms treated by several drugs such as praziquantel (CitationAwadalla et al., 1991; CitationShuhua et al., 2000a; CitationWilliam et al., 2001), oxamniquine (CitationPopeil & Erasmus, 1984), astiban (CitationLeitch & Probert, 1990) and artemether (CitationShuhua et al., 2000b; CitationXiao et al., 2000). These studies revealed that the tegument is a key target of tested drugs. Tegumental alterations included swelling, fusion of tegumental ridges, vacuolization, peeling, erosion and sometimes collapse of the tegument (CitationXiao et al. 2000). The ultrastructural changes are proportional to the concentration of the drug and time elapsed post-treatment (CitationMohamed & Fawzi, 1997). The present experiment provides more support to the negative results of MZ. Thus it increases the doubt about the real effect of this drug. A possible explanation for controversy of various reports about this drug may be the unevenness of its structure, being a product obtained from a natural source.
Acknowledgements
This work has been carried out in the Schistosome Biological Supply Center at Theodor Bilharz Research Institute. The authors thank the scientific and technical team of this facility for the kind help they provided.
Declaration of interest
This work was funded by Theodor Bilharz Research Institute, Warrak ElHadar, Imbaba, P.O.Box 30,Giza 12411, Egypt.
References
- Abo-Madyan AA, Morsy TA, Motawea SM (2004): Efficacy of myrrh in the treatment of schistosomiasis (haematobium and mansoni) in Ezbet El-Bakly, Tamyia Center, EL-Fayoum Governorate Egypt. J Egypt Soc 34: 423–446.
- Audouin V (1827): Explication sommaire des planches des Mollusques dont les dessins ont ete fournis.M.J.C. Savigny pour “L Histoire Naturelle de L ouvrage”, in Description de I Egypt. 2nd ed., XXII,112–117.
- Awadalla HN, El Azzouni MZ, Kalil AI, El Mansoury ST (1991): Scanning electron microscopy of normal and praziquantel-treated S. haematobium worms (Egyptian strain). J Egypt Soc Parasitol 21: 715–722.
- Badria F, Abou-Mohamed G, El-Mowafy A, Massoud A, Salama O (2001): Mirazid: A new schistosomicidal drug. Pharm Biol 39: 127–131.
- Barakat R, Elmorshedy H, Fenwick A (2005): Efficacy of myrrh in the treatment of human schistosmiasis mansoni. Am J Trop Med Hyg 73: 365–367.
- Bilharz T, Bir Beity Zur (1852): Helminthology. Zsc.f.wiss.Zoo 4: 53–72.
- Blagg W, Mansour NS, Khalf GL (1955): A new concentration technique for the demonstration of protozoa and helminthic eggs in feces. Am J Trop Med Hyg 14: 23–28.
- Botros S, William S, Ebeid F, Cioli D, Katz N, Day TA, Bennett JL (2004): Lack of evidence for an antischistosomal activity of myrrh in experimental animals. Am J Trop Med Hyg 71: 206–210.
- Botros S, Sayed H, EL-Dusoki H, Sabry H, Rabie I, El Ghannam M, Hassanein M, EL-Wahab YA, Engels D (2005): Efficacy of Mirazid in comparison with praziquantel in Egyptian Schistosoma mansoni – infected school children and households. Am J Trop Med Hyg 72: 119–123.
- Bricker CS, Depenbusch JW, Bennett JL (1983): Tegumental disruption of Schistosoma mansoni caused by praziquantel and Ro 11-3128. Z fur Parasit 69: 61–71.
- Cheever AW (1970): Quantitative comparison of the intensity of Schistosoma mansoni infection in man and experimental animals. Trans R Soc Trop Med Hyg 63: 781–795.
- Duvall RH, Dewitt WB (1967): An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg 16: 483–486.
- El Baz MA, Morsy TA, El Bandary MM, Motawea SM, (2003): Clinical and parasitological studies on the efficacy of Mirazid in treatment of schistosomiasis haematobium in Tatoon, Etsa Center, El Fayoum Governorate. J Egypt Soc Parasitol 33: 761–776.
- Guirguis FR, Mahmoud SS (2003): On the efficacy of a new antischistosomicidal drug (Mirazid) against Schistosoma haematobium and S. mansoni, an in vivo study. J Egypt Ger Zool 42: 89–98.
- Leitch B, Probert AJ (1990): Schistosoma haematobium: The effect of astiban on the cell composition and ultrastructure of the tegument and gastrodermis. J Helminthol 64: 65–69.
- Massoud AM (1999a): Efficacy of myrrh as a new schistosomicide: An experimental study. Ain Shams Med J 50: 1287–1298.
- Massoud AM (1999b): Mrryh: A schistosomicide for human schistosomiasis haematobium. Ain Shams Med 50: 1383–1399.
- Massoud AM (1999c): Mrryh: A schistosomicide for human schistosomiasis mansoni. Ain Shams Med 50: 1401–1417.
- Massoud AM, El Ebiary FH, Abou-Gamra MM, Mohamed GF, Shaker SM (2004): Evaluation of schistosomicidal activity of myrrh extract: Parasitological and histological study. J Egypt Soc Parasitol 34: 1051–1076.
- Massoud AM, El Ebiary FH, Ibrahim SH (2005): Light microscopic study of effect of new antischistosmal drug (myrrh extract) on the liver of mice. J Egypt Soc Parasitol 35: 971–988.
- Mohamed SH, Fawzi SM (1997): Scanning electron microscopy on adult of Schistosoma mansoni treated in vivo with praziquantel and Ro 15-5458. Qatar Univ Sci J 17: 349–358.
- Pellegrino J, Faria J (1965): The oogram method for the screening of drugs in schistosomiasis mansoni. Am J Trop Med Hyg 14: 363–369.
- Popeil I, Erasmus DA (1984): Schistosoma mansoni: Ultrastructure of adults from mice treated with oxamniquine. Exp Parasitol 58: 254–262.
- Sambon LW (1907): New or little known African entozoa. J Trop Med Hyg 10: 117.
- Sheir Z, Nasr AA, Massoud A (2001): A safe, effective, herbal antischistosomal therapy derived from myrrh. Am J Trop Med Hyg 65: 700–704.
- Shuhua X, Binggui S, Chollet J, Tanner M (2000a): Tegumental changes in adult Schistosoma mansoni harbored in mice treated with praziquantel. Acta Trop 70: 107–117.
- Shuhua X, Binggui S, Chollet J, Utzinger J, Tanner M (2000b): Tegumental changes in adult Schistosoma mansoni harbored in mice treated with artemether. Parasitology 86: 1125–1132.
- Soliman OE, El-Arman M, Abdul-Samie ER, El-Nemr HI, Massoud A (2004): Evaluation of myrrh (Mirazid) therapy in fascioliasis and intestinal schistosomiasis in children: Immunological and parasitological study. J Egypt Soc Parasitol 34: 941–966.
- William S, Botros S, Ismail M, Farghally A, Day TA, Bennett JL (2001): Praziquantel-induced tegumental damage in vitro is diminished in schistosomes derived from praziquantel-resistant infections. Parasitology 122: 63–66.
- Xiao S, Binggi S, Chollet J, Tanner M (2000): Tegumental changes in 21-day-old Schistosoma mansoni harbored in mice treated with artemether. Acta Trop 75: 341–348.
