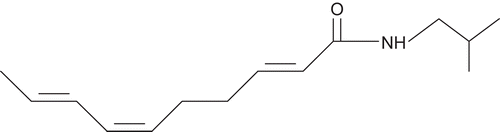
Abstract
Heliopsis longipes S.F. Blake (Asteraceae: Heliantheae) (chilcuague) is used in Mexican traditional medicine against parasites and to alleviate tooth and muscle pains. Its biocide effect has already been experimentally demonstrated; however, its analgesic action and its action on the nervous system (NS) have not been investigated yet. The objectives of this study were to evaluate the analgesic action of affinin and the H. longipes root ethanol extract, as well as their effects on the NS using an animal model. The ethanol extract was obtained by maceration, and affinin was purified from it through chromatographic techniques. Chemical and thermal analgesia were used to assess their analgesic proprieties. Irwin’s test was used to evaluate their stimulating or depressing effects. The ethanol extract and affinin displayed analgesic action similar to ketorolac and stimulating effect comparable to caffeine on the nervous system of adult mice.
Introduction
Heliopsis longipes S.F. Blake (Asteraceae) is a perennial herb endemic to Sierra Gorda and Sierra Alvarez, a mountainous region located in central eastern Mexico, where its root is used as a condiment in sauces and spicy meals because it has a flavor similar to chili; in addition, it is used in traditional medicine to alleviate tooth and muscle pains, and also as an insecticide (CitationLittle, 1948; CitationMartínez, 1967). This species is commonly called “chilcuague”, “chilcuán”, “pelitre”, golden root and Aztec root (CitationMartínez, 1967). An alkamide (), the N-isobutyl-2E,6Z,8E-decatrienamide, named affinin, was isolated from this root (CitationAcree et al., 1945). Since the isolation of this molecule, several studies have been conducted with different species of the same genus, demonstrating that root extracts of H. scabra Dunal, H. parvifolia A. Gray and H. gracilis Nutt. are toxic to the domestic fly (CitationGersdorff & Miltin, 1950). It has been shown that other species of this genus, such as H. aff. novogaliciana B.L. Turner, H. procumbens Hemls. and H. annua Hemls., also contain alkamides in their roots (CitationGarcía-Chávez et al., 2004).
Further studies have evaluated the biocide properties of H. longipes, such as its insecticidal action against the domestic fly and common bean weevil (CitationJacobson et al., 1947; CitationDomínguez et al., 1958). Furthermore, affinin has been found to be effective against mollusks (CitationJohns et al., 1982) and fungi (CitationRamírez et al., 2000), and H. longipes crude root extracts kill a number of bacteria and fungi (CitationGutierrez-Lugo et al., 1996). In contrast to the solid evidence in favor of its biocide action, the use of this root in traditional medicine as local anesthetic has not been experimentally verified. Therefore, in this study we evaluated the analgesic action of affinin and ethanol extract of roots of H. longipes, as well as the effect of affinin and ethanol extract on the nervous system (NS).
Materials and methods
Plant material collection and identification
Heliopsis longipes specimens were gathered in June 2004 in an oak forest (Quercus spp.) in Rioverde, San Luis Potosí, Mexico, at 1,795 m above sea level. The species was authenticated by taxonomist Jose García Pérez, SLPM herbarium curator, and the reference specimen was deposited in the same herbarium (specimen number 41523).
Extract preparation and affinin purification
Roots (100 g) were dried, milled and then macerated with absolute ethanol (Merck, Germany) for one week, all under laboratory conditions. The ethanol of the extract was evaporated in a rotary evaporating device at 60°C under reduced pressure. This extract (3.9 g) was then purified by column chromatography, using silica gel (60 G Merck, Darmstadt, Germany) as adsorbent, and several mixtures of hexane and ethyl acetate (80:20, 70:30, 60:40, 40:60, 50:50 v/v) as solvents. Affinin purification was carried out by means of thin layer chromatography (20 × 20 cm silica gel on glass plates) with a solvent system hexane:ethyl acetate (2:1 v/v). A dark band with Rf 0.5 was collected and re-extracted with ethyl acetate (1.4 g). Affinin presence was demonstrated by thin layer chromatography, and matched with a chemically pure control; the adsorbent used was alumina (60 GF) as fluorescence indicator, along with the solvent system hexane:ethyl acetate 2:1 v/v (CitationMolina-Torres et al., 1996; Citation1999).
Animals
Male albino mice (30 to 33g) were kept under controlled temperature (23 ± 2°C), 12/12 inverted light-darkness cycle, and with free access to water and food. Animals were allowed to adapt to this environment for two weeks before beginning the experiments. Animal handling and care was performed according to the Mexican Official Standard NOM-062-ZOO-1999, which provides technical specifications for the production, care and use of laboratory animals. A different set of animals was used for each test.
Drugs
Chlorpromazine hydrochloride (Rhone-Poulene Pharma, Mexico), ketorolac (Precimex, Mexico), caffeine and acetic acid (Merck), and isotonic solution (Abbott, Mexico) were used.
Analgesic action
Chemical stimulus
The acetic acid method was employed to induce pain in the animals, which is manifested by abdomen stretching and contractions (CitationKoster et al., 1959). Male albino mice were used in groups of seven animals per treatment. Four treatments were evaluated, affinin (1 mg/kg), ethanol extract (10 mg/kg), ketorolac (6 mg/kg) or isotonic solution were administered intraperitoneally (i.p.). Thirty minutes after the administration of the treatments, 3% acetic acid solution was injected i.p., and the number of stretches that occurred over 20 min was recorded. Affinin and ethanol extract doses were chosen based on preliminary toxicity testing.
Thermal stimulus
The hot-plate test consists of the observation of two possible pain reactions in mice after contact with a hot surface: paw licking and/or jumping (CitationEddy & Leimbach, 1953). Male albino mice were used in groups of seven animals per treatment. Four treatments were evaluated, affinin (1 mg/kg), ethanol extract (10 mg/kg), ketorolac (6 mg/kg) or isotonic solution (0.9% NaCl) were administered i.p. The pain reaction to the thermal stimulus was recorded at successive intervals (15, 30, 45, and 60 min) after administration of the treatments. The time elapsed until the first manifestation of pain (paw licking and/or jumping) was recorded after placing them on a plate heated at 55° ± 1°C; the maximum time allowed to the onset of the pain symptom was 30 s.
Irwin’s test
This test (CitationIrwin, 1968) was conducted to assess the stimulating or depressing effects on the NS caused by affinin and the ethanol extract. This is a preliminary neuropharmacological test which allows an indirect quantitative evaluation of a number of neurological, autonomous or toxic changes produced by drugs in each animal. Male albino mice were used in groups of ten animals per treatment. Five treatments were evaluated, affinin (1 mg/kg), ethanol extract (10 mg/kg), caffeine (10 mg/kg) and chlorpromazine (3 mg/kg) as positive controls for stimulating and depressing effects, respectively, and isotonic solution (0.9% NaCl) was administered as a control. The treatments were administered i.p. Each animal was systematically observed and manipulated to measure its reaction to treatments through the presence, duration and intensity of each activity assessed. The observations were performed at 0.5, 1.5, 3.0, 6.0, and 24.0 h after treatment administration; the experiment was carried out during 10 days. The animals were placed in individual cages throughout the evaluation period.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey test for multiple comparisons. P < 0.05 was considered as significant.
Results
Analgesic activity
Chemical stimulus
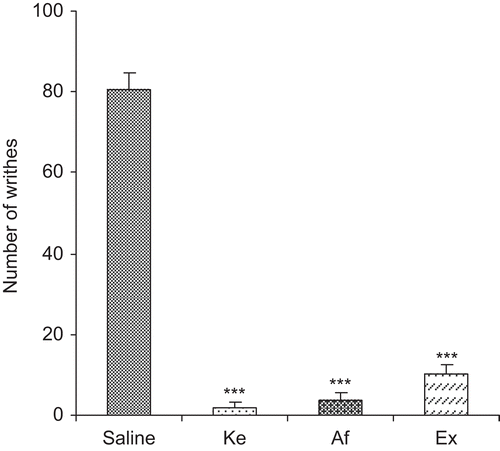
Affinin (1 mg/kg) and the ethanol extract (10 mg/kg), displayed analgesic effect similar to ketorolac (6 mg/kg) by significantly reducing (p < 0.001) the number of abdominal stretches caused by the i.p. administration of 3% acetic acid during the 20-min observation. Both affinin and the ethanol extract inhibited the number of stretches by 95 and 87%, respectively, compared with the control treatment ().
Figure 2. Effect of the i.p. administration of 1 mg/kg affinin (Af) and 10 mg/kg ethanol root extract (Ex) on stretches induced by the i.p. administration of 3% acetic acid in mice (n = 7). Control was administered using 0.9% saline, and the reference analgesic was treated with 6 mg/kg ketorolac (Ke). Results are shown as mean ± SEM. Stretch inhibition and the statistical difference compared to the control is shown as *** p < 0.001.
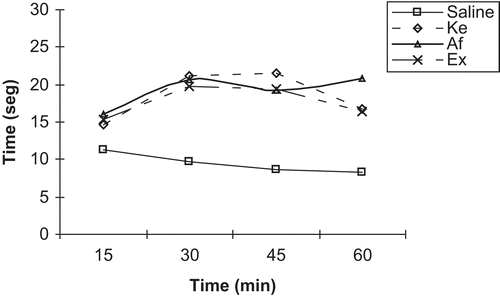
Thermal stimulus
Affinin (1 mg/kg) and the ethanol extract (10 mg/kg) displayed analgesic action to thermal stimuli, since both increased the time to the onset of pain symptoms. This analgesic effect was evident 15 min after its administration, increasing 30 min post-treatment and continuing throughout the duration of the experiment (60 min) with only a slight decline ().
Irwin’s test
Some significant differences were observed in the behavior of animals with the i.p. administration of affinin and the ethanol extract, compared to control, caffeine (stimulant) and chlorpromazine (depressant). Thus, 1.5 h after the administration of affinin and the ethanol extract, an increase in the spontaneous motor activity, alertness, irritability, uneasiness, exophthalmus, palpebral opening and passivity was observed. These differences peaked in animals which received affinin compared with those treated with the ethanol extract. In parallel, a decrease in the reaction to touch and noise in animals administered with affinin and ethanol extract was noted. These effects were not observed at 24 h after treatment administration ().
Table 1. Overall activity of mice observed during Irwin’s test.
Discussion
In Mexico, the root of H. longipes is used as a condiment and to ease tooth and muscular pains in traditional medicine; our results confirm that both affinin and the ethanol extract showed analgesic and also stimulating actions.
The antinociceptive effect of H. longipes was evaluated with the acetic acid and hot-plate tests, which are used to detect narcotic and non-narcotic analgesia, respectively. Since the acetic acid test is used to evaluate peripheral analgesic effects of drugs (CitationKoster et al., 1959), this study gives evidence that affinin and the ethanol extract have analgesic action by significantly reducing the nociceptive reactions (abdominal stretching) caused by the chemical stimulus. The administration of acetic acid causes pain and inflammation in the peritoneal area (CitationFerreira et al., 2004), which in turn induces the abdominal stretching syndrome due to the rise in the release of prostaglandins E2 and F2 (CitationDeraedt et al., 1980; CitationFerreira et al., 2004; CitationBentley et al., 1983; CitationBerkenkopf & Weichman, 1988), and of catecholamine neurotransmitters (CitationDuarte et al., 1988) in the peritoneal fluid after the administration of acetic acid. A possible action of the treatments is that these chemical mediators are altered. In this respect, affinin is an alkamide, and it has been found that alkamides can inhibit the production of PGEs (Prostaglandins) (CitationLaLone et al., 2007). Given that the abdominal contraction relates to the sensitization of prostaglandin-nociceptive receptors and of sympathetic nervous system mediators, it is likely that affinin and the ethanol extract inhibit either their synthesis or the contact with their receptors, thereby producing the analgesic effect. The analgesic effect of H. longipes has been previously assessed by the acetic acid test (CitationOgura et al., 1982), finding a maximum inhibition (50.3%) with 50 mg/kg of ethanol extract, and with 10 mg/kg of affinin (68% inhibition). In this work, higher inhibition (95% with affinin and 87% with the ethanol extract) was recorded at lower doses, probably due to the i.p. route of administration.
The analgesic effect on the NS was also recorded by means of the hot-plate test, since in this test an analgesic effect is observed only when central pain receptors are inhibited (CitationEddy & Leimbach, 1953; CitationOjewole, 2006). Affinin and ethanol extract displayed an analgesic effect by delaying the reaction to pain in the mouse (paw licking and/or jumping) after being exposed to a thermal stimulus; affinin produced a higher analgesic effect than ethanol extract, by achieving longer delays in the onset of pain signals. Recently, it has been found that affinin can induce GABA (gamma-Aminobutyric acid) release in mouse brain slices (CitationRíos et al., 2007). Thus, the analgesic effect of affinin in the hot-plate test may be due to its action on the spinal cord, on higher central nervous system levels, or by an indirect mechanism (CitationYaksh & Rudy, 1977).
Finally, CitationIrwin’s test (1968) is a sensitive assay for quantifying a wide variety of drug-related behavioral changes in experimental animals, which may be neurological, autonomic or toxic. To test these effects, animals are systematically observed and handled to measure the duration and intensity of any changes caused by the test substance (CitationMorales et al., 2001). In this test, the H. longipes ethanol root extract and affinin showed a stimulating effect, by increasing mouse activity, as well as a depressing effect, by reducing the reaction to touch and noise. These reactions offer an explanation for the traditional use of H. longipes root as a condiment and in chili sauces; given that it has a pungent taste which stimulates salivary secretion, while reducing the sensitivity of the tongue at the same time (CitationLittle, 1948; CitationMartínez, 1967).
It has been postulated that affinin is responsible for the traditional uses of H. longipes (CitationRamírez et al., 2000); affinin belongs to alkamides which are used as flavoring agents and as medicinal compounds (CitationMolina-Torres & García, 2001). Alkamides are present in some species of Asteraceae, especially in the Heliantheae and Anthemidae tribes, as well as in some species of Solanaceae and Piperaceae (CitationHegnauer, 1977; CitationMolina-Torres & García, 2001). All alkamides present in Asteraceae show similar characteristics, possess insecticidal properties, have pungent flavor, stimulate salivary secretion, and are used in traditional medicine for their analgesic action. Such is the case of affinin present in Wedelia parviceps Blake, Spilanthes oleraceae Jacq (CitationJohns et al., 1982), Acmella ciliata H.B.K., A. oleracea L., A. oppositifolia (Lam.) Jansen and H. longipes S.F. Blake (CitationJacobson et al., 1947; CitationJohns et al., 1982; CitationMolina-Torres et al., 1999), of spilanthol present in some species of the genus Sphilantes (CitationJohns et al., 1982), and of pellitorine present in Anacyclus pyrethrum DC (CitationGulland & Hopton, 1930). All these Asteraceae species are used as analgesics in traditional medicine in Mexico (CitationLittle, 1948; CitationMartínez, 1967), Belize (CitationArson et al., 1980), the Middle East (CitationJohns et al., 1982), and Africa (CitationGulland & Hopton, 1930).
In conclusion, this work provides evidence that affinin and ethanol extract of H. longipes roots display analgesic and also stimulating effects on NS. These findings would explain the use of H. longipes root as a condiment and as an anesthetic in traditional medicine in Mexico.
Acknowledgements
The authors thank S. Jasso-Espino for technical assistance, and Esther Jiménez-Capdeville, J.C. García López and R.I. Yeaton, who suggested improvements to earlier versions. V.G. Cilia-López was the recipient of a scholarship (172443) from CONACYT (Consejo Nacional de Ciencia y Tecnología) (Mexico).
Declaration of interest: The authors have no conflict of interest. This research was funded by Universidad Autónoma de San Luis Potosí.
References
- Acree F, Jacobson M, Haller HL (1945): An amide possessing insecticidal properties from the roots of Erigeron affinis DC. J Org Chem 10: 236–242.
- Arson T, Uck F, Lambert J, Hebda R (1980): Maya medicinal plants of San Jose Succotz, Belize. J Ethnopharmacol 2: 345–364.
- Bentley GA, Newton SH, Starr J (1983): Studies on the antinociceptive action of α-agonist drugs and their interactions with opioid mechanisms. Br J Pharmacol 79: 125–134.
- Berkenkopf JW, Weichman BM (1988): Production of prostacyclin in mice following intraperitoneal injection of acetic-acid, phenylbenzoquinone and zymosan; its role in the writhing response. Prostaglandins 36: 693–709.
- Deraedt R, Jougney S, Delevalcee F, Falhout M (1980): Release of prostaglandin E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 51: 17–24.
- Domínguez JA, Leal DG, Viñales DMA (1958): Síntesis de N-isopropil y N-isobutilamida de algunos ácidos y comparación de su acción insecticida con la afinina. Ciencia 17: 213–216.
- Duarte JDG, Nakamura M, Ferreira SH (1988): Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res 21: 341–343.
- Eddy NB, Leimbach D (1953): Synthetic analgesic II. Dithienylbuteryl and dithienylamines. J Pharmacol Exp Ther 107: 385–393.
- Ferreira MAD, Nunes ODRH, Fontenele JB, Pessoa ODL, Lemos TLG, Viana GSB (2004): Analgesic and anti-inflammatory activities of a fraction rich in oncocalyxone A isolated from Auxemma oncocalyx. Phytomedicine 11: 315–322.
- García-Chávez A, Ramírez-Chávez E; Molina-Torres J (2004): El género Heliopsis (Heliantheae: Astercaeae) en México y las alcamidas presentes en sus raíces (The genus Heliopsis (Heliantheae: Asteraceae) in Mexico and the alkamides of its roots). Act Bot Mex 69: 115–131.
- Gersdorff WA, Miltin N (1950): Insecticidal action of American species of Heliopsis. J Econ Ent 43: 554–555.
- Gulland JM, Hopton GU (1930): Pellitorine, the pungent principle of Anacyclus pyrethrum. J Org Chem 132: 6–11.
- Gutierrez-Lugo MT, Barrientos BT, Luna, B, Ramírez GRM, Bye R, Linares E, Mata R (1996): Antimicrobial and cytotoxic activities of some crude drug extracts from Mexican medicinal plants. Phytomedicine 2: 341–347.
- Hegnauer R (1977): The chemistry of the Compositae, in: Heywood VH, Harborne JB, Turner BL, eds, The Biology and Chemistry of the Compositae. New York, NY, USA: Academic Press. pp. 283–335.
- Irwin S (1968): Comprehensive observational assessment. 1a. A systematic quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacology 13: 22–257.
- Jacobson M, Acree F Jr, Haller HL (1947): Correction of source affinin (N-isobutyl-2, 6, 8-decatrienoamide). J Org Chem 12: 731–732.
- Johns T, Graham K, Towers GHN (1982): Molluscicidal activity of affinin and other isobutylamides from the Asteraceae. Phytochemistry 21: 2737–2738.
- Koster R, Anderson M, De Beer EJ (1959): Acetic acid for analgesic screening. Fed Proc 18: 412–417.
- LaLone CA, Hammer KDP, Wu L, Bae J, Leyva N, Liu Y, Solco AKS, Kraus GA, Murphy PA, Wurtele ES, Kim OK, Seo KII, Widrlechner MD, Birt DF (2007): Echinacea species and alkamides inhibit prostaglandin E2 production in RAW264.7 mouse macrophage cells. J Agric Food Chem 55: 7314–7322.
- Little EL (1948): Heliopsis longipes a Mexican insecticidal plant species. J Wash Acad Sci 38: 269–274.
- Martínez M (1967): Las Plantas Medicinales de México. México, Botas, pp. 113–115.
- Molina-Torres J, Salgado-Garciglia R, Ramírez-Chávez E, del Río RE (1996): Purely olefinic alkamides in Heliopsis longipes and Acmella (Sphilanthes) oppositifolia. Biochem Syst Ecol 24: 43–47.
- Molina-Torres J, García CA, Ramírez CE (1999): Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: Affinin and capsaicin. J Ethnopharmacol 64: 241–248.
- Molina-Torres J, García CA (2001): Alcamidas en plantas: Distribución e importancia. Avance y Perspectiva 20: 377–387.
- Morales CM, Gómez SP, Iglesias I, Villar FAM (2001): Neuropharmacological profile of ethnomedicinal plants of Guatemala. J Ethnopharmacol 76: 223–228.
- Ogura M, Cordell GA, Quinn ML, Leon C, Benoit PS, Soejarto DD, Farnsworth NR (1982): Ethnopharmacologic studies. I. Rapid solution to a problem oral use of Heliopsis longipes by means of a multidisciplinary approach. J Ethnopharmacol 64: 215–219.
- Ojewole JAO (2006): Antinociceptive, anti-inflammatory and antidiabetic properties of Hypoxis hemerocallidea Fisch. & C.A. Mey. (Hypoxidaceae) corm [“African potato”] aqueous extract in mice and rats. J Ethnopharmacol 103: 126–134.
- Ramírez CE, Lucas V L, Virgen C G, Molina TJ (2000): Actividad fungicida de la afinina y del extracto crudo de raíces de H. longipes en dos especies de Sclerotium. Agrociencia (Fungicide action of affinin and the H. longipes raw extract on two species of Sclerotium). 3: 207–215.
- Ríos MY, Aguilar-Guadarrama B, Gutiérrez MC (2007): Analgesic activity of affinin, an alkamide from Heliopsis longipes (Compositae). J Ethnopharmacol 110: 364–367.
- Yaksh TL, Rudy TA (1977): Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther 2002: 411–428.