Abstract
A simple one-step purification using liquid–liquid extraction for preparing pomegranate peel extract rich in ellagic acid has been demonstrated. The method involved partitioning of the 10% v/v water in methanol extract of pomegranate peel between ethyl acetate and 2% aqueous acetic acid. This method was capable of increasing the ellagic acid content of the extract from 7.06% to 13.63% w/w. Moreover, the antioxidant activity of the extract evaluated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay was also increased (ED50 from 38.21 to 14.91 μg/mL). Stability evaluations of the ellagic acid-rich pomegranate peel extract in several conditions through a period of four months found that the extracts were stable either kept under light or protected from light. The extracts were also stable under 4° ± 2°C, 30° ± 2°C and accelerated conditions at 45°C with 75% relative humidity. However, study on the effect of pH on stability of the extract in the form of solution revealed that the extract was not stable in all tested pH (5.5, 7 and 8). These results indicated that the ellagic acid-rich pomegranate peel extract was stable when it was kept as dried powder, but it was not stable in any aqueous solution.
Introduction
Pomegranate [Punica granatum L. (Punicaceae)] fruit peel, a by-product of the pomegranate juice industry, is a rich source of hydrolysable tannin belonging to ellagitannins. It is therefore an inexpensive and abundant source of ellagitannins. A wide range of clinical applications of pomegranate fruit peel extract for the treatment and prevention of cancer, as well as other diseases where chronic inflammation is believed to play an essential etiologic role, is suggested (CitationLansky & Newman, 2007). The reports have revealed that pomegranate fruit peel extract is a potential antioxidant agent for nutraceutical and cosmetic applications (CitationAguilar et al., 2008). Ellagic acid is considered as an indicative marker for standardization of the fruit peel extract (CitationZhou et al., 2008) and as a biomarker for human bioavailability studies involving consumption of ellagitannins from food sources (CitationSeeram et al., 2004). In addition, ellagic acid also has the benefit of being anti-inflammatory (CitationOgawa et al., 2002), anti-allergy (CitationRogerio et al., 2008), anti-cancer (CitationLarrosa et al., 2006; CitationMertens-Talcott & Percival, 2005), reducing microbial growth (CitationZambuchini et al., 2008), inhibiting many toxic effects caused by phospholipase A2 (CitationDa Silva et al., 2008) and a protective effect against testicular toxicity (CitationTurk et al., 2008). Thus, a method for ellagic acid enrichment in pomegranate fruit peel extract is needed. As part of our interest in a simple purification method that can improve the ellagic acid content in pomegranate fruit peel extract, liquid–liquid extraction method was used to prepare ellagic acid-rich pomegranate fruit peel extract. Stability of the extract was also studied in order to get useful information for future studies on development of herbal products from the extract.
Materials and methods
Plant material
Pomegranate fruits were collected from Mengzhi pomegranate garden, Yunnan, China, in August 2006. The voucher specimen (no. SKP 158 16 07 01) was identified by Pharkphoom Panichayupakaranant and deposited at the herbarium of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand. The fruit peels were dried at 50°C for 24 h in a hot air oven and were reduced to powder using a grinder and a no. 45 sieve.
Chemicals and reagents
Standard ellagic acid and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Fluka (Buchs, Switzerland). Methanol (High Performance Liquid Chromatography (HPLC) grade and analytical grade) and ethyl acetate (analytical grade) were purchased from Labscan Asia (Bangkok, Thailand). Acetic acid was from J.T. Baker (Phillipsburg, NJ, USA). Water was purified in a Milli-Q system (Millipore, Bedford, MA).
Preparation of ellagic acid-rich pomegranate fruit peel extract
The dried powder of pomegranate fruit peel (0.5 kg) was extracted with methanol containing 10% (v/v) water (2 L) under reflux conditions for 1 h (× 2). The pooled extracts were dried in vacuo. The extract (4 g) was then suspended in 2% aqueous acetic acid (400 mL) and partitioned with ethyl acetate (400 mL × 4). The pooled ethyl acetate fractions were then evaporated to dryness in vacuo.
Quantitative analysis of ellagic acid
HPLC conditions and calibration curves
HPLC analysis was carried out using Agilent 1100 series equipped with Photodiode-array detector (PDA) and autosampler. Data analysis was performed using Agilent 3D ChemStation software (Agilent, Santa Clara, CA, USA). Separation was achieved at 25°C on a 150 mm × 4.6 mm TSK-gel ODS-80Tm column. The mobile phase consisted of methanol and 2% aqueous acetic acid with gradient mode elution (0-15 min, 40-60% v/v methanol and 15-20 min, 60% v/v methanol) at a flow rate of 1 mL/min. The injection volume was 20 μL. The quantitation wavelength was set at 254 nm. The calibration curve was established from the standard ellagic acid at a concentration between 3-50 μg/mL. The linear equation of Y = 139159X + 26.146 (r2 = 0.9995) corresponds to ellagic acid.
Sample preparation
The extracts were accurately weighed to 5 mg and diluted to 10 mL volumetric flask with methanol. The solutions were filtered through 0.45 μm membrane filter and subjected to HPLC analysis.
In vitro antioxidant assay
The antioxidant activity of the extracts and fractions was determined according to DPPH radical scavenging assay (CitationYamasaki et al., 1994). Briefly, the stock solution (1 mg/mL) of the sample was prepared in absolute ethanol. Five concentrations of the sample were produced by two-fold dilutions. A portion of the sample solution (0.1 mL) was mixed with the same volume of 6 × 10−5 M DPPH in absolute ethanol. After the mixture had been allowed to stand for 30 min at room temperature, its absorbance was measured at 520 nm using a spectrophotometer (Milton Roy Spectronic Genesys 5, Champaign IL, USA). The scavenging activity of the sample against DPPH radicals was calculated according to the following equation: DPPH radical scavenging activity (%) = (1 − absorbance of sample/absorbance of control) × 100. A mixture of absolute ethanol (500 μL) and 6 × 10−5 M DPPH in absolute ethanol (500 μL) was used as the control. Te dose response curve was plotted between inhibition and concentrations. Linear regression analysis was carried out calculating the effective concentration of the sample required to scavenge DPPH radical by 50% (EC50). Ellagic acid and quercetin (Sigma, St. Louis, MO, USA) were used as the positive control. All tests were carried out in triplicate.
Stability evaluation
Effect of light on stability of the extract
The ellagic acid-rich pomegranate fruit peel extracts were weighed to 100 mg and kept in well-closed containers. The extracts were then stored at room temperature (30° ± 2°C) either protected from light or exposed to light for 4 months. An aliquot of each sample was taken at 0, 1, 2, 3, 4, 6, 8, 12, and 17 weeks and subjected to quantitative analysis of the ellagic acid content using HPLC. The experiments were done in triplicate.
Effect of temperature on stability of the extract
The ellagic acid-rich pomegranate fruit peel extracts were weighed to 100 mg and kept in well-closed containers and protected from light. The extracts were then stored at 4° ± 2°C and at room temperature (30° ± 2°C) for 4 months. An aliquot of each sample was taken at 0, 1, 2, 3, 4, 6, 8, 12, and 17 weeks and subjected to quantitative analysis of the ellagic acid content using HPLC. The experiments were done in triplicate.
Effect of accelerated conditions for stability of the extract
The ellagic acid-rich pomegranate fruit peel extracts were divided into aliquots of 100 mg and were kept in well-closed containers and protected from light. The extracts were then stored in a stability chamber at 45°C, 75% humidity for 4 months. An aliquot of each sample was taken at 0, 1, 2, 3, 4, 6, 8, 12, and 17 weeks and subjected to quantitative analysis of the ellagic acid content using HPLC. The experiments were done in triplicate.
Effect of pH on stability of the extract
The ellagic acid-rich pomegranate fruit peel extracts were accurately weighed into aliquots of 100 mg and dissolved in phosphate buffer solution to achieve pH value of 5.5, 7, and 8. The sample solutions were kept in well-closed containers and protected from light and stored at room temperature (30° ± 2°C) for 4 months. An aliquot of each sample was taken at 0, 1, 2, 3, 4, 6, 8, 12, and 17 weeks and subjected to quantitative analysis of the ellagic acid content using HPLC. The experiments were done in triplicate.
Statistics
Values are expressed as mean ± SD. Data were analyzed by student t-test. The level of statistical significance was taken as P < 0.05.
Results and discussion
Preparation of ellagic acid-rich pomegranate fruit peel extract
After fractionation of the pomegranate fruit peel extract using liquid–liquid extraction between ethyl acetate and 2% aqueous acetic acid, the ellagic acid-rich extract was obtained from the ethyl acetate fraction with a yield of 16.56 ± 1.132% w/w compared to the weight of dried powder (). The appearance of the extract was changed from a dark brown semisolid of the crude extract to a brown powder of the ellagic acid-rich extract. On the basis of HPLC analysis, only ellagic acid was found as the major compound in the extract (). This method was capable of increasing the ellagic acid content in the extracts from 7.06 ± 0.025% to 13.63 ± 0.89% w/w as well as the antioxidant activity (). Preliminary studies on fractionation of the pomegranate fruit peel extract found that liquid–liquid extraction between ethyl acetate and water was an appropriate method for a preparation of the high antioxidant potency extract of pomegranate fruit peel. However, the result from this study indicated that the use of 2% aqueous acetic acid instead of water in the liquid–liquid extraction process produced an extract with a higher ellagic acid content and antioxidant activity (). This is due to a suppression of ionization of ellagic acid in aqueous acidic conditions that was capable of decreasing the water solubility of ellagic acid. Hence it is more soluble in ethyl acetate. According to the satisfactory antioxidant activity of the ellagic acid-rich pomegranate extract, the extracts used in further studies were standardized to an ellagic acid content not less than 13% w/w.
Figure 1. HPLC-chromatograms of the solution of ellagic acid-rich pomegranate extract (pH 8) at the initial time (A) and after keeping for 10 weeks (B).
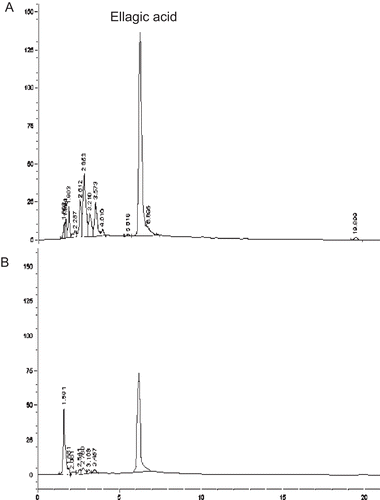
Table 1. Extraction yield, ellagic acid content and antioxidant activity of the ellagic acid-rich pomegranate fruit peel extract.
Table 2. Ellagic acid content and antioxidant activity of the ethyl acetate fractions obtained from liquid-liquid extractions.
Stability evaluation
Ellagic acid was used as the marker for monitoring the chemical stability of the ellagic acid-rich pomegranate fruit peel extract.
Study on the effect of light on the stability of the extract demonstrated that the physical appearances (color and solidity) of the extracts were similar, and were unchanged throughout the period of 4 months. In addition, the ellagic acid contents of the extracts kept in both conditions were not significantly decreased through the period of 4 months. Normally, antioxidant compounds can degrade in light conditions, because the antioxidant compounds are oxidized when they are exposed to light. From this result, light has no effect on the ellagic content of the extracts. This may be due to the other polyphenolic compounds or ellagitannins in the extract that may play an important role as antioxidant for ellagic acid.
Study on the effect of temperature on the stability of the extract showed that both tested temperatures did not affect either the physical appearance of the extracts or ellagic acid content through the period of 4 months. It also implies that the ellagic acid-rich pomegranate fruit peel extract is stable at temperatures between 4°C and 30°C at least in the period of 4 months.
Study of the effect of accelerated conditions on the stability of the extracts showed that their physical appearances as well as the ellagic acid contents of the extracts were unchanged even when stored under accelerated conditions in the period of 4 months. This result implies that the ellagic acid-rich pomegranate fruit peel extract should be stable for at least two years when kept in a well-closed container and stored at room temperature.
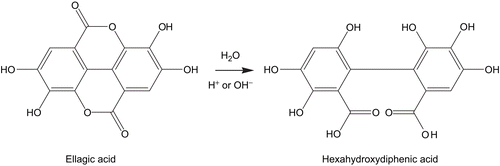
The acid-base stability evaluation of the ellagic acid-rich pomegranate fruit peel extract in the solution was determined at three different pH including 5.5, 7, and 8. It was found that at pH 5.5 the solution was yellow, at pH 7 the solution was brownish-yellow and at pH 8 the solution was brown. Unfortunately, in all tested pH, the ellagic acid content of the extract was significantly decreased after keeping for four weeks (). The remaining percentage of ellagic acid in the extract after 4 weeks of storage was significantly lower than the initial time. In these conditions, HPLC chromatograms showed the main peak of the degraded polar compound with the retention time of 1.6 min (). The instability of ellagic acid in the solution should be considered as their hydrolysis of the ellagic acid (). The ester group of ellagic acid was hydrolyzed to hexahydroxyphenic acid in aqueous, acid and base solution. Thus, application of the ellagic acid-rich pomegranate fruit peel extract in an aqueous solution should be performed carefully.
Table 3. Ellagic acid content of the ellagic acid-rich pomegranate fruit peel extract in the solution at pH 5.5, 7 and 8.
The results from these stability tests indicate that the ellagic acid-rich pomegranate fruit peel extract possesses satisfactory stability for further development of herbal medicines. However, an application as aqueous solution should be avoided.
Acknowledgements
The authors wish to thank the National Research Council of Thailand and the Graduate School, Prince of Songkla University, for support in the form of a research grant.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aguilar CN, Aguilera-Carbo A, Robledo A, Ventura J, Belmares R, Martinez D, Rodríguez-Herrera R, Contreras J (2008): Production of antioxidant nutraceuticals by solid-state cultures of pomegranate (Punica granatum) peel and creosote bush (Larrea tridentata) leaves. Food Technol Biotechnol 46: 218–222.
- Da Silva SL, Calgarotto AK, Chaar JS, Marangoni S (2008): Isolation and characterization of ellagic acid derivatives isolated from Casearia sylvestris SW aqueous extract with anti-PLA2 activity. Toxicon 52: 655–666.
- Lansky EP, Newman RA (2007): Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol 109: 177–206.
- Larrosa M, Tomas-Barberan FA, Espin JC (2006): The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem 17: 611–625.
- Mertens-Talcott SU, Percival SS (2005): Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett 218: 141–151.
- Ogawa Y, Kanatsu K, Iino T, Kato S, Jeong Y, Shibata N, Takada K, Takeuchi K (2002): Protection against dextran sulfate sodium-induced colitis by microspheres of ellagic acid in rats. Life Sci 71: 827–839.
- Rogerio AP, Fontanari C, Borducchi E, Keller AC, Russo M, Soares EG, Albuquerque DA, Faccioli LH (2008): Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur J Pharmacol 580: 262–270.
- Seeram NP, Lee R, Heber D (2004): Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta 348: 63–68.
- Turk G, Ateşşahin A, Sonmez M, çeribaşi AO, Yuce A (2008): Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil Steril 89: 1474–1481.
- Yamasaki K, Hashimoto A, Kokusenya Y, Miyamoto T, Sato T (1994): Electrochemical method for estimating the antioxidative effects of methanol extracts of crude drugs. Chem Pharm Bull 42: 1663–1665.
- Zambuchini B, Fiorini D, Verdenelli MC, Orpianesi C, Ballini R (2008): Inhibition of microbiological activity during sole (Solea solea L.) chilled storage by applying ellagic and ascorbic acids. LWT - Food Sci Technol 41: 1733–1738.
- Zhou B, Wu Z, Li X, Zhang J, Hu X (2008): Analysis of ellagic acid in pomegranate rinds by capillary electrophoresis and high- performance liquid chromatography. Phytochem Anal 19: 86–89.